Abstract
Transitional areas between ecosystem types are often active biogeochemically due to resource limitation changes. Lotic-to-lentic transitions in freshwaters appear active biogeochemically, but few studies have directly measured nutrient processing rates to assess whether processing within the rivermouth is important for load estimates or the local communities. We measured oxic fluxes of inorganic nitrogen and phosphorus and dissolved organic carbon (DOC) from sediments in two rivermouths of Green Bay (Lake Michigan, USA). Soluble reactive phosphorus (SRP) flux was positive in most cases (overall mean 1.74 mg SRP m− 2 day− 1), as was ammonium (NH4) flux (40.6 mg NH4 m− 2 day− 1). Partial least square regression (PLSR) indicated a latent variable associated with both sediment [loosely bound phosphorus (P), iron bound P, organic content] and water column properties [temperature, DOC:dissolved inorganic nitrogen (DIN) and DOC:SRP ratios (negatively)] that was moderately associated with variation in SRP flux. PLSR analysis also indicated several sediment characteristics were moderately related to NH4 flux, especially organic content, density (negative), and porosity. Flux of nitrates/nitrites (NOX) and DOC were positively associated with the water column concentrations of NOX and DOC and qualitative estimates of the labile, non-humic types of DOC. In early summer, water column NOX and DOC concentrations were high and labile DOC may have fueled denitrification, resulting in net flux into sediments of both NOX and DOC. By late summer, water column NOX and DOC were very low and both these constituents were fluxing out of sediments into the water column. Based on our estimates for the entire period from April through September, rivermouth sediments were a net source of SRP and DIN, with a DIN:SRP ratio of ~ 44 and a NH4:NOX > 1. We estimated that the sediments in the Fox rivermouth probably contributed a small proportion of the total Fox River load during the growing season 2016 (< 5%), but at times may have contributed as much as 14% of the daily load. Despite the small size of the Fox rivermouth (< 0.5% of the watershed area), these results indicate that at times sediments can contribute substantially to the overall delivery of nitrogen and phosphorus to the nearshore zone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transitional areas between ecosystems are often locations of high biological activity (Schade et al. 2005). Rivermouths and freshwater estuaries are zones of transition from lotic to lentic conditions that experience abrupt shifts in environmental conditions. Primary production in rivers is often limited by light (due to shading from in-stream turbidity and riparian vegetation) or flow conditions (due to short residence times and disturbance) (Hilton et al. 2006). By contrast, nutrients often limit productivity in lentic ecosystems, especially large lakes (Wetzel 2001; Sterner 2008). Rivermouths sit in the transition zone between rivers and lentic systems, where sediments are deposited due to slowing water velocities but soluble nutrient concentrations remain high (Dila and Biddanda 2015; Larson et al. 2016).
In the Laurentian Great Lakes, regions experiencing the most obvious signs of cultural eutrophication (e.g., Green Bay, Saginaw Bay, western Lake Erie) drain agricultural watersheds (see discussions in Great Lakes Interagency Task Force 2014; Annex 4 Task Team 2015). These agricultural watersheds are the source of elevated loads of important nutrients such as nitrogen (N), phosphorus (P) and organic carbon (OC), which appear to drive annual variation in cyanobacterial bloom frequency and intensity (Bertani et al. 2016; Newell et al. 2019). By contrast, the open waters of these large lakes have become increasingly nutrient-poor, creating steep gradients in solute concentrations across the river-to-lake transition zone (Evans et al. 2011; Larson et al. 2013, 2016). In addition to being the location of these steep gradients in solute concentrations, rivermouths themselves appear to transform, retain, and release nutrients (Krieger 2003; Morrice et al. 2004; Marko et al. 2013). In a survey of 23 rivermouths, Larson et al. (2016) used water mixing models and found that 8 rivermouths had a substantial impact on N, P, or dissolved organic carbon (DOC) concentrations. These ‘high effect’ rivermouths drained watersheds with high amounts of row-crop agriculture. However, most river load estimates are made at gages that occur well upstream from the rivermouth (by design) and so do not capture these rivermouth-induced changes in nutrient loads. One exception is the Fox rivermouth entering Green Bay, where load estimates are made at the coastline and account for seiche-induced bi-directional flows (Robertson et al. 2018).
Release rates of P from sediments have been reported in rivermouths of the Great Lakes (Steinman and Ogdahl 2012; Steinman et al. 2012, 2014) particularly as they relate to potential for eutrophication. On the other hand, measurements of sediment release rates of ammonium (NH4), nitrates/nitrites (NOx) and DOC are less commonly reported. Even if nutrient release from sediments is a small portion of the total flux to the nearshore zone, the transient storage of watershed-derived sediment in rivermouth areas and subsequent release to the overlying water column may influence the ecology of rivermouth and nearshore areas (Withers and Jarvie 2008; Sharpley et al. 2013). Harmful algal blooms, for example, occur in late summer when tributary loading is low, so sediment nutrient fluxes may make up a larger proportion of the available nutrients at that time (Robertson et al. 2018). The availability and form of N and P seems to strongly influence the development of phytoplankton communities and the ability of cyanobacteria to produce cyanotoxins (Davis et al. 2010; Glibert 2017). At the same time, dissolved organic matter (DOM) quantity and quality regulate light availability and competitive interactions between heterotrophs and autotrophs (Williamson et al. 1999; Stutter et al. 2018). Here, our objective was to examine fluxes of soluble reactive phosphorus (SRP), NH4, NOX and DOC between sediments and the overlying water column in and around the mouth of the Fox River, which is the major source of P and other nutrients to Green Bay (Lake Michigan; Klump et al. 1997). We also qualitatively estimated changes in DOM composition using optical properties to assess the types of DOM that were being altered by sediment flux. By studying these elements together, we hope to improve our stoichiometric understanding of how processing of these elements are linked (Stutter et al. 2018).
Methods
Study sites
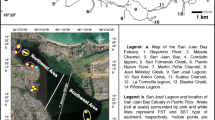
The Fox River flows northeast through Wisconsin before entering Lake Michigan at the southern end of Green Bay. The Fox rivermouth occurs in the City of Green Bay, Wisconsin, and has been designated an Area of Concern due to a legacy of industrial pollution (https://dnr.wi.gov/topic/greatlakes/greenbay.html). Like many other rivers draining into eutrophic nearshore waters in the Great Lakes (e.g., the Maumee River, the Saginaw River), the Fox River drains a very agricultural watershed. Sediment delivery to the Fox rivermouth is affected by a series of upstream dams and by a large inland lake (Lake Winnebago). While dams are common in Great Lakes tributaries (Moody et al. 2017), the Fox River is unusual in also draining Lake Winnebago, one of the largest inland lakes in the entire Great Lakes Basin.
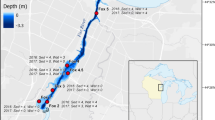
The focal area of our study was the Lake Michigan-affected portion of the Fox rivermouth that is downstream from the De Pere Dam (44.448256 N, 88.064133 W) and upstream from the open area of Green Bay (approximately 44.539571 N, 88.004666 W). This is a linear distance of approximately 11.9 km along the river’s path with an approximate surface area of 4.86 km2. Six locations were sampled repeatedly from April through September in 2016 along the Fox rivermouth (Table S1; Fig. 1). Adjacent to the Fox watershed, but slightly to the north and west lies the Duck Creek watershed, which also has substantial upstream agricultural and urban development and small dams (similar to the De Pere Dam), but has a large wetland at the rivermouth. Two sites were sampled repeatedly April through September in 2016 in the Duck Creek rivermouth to give some idea of how sediment nutrient release might differ in another rivermouth (Table S1; Fig. 1).
For estimating whole rivermouth nutrient release, the Fox rivermouth was broken into three sections based on the location of tributary inputs: Upper, Middle and Lower. The Upper portion starts at the De Pere Dam and extends longitudinally until the Ashwaubenon Creek enters the Fox River (1.31 km2) and includes sites Fox 1–3 in this study. The Middle portion starts at the confluence with Ashwaubenon Creek and runs downstream to the confluence with the East River (3.0 km2) and includes sites Fox and Fox 4.5 in this study. The Lower portion begins at the confluence with East River and ends where the rivermouth intersects the coastline (0.55 km2) and includes site Fox 5 in this study (Fig. 1).
Sediment core collection and incubation experiments
Three replicate sediment core samples were collected at each site for experimental purposes in April, June, August, and September 2016 (92 cores total). Data from 92 sediment incubation experiments using sediment cores collected for this study are available in an associated data release (https://doi.org/10.5066/P9LVTWS8). Intact sediment cores were collected at most stations using a gravity coring device (Aquatic Research Instruments, Hope, Idaho) equipped with acrylic liners (20 cm height, 6.5 cm inside diameter). A box corer (Wildco Wildlife Supply Co., Saginaw, Michigan) containing an acrylic insert (15 × 15 × 15 cm) was used to collect coarser-grained sediments located at Fox 4.5 and 5. Intact cores were extracted from the insert using the smaller 6.5 cm × 20 cm acrylic liners. Sediment cores with overlying water were sealed with stoppers, stored on ice in a cooler during transport to the laboratory, and processed within 24 h. River water, collected at Fox 3, was stored on ice for transport and served as overlying water for the sediment incubation systems and to quantify SRP, NH4, NOX, and DOC (see below).
At the laboratory (University of Wisconsin-Stout), overlying water was carefully siphoned from each core and the thickness of the sediment was adjusted to ~ 10 cm within the acrylic liner. The additional water collected from the Fox River was filtered through a glass fiber filter (Gelman A/E glass fiber), and 300 mL (i.e., ~ 10 cm in height in the core tube) was carefully siphoned onto the intact core without causing sediment disturbance and resuspension to serve as overlying water. The cores were placed in a darkened environmental chamber and incubated at ambient collection temperature (12 °C April, 22 °C June, 26 °C August, 18 °C September). Dissolved oxygen sensors deployed during 2016, along with longitudinal transects of dissolved oxygen throughout the rivermouth, never indicated low oxygen conditions, so we incubated sediment cores from all sites and dates in oxic conditions. Aerobic conditions were maintained by gently bubbling air through an air stone (spherical gas diffusion stone, 60 µm nominal poresize) placed ~ 1 cm above the sediment–water interface. On five site–date combinations (Fox 2 June, Fox 2 August, Fox 2 September, Fox 4 September and Fox 5 August), in addition to oxic incubations of sediment cores, we also performed three core incubations in anoxic conditions, created by bubbling N2 gas through the air stone. Constant gentle bubbling was maintained in each incubation system with individual air valves. A water sample (40 mL) was collected daily from each incubation system. Daily water samples were analyzed for SRP, and approximately half of the daily samples were analyzed for NH4, NOX and DOC.
Samples for SRP, NH4, and NOX were filtered through a 0.45-µm syringe filter (Fisher 09-719-004) prior to analysis. SRP was immediately analyzed using the ascorbic acid method (APHA 2011, 4500-P E., detection limit = 0.005 mg/L). Samples for dissolved inorganic nitrogen (DIN) were preserved with sulfuric acid to pH < 2.0, and analyzed following methods described in Soballe and Fischer (2004, Sect. 5.3–5.5, 5.7–5.8, detection limit = 0.01 mg/L NOX and 0.008 mg/L NH4), except that sample volumes were smaller (12 mL). While SRP was measured in each of the daily water samples, DIN was only measured in water collected on 3 days of the incubation (usually the first, last, and one day in between).
Samples for DOC and DOM were filtered through a pre-rinsed 0.22-µm polycarbonate membrane filter and stored in the dark in glass scintillation vials. Filtrate was then shipped to Trent University overnight in coolers with ice packs for analysis of DOC concentration and DOM optical character. DOM was not measured in enough of the April incubations to be used in this analysis.
DOC concentration (mg C L− 1) was measured using an OI Aurora TOC Analytical Analyzer after acidification of the sample with persulfate. We characterized DOM composition using spectrofluorometric characteristics. DOM absorbance was measured from 800 to 230 nm using a Perkin Elmer Lambda 25. UV–Visible 3D excitation–emission matrix (EEM) scans were conducted on each water sample using a Varian Cary Eclipse Fluorometer. Scans were made from 600 to 270 nm emission at a 2-nm interval and 500- to 230-nm excitation at a 5-nm interval with 5-nm band widths at 0.25 nm s− 1 (Williams et al. 2010; Murphy et al. 2010). EEMs were corrected for daily Milli-Q blank scans, instrument bias using manufacturer supplied factors, and inner filter effects (Cory et al. 2010; Murphy et al. 2010). The area under the Milli-Q blank Raman scatter peak at 350-nm excitation was used to standardize EEM fluorescence to Raman Units (RUs). The following standard DOM composition indices were calculated: (1) a spectral slope ratio used as a proxy for molecular size and photooxidation (SR; Helms et al. 2008), (2) the molar absorptivity at 280 nm used as a correlate for aromaticity, analogous to specific UV absorbance [ε280; correlation between ε280 and specific UV absorbance at 254 (SUVA254) was > 0.98 in these samples; Weishaar et al. 2003], (3) a modified humification index (HIX; Zsolnay et. al. 1999; Ohno 2002), and (4) the beta–alpha ratio (also called the freshness index) used as an indicator of the extent of degradation (BA ratio; Parlanti et. al. 2000; Wilson and Xenopoulos 2009). In addition, the ratio of emission at 470 nm to emission at 520 nm (at 370 nm excitation) was calculated as an indication of DOM source (i.e., a slightly modified version of the fluorescence index; FI; McKnight et. al. 2001; Cory et al. 2010). Due to variability in the Varian Cary Eclipse Fluorometer at these wavelengths, emission wavelengths 470 ± 4 and 520 ± 4 nm were averaged prior to division. Table 1 explains how these individual DOM optical properties are usually interpreted (slightly modified from Larson et al. 2014). A few duplicate samples (~ 10) had obvious signs of contamination (e.g., unrealistic FI values > 4) and were discarded. Otherwise duplicate values were generally similar (< 10% difference between duplicates) and were averaged at each location.
Sediment characteristics
The upper 5-cm section of an additional sediment core collected at each station on the same dates was analyzed for various physical-textural and chemical characteristics. Homogenous subsamples were dried at 105 °C for determination of bulk density, moisture content, and porosity then combusted at 550 °C for estimation of loss-on-ignition organic matter using methods and equations described in Avnimelech et al. (2001) and Håkanson and Jansson (2002). Sediment particle-size distribution was determined on wet sediment using a combination sieve and separation via settling in 1-L columns according to Plumb (1981). Particles greater than 62.5 µm (i.e., sand fraction) were separated by washing a known mass of sediment through a 62.5-µm stainless steel mesh sieve and quantitatively transferring the captured material to a crucible for dry mass determination. Separation of particles between 62.5 and 1.95 µm (i.e., silt) and less than 1.95 µm (i.e., clay) was conducted by transferring sediment that passed through the 62.5-µm mesh to a standard 1-L settling column. The slurry was subjected to 10 mL of a 1% Calgon solution to reduce flocculation, mixed thoroughly via inversion, and sampled using a 20-mL pipette before and after settling to determine the dry mass of particles less than 62.5 µm and particles less than 1.95 µm, respectively. Settling times required to capture particles less than 1.95 µm, were based on temperature and Stokes law as indicated in Plumb (1981). Samples were placed in crucibles and dried at 105 °C for mass determination.
Phosphorus fractionation was conducted according to Psenner and Puckso (1988) for the determination of ammonium-chloride-extractable P (1 M NH4Cl, loosely-bound P), bicarbonate–dithionite-extractable P (0.11 M NaHCO2–dithionate, iron-bound P or Fe–P), sodium hydroxide-extractable P (1 M NaOH, aluminum-bound P or Al-bound P), and hydrochloric acid-extractable P (0.5 N HCl, calcium-bound P or Ca–P). A subsample of the sodium hydroxide extract was digested with potassium persulfate to determine nonreactive sodium hydroxide-extractable P (Psenner and Puckso 1988). Labile organic P (LOP) was calculated as the difference between reactive and nonreactive sodium hydroxide-extractable P. The dry mass equivalent of 25 mg wet sediment was sequentially extracted. Each extract was filtered through a 0.45-µm filter, neutralized to pH 6–8, and analyzed for soluble reactive P using the ascorbic acid method (APHA 2011). Concentrations were calculated as mg P/g dry mass of sediment.
A principal components analysis (PCA) was used as an exploratory analysis to identify common axes of variation among sediment characteristics. The PCA was completed using the princomp() function in R and enough parameters to explain > 80% of the variation were retained (R Development Core Team 2014).
Data analysis: individual nutrient flux calculations
Concentrations in the water above the sediments in the incubation chambers were used to calculate nutrient flux (SRP, NH4, NOX and DOC) using mass balance equations. Details of these equations and accompanying statistical code are in the Statistical Appendix, but these largely follow methods used in other studies (James 2017). On occasion, concentrations of SRP were below the detection limit of the analytical methods. In these cases, multiple imputation was used to determine whether reasonable inferences about SRP flux could be made despite the censored data (Helsel 2005). For each sample with a concentration below the detection limit (106 occasions), we artificially inserted a value drawn from a uniform distribution between the detection limit and zero. With these inserted values, we calculated a conditional SRP flux as described in the code in the Statistical Appendix. This was repeated a total of 500 times. If the coefficient of variation of these 500 conditional SRP fluxes was > 10%, then we excluded that estimate of SRP flux from any further consideration (28 of the 106 occasions when SRP flux dropped below the detection limit). Otherwise, the mean of the 500 conditional SRP flux estimates was reported as the SRP flux (indicating the censored value has little influence on our estimates of nutrient flux). Detailed code for this procedure is available online (https://doi.org/10.5066/P995SMVW). This approach was also used for NOX (which was occasionally below the detection limit) and NH4 and DOC (which were never below the detection limit). Only the first 1 or 2 days of the incubation was used for NOX, NH4, and DOC because this was the period when changes appeared to be linear and also because we did not measure these solutes at every sampling event. Therefore, estimating the release of NOX, NH4, and DOC after day 2 would require multi-day interpolation during potentially non-constant fluxes, greatly increasing estimation uncertainty.
For DOM optical properties (SR, ε280, HIX, BA ratio, FI), we calculated the difference between the initial optical property value (which was measured just once) and the value measured after 1 or 2 days. To assess whether these differences were greater or less than zero, we calculated the 90% credible interval of the difference between these DOM properties after 1 or 2 days and the initial value (Kruschke 2011), as implemented in the bayestestR package (Makowski et al. 2019) in R. If the 90% credible intervals did not encompass zero, then we concluded there was support for change over the 1 or 2 days of incubation.
To evaluate associations between sediment characteristics, water column nutrients and sediment nutrient release, we used a partial least squared regression (PLSR) analysis (Carrascal et al. 2009). Unlike PCA, PLSR has a predictor–response framework. PLSR identifies latent components from predictor variables that maximize the explained variance in a dependent variable. We used the PLS package in R (Mevik and Wehrens 2007). We ran individual PLSR analyses for each solute flux rate (SRP, NH4, NOX, and DOC). Cross-validation was completed using the default method employed in the plsr() function (which divides the data into 10 segments). We used root mean square error of prediction (RMSEP) from the cross-validation to inform our understanding of how many latent variables (i.e., components) to include, although we did not focus our interpretation on latent variables that explained less that 5% of the variation in the flux rates.
Data analysis: whole-rivermouth nutrient flux calculations
Sediment fluxes were simulated for the entire Fox rivermouth from April to September by multiplying the area of each portion of the rivermouth by the nutrient release rate (e.g., mg SRP m− 2 day− 1). Daily nutrient fluxes were drawn from a normal distribution estimated from all the incubations completed within that portion of the rivermouth and interpolated between sampling dates. For example, if three incubations were done in the lower portion with estimated SRP fluxes of 1, 2, and 3 mg SRP m− 2 day− 1 on Day 1, then a release rate for Day 1 was drawn from a normal distribution with mean 2 and standard deviation of 1 for Day 1. If the next incubations were completed on Day 5 and were 4, 5, and 6 mg SRP m− 2 day− 1, then the Day 5 flux was drawn from a normal distribution with mean 5 and standard deviation of 1. To estimate the flux on other days (e.g., on Day 3 in this example), an estimate was drawn from each of these distributions, and then weighted by the number of days between the two estimated release rates using the following equation:
EN = EI * (1- tI/tTOT) + EF * (1 – tF/tTOT),
where EN is the estimated flux at time N, EI is the flux from the previous incubation experiment, EF is the flux from the next incubation experiment, tI is the time since the previous incubation experiment, tF is the time until the next incubation experiment and tTOT is the total time that passed between these two incubation experiments. These estimated daily fluxes were then multiplied by the area of that particular rivermouth section (Upper, Middle or Lower). Detailed code for this procedure is available (https://doi.org/10.5066/P995SMVW). Using these whole rivermouth daily fluxes, we calculated the percent of the daily flux to Lake Michigan that was potentially derived from the sediment (i.e., daily rivermouth flux divided by daily flux to Lake Michigan), based on the estimated fluxes from the models of Robertson et al. (2018; projected for the 2016 water year). The Fox River is one of the few locations where flux to Lake Michigan is calculated at the end of the rivermouth, and therefore one of the few locations where this estimate can be made.
Results
Sediment and water column conditions
Sediment and water column characteristics varied substantially among sampling dates and sites (Tables 2, 3, S2; Fig. 2). NH4 and NOX concentrations were highest in both the Fox and Duck in April and lowest in August, whereas SRP was much higher in June than other months (Table 2). DIN:SRP ratios were very high in April but still indicated P limitation in in other months. DOC was highest in June and declined thereafter (DOC was not measured in April). DOM composition varied less, with no obvious trend (Table 2).
Even sampling from approximately the same location, we saw substantial changes in sediment properties. For example, at Fox 1 organic content was 14.6% in April and just 7.3% in August, bulk density (dry) was 0.205 mg/mL in April and 0.555 mg/mL in August, sand content shifted from 32.1 to 78.5% (Table 3). Most of the sediment-bound P appeared to be bound to either iron (Fe), calcium (Ca), or as part of LOP (Table S2). Total sediment P varied substantially over time (e.g., at Fox 1, ranged from 1.076 mg/g in April to 0.284 mg/g in August; Table S2; Fig. 2).
To characterize co-variation in sediment characteristics, we extracted two components in a PCA that together explained over 80% of the total variation. The first axis explained ~ 74% of the variation in the sediment characteristics with positive associations between moisture content, porosity, silt, and organic matter content and 4 of the 5 forms of sediment-bound P (Fe, Al, loosely bound and LOP; Table 4). Ca-bound P was inversely related to clay content, which was the main association identified in PCA Axis 2 (explaining 10% of the variation; Table 4).
Nutrient fluxes
SRP flux ranged from 9.47 mg SRP m− 2 day− 1 in April at Fox 2 to a low of − 0.56 mg SRP m− 2 day− 1 at Duck 1 in June with an overall mean of 1.74 mg SRP m− 2 day− 1 (Fig. 3). A key feature of the measured SRP flux was high variability over space and time, by which we mean the standard deviation was greater than the mean when averaging across space or time (Table S3). Averaged over all the sites, this was the case in 2 of the 4 months sampled and averaged across months this was the case for 5 of the 8 sites sampled (Table S3). On the other hand, replicates within a single site–month combination had less variability, with the standard deviations being greater than the mean just 7 of 31 times (Table S3). In other words, multiple cores from a single site generally had less variability than comparing among sites or through time. The five core incubations performed in anoxic conditions had much higher SRP flux than the oxic cores (overall mean of anoxic cores was 17.66 mg SRP m− 2 day− 1, Table S4).
Box and whisker plots showing the range of solute fluxes (mg m− 2 day− 1) in the lower Fox River and Duck Creek, Green Bay, Wisconsin, 2016 from different sampling events. Eight sites were sampled during the June, August and September sampling dates and seven sites were sampled during the April sampling. Boxes encompass the first and third quartile around the median (central line). The lines (whiskers) show the largest or smallest observation that falls within 1.5 times the box size. Individual observations are offset slightly to the right to show spatial differences. SRP soluble reactive phosphorus, NH4 ammonium, NOX nitrates plus nitrites, DOC dissolved organic carbon, F1–5 site Fox 1–Fox 5, D1–2 site Duck 1 or 2
Most sediments had a positive NH4 flux (mean 40.6 mg NH4 m− 2 day− 1; Table S3; Fig. 3), although NH4 flux varied among sites. Fox 1, Fox 2 and Fox 4 had mean NH4 flux that were clearly higher than Duck 1 and Fox 5 (Table S3). Anoxic cores released NH4 at flux rates that were usually within 1–2 standard deviations of the oxic flux rates, although this was not the case in September incubations (Table S4). For NOX, sediments tended to be net sinks in April and June and net sources in August and September (Table S3; Fig. 3). Monthly mean April and June NOX flux was − 18.92 mg NOX m− 2 day− 1 and − 18.03 mg NOX m− 2 day− 1 (i.e. negative rate = influx to the sediment), respectively, and these mean fluxes climbed to 6.50 mg NOX m− 2 day− 1 in August before dropping back to 0.96 mg NOX m− 2 day− 1 in September (i.e., positive rate = efflux from the sediment, Table S3). In anoxic conditions, NOX concentrations in the sedimentation chambers were always below the detection limit and so estimates of NOX flux are qualitatively negative but quantitative estimates have extremely high uncertainty. This is consistent with denitrification occurring under low oxygen conditions and a lack of oxygen for nitrification.
In June, DOC was removed from the water column in the incubation experiments, but in August and September the sediments appeared to be a net source of DOC (Table S3; Fig. 3). In anoxic conditions in August, DOC flux was reversed, and the sediments appeared to be a net sink (Table S4). In September, anoxic DOC flux was similar to oxic conditions (Table S4). In June, when DOC was removed from the water column, molar absorptivity (ε280; median change 50.4, 90% credible interval [41.9, 60.3]) and HIX (1.56 [0.1, 3.9]) increased, while the spectral slope declined (− 0.05 [− 0.09, − 0.01]). In August, when the sediments appeared to be releasing DOC, water column HIX decreased (− 1.58, [− 2.8, − 0.3]), indicating less humic material entering the water column. In September sediments again appeared to be releasing DOC, and ε280 (− 19.0 [− 29.4, − 6.8]) and HIX (− 0.91 [− 3.0, − 0.1]) decreased, indicating less aromatic and less humic DOM entering the water column. FI and BA tended to vary around the initial values in all cases (Figs. S1, S2 for the Fox rivermouth sites).
We simulated whole rivermouth nutrient flux for the period from April 21 to September 20, 2016, in the Fox rivermouth, weighted by the three areas identified in Fig. 1. Over 1000 simulations, there was an mean SRP flux of 1559.9 kg (standard deviation 44.63), NH4 flux of 35,872 kg (1513.5), and sediments were a net sink for NOX of 4200 kg (168.45; Fig. 4). From June to September (no April data are available for DOC), the rivermouth sediments appeared to be a net sink of DOC, removing 2647 kg DOC from the water column (standard deviation 284.9; Fig. 4).
Estimated daily sediment nutrient release or uptake rates during the summer of 2016 in the Fox rivermouth, Green Bay, Wisconsin, 2016. Solid circles are the mean estimate over 1000 simulations for a given day and dashed lines indicate 1 standard deviation around that mean. Values depicted here represent the total nutrient release or uptake summed across the Upper, Middle, and Lower rivermouth sections. No dissolved organic carbon measurements were made before June. a SRP: soluble reactive phosophorus, b NH4: ammonium N, c NOX: nitrate plus nitrite N, and d DOC: dissolved organic carbon
For SRP and NOX, we compared these fluxes to loading estimates over the same period as estimated by models from Robertson et al. (2018; Fig. 5). The mean SRP flux from sediments was 2.9% of the daily orthophosphate load (the load estimate mostly closely associated with SRP; range 0.3–15.5%; Fig. 5). For NOX, the overall mean was − 0.15% (corresponding to the sediments being an overall net sink during this sampling period; Figs. 3, 5), but during the early season up to 12% of the overall daily flux was moving into the sediments. By contrast in late summer (when NOX concentrations in the water column were low), the sediments were the source of (at most) 19.8% of the NOX.
Percentage of the total daily soluble reactive phosphorus (SRP) and nitrate plus nitrite (NOX) load from the Fox River (Wisconsin) that is estimated to come from sediment nutrient release in the Fox rivermouth during the 2016 growing season. A negative percentage indicates that NOX was being removed from the water column. On 3 days, large seiche events made it impossible to estimate a daily load, so those values are excluded here
During our August sampling event all the measured fluxes were positive (indicating movement from sediments to the water column; Table S3). DIN fluxes to the water column were dominated by NH4 rather than NOX (in the Fox River) and the DIN:SRP ratio of flux was above the Redfield ratio in 4 of 6 sites (Table 5). This was occurring while water column SRP and NOX were below the detection limits and NH4 was at its lowest observed values (Table 2). For the entire Fox rivermouth during this season, the DIN:SRP ratio of nutrients released from the sediments was 45.0, DOC:DIN ratio was 0.10, and the DOC:SRP ratio was 4.4.
Associations between sediment and water column properties and nutrient release
PLSR models revealed several components related to each of the estimated nutrient fluxes that was supported by cross validation (Table S5, S6, S7, S8). However, while multiple components were supported by cross validation only the first components explained more than 15% of variation in response data, and so we have focused on each first component here (Table 6, see full results in Tables S5, S6, S7, S8).
The first PLSR component (i.e., latent variable) for SRP was correlated with the loosely bound and Fe-bound P content as well as with the moisture content, porosity, and organic matter content of the sediments. The sediment characteristics of this component seems qualitatively similar to the main axis of variation in the sediment data as evidenced from the PCA analysis (Table 6; PCA Axis 1), and indicates that more sediment P (in certain forms) corresponded to higher P flux. This latent variable was also positively correlated with water column variables of temperature, surface water concentrations of NOX and DOC and negatively correlated with dissolved nutrient ratios (Table 6). The loadings (i.e., correlations to the component) of individual predictor variables are all fairly low (< 0.3), and overall the first component explains about 41.3% of the variation in the data. Plotting the model predictions (including all supported components) against the actual measurements revealed some apparent non-linearity (Fig. 6a), but log transformation did not substantially improve this fit or change the results (results not shown).
Predicted solute flux from sediments (mg m− 2 day− 1) in the lower Fox River and Duck Creek, Green Bay, Wisconsin, 2016 generated from cross validation of partial least squared regression (PLSR) analysis compared to actual measurements for a soluble reactive phosphorus (SRP), b ammonium (NH4), c nitrates plus nitrites (NOX), and d dissolved organic carbon (DOC). Positive values indicate release from sediments
While the first component for SRP was associated with a mix of sediment and water column characteristics, other nutrient fluxes were associated with only one or the other. The first PLSR component for NH4 flux was associated with a sediment component that again was very similar to PCA Axis 1 (Table 6). This axis explained about 49% of the variation in the NH4 flux (Table 6). As with SRP, plotting actual NH4 fluxes against modeled NH4 flux indicates some non-linearity that could not be resolved by transformation. For NOX flux, by contrast, component 1 explained ~ 75% of the variation and was correlated to water column properties (Table 6; Fig. 6c). Specifically, the component associated with NOX flux was negatively correlated to temperature, SRP, NOX, NH4 and DOC and positively correlated to NH4:NOX, DOC:SRP and DOC:DIN ratios (Table 6). For DOC flux, water column properties again were strongly associated with component 1, in almost the exact same fashion as for NOX flux (Table 6). The DOC flux used in this analysis clustered in two very different groups (Fig. 6d). Component 1 explained ~ 95% of the variation in DOC flux.
Discussion
Are nutrient flux rates in the Fox rivermouth high compared to other aquatic ecosystems?
Compared to other studies on lakes and rivermouths, the SRP fluxes measured here in the Fox rivermouth are relatively high. Using the conversion from SRP to total P (TP) that Orihel et al. (2017) developed, the overall mean from the Fox and Duck sites in the incubations reported here was 1.98 mg TP m− 2 day− 1 (range − 0.58 to 10.55 mg TP m− 2 day− 1). Steinman and Ogdahl (2012) and Steinman et al. (2009) measured oxic and anoxic P release in two drowned rivermouth lakes entering southern Lake Michigan, and found oxic P flux ranged between − 0.33 to 1.54 mg TP m− 2 day− 1. In a review of Canadian freshwaters (mostly lakes), Orihel et al. (2017) found that the median reported oxic TP flux was 0.62 mg TP m− 2 day− 1. The mean for the western basin of Lake Erie (much of which is arguably part of the Maumee and Detroit rivermouths) was 1.35 mg P m− 2 day− 1 in a study that used several different methodological approaches (Matisoff et al. 2016). The rates in the Fox and Duck rivermouths were similar to the range observed in Ontario (Canada) rivers: − 0.73 to 5.24 mg TP m− 2 day− 1 (Juckers et al. 2013). More eutrophic systems, or those with more agricultural and urban watersheds, appear to generally have higher sediment P flux rates (Juckers et al. 2013; Orihel et al. 2017), and the Fox and Duck watersheds include substantial agricultural and urban land use.
The mean August and September sediment DOC fluxes measured here (~ 21 and ~ 55 mg m− 2 day− 1) are similar to estimated values from the St. Lawrence estuary (15.6–46.2 mg m− 2 day− 1; Alkhatib et al. 2013), but much lower than the upper estimates from smaller rivers in Ontario, Canada (up to 160 mg m− 2 day− 1; Juckers et al. 2013). Outside of the St. Lawrence watershed, estimates from freshwater-freshwater rivermouths appear to otherwise be lacking. Peter et al. (2016) used methods similar to those used here and found that oxic sediments were a net sink of DOC in a small lake (− 34.8 mg m− 2 day− 1), while anoxic sediments released DOC at a rate similar to what we observed (~ 39 mg m− 2 day− 1). In that study, colored and aromatic DOM were being released (Peter et al. 2016). In this study, we found it was the microbial-like DOM being taken up or released. Peter et al. (2016) concluded that formation and dissolution of DOM–Fe complexes were dictating flux in the lake sediments they studied, whereas studies in estuaries and marine sediments have shown microbiota and attached autotrophs have a large influence on DOC flux (Aguilar and Thibodeaux 2005; Maher and Eyre 2010). Given that it was the non-humic, non-aromatic and therefore more labile portions of the DOM pool that were moving into and out of the sediment in this study, we suspect these fluxes were primarily driven by bacteria near the sediment water interface responding to changes in water-column conditions.
We found that water column conditions were strongly associated with the flux of NOX and DOC from sediments. When NOX and DOC were more abundant in the water column, the sediments removed these solutes, and when NOX and DOC decreased in late summer the sediments began releasing these solutes. Previous studies have demonstrated that increasing availability of nitrate results in increased denitrification, which is consistent with what we observed (Richardson et al. 2004). Juckers et al. (2013) found that labile DOM increased NO3 flux into stream sediments. In our study, labile DOM appeared to be removed in June, which was a time when there was NOX flux into the sediment, consistent with Juckers et al.’s (2013) observation that DOM was being used by biota removing NOX from the water column. When net NOX flux was to the water column in late summer, DOC concentrations were much lower, and DOC flux was also towards the water column. Overall, fluxes of NH4 and SRP were weakly correlated to water column characteristics in this study and were much less strongly related to any of the environmental characteristics we measured than NOX and DOC flux.
The redox conditions of the sediments and surficial waters have long been thought of as strongly influencing P release from sediments (Hupfer and Lewandowski 2008). In the Fox rivermouth, water column dissolved oxygen rarely drops to hypoxic or anoxic conditions for extended periods (Fitzpatrick unpublished data). Presumably low DO does occur in sediments, and SRP may be dissolved into sediment porewaters and exchanged with surface waters via that mechanism (Orihel et al. 2017). Mineralization of P from decaying organic material much closer to the sediment-water interface may also be an important source of SRP during oxic conditions, especially in eutrophic conditions (Orihel et al. 2017). Because organic P was often a non-trivial contribution to the total sediment P pool in these sediments, this may be an important mechanism. Based on our very limited sampling of flux rates in anoxic conditions, there is potential for much higher P release during hypoxic events in the Fox rivermouth.
The Fox tributary system is unusual among Great Lakes tributaries due to the presence of a large inland lake (Lake Winnebago) and it is difficult to know the extent to which this influences sediment nutrient fluxes. Approximately 91% of the total Fox River watershed occurs upstream from Lake Winnebago, therefore sediments draining into the landscape are likely intercepted or transformed by this large inland lake (https://dnr.wi.gov/topic/Watersheds/basins/lowerfox/). Other than connecting channels (e.g., the Detroit River), no other Great Lakes rivermouth ecosystems have such a large surface water body in proximity upstream. The only other rivermouth sampled as part of this study was the Duck Creek rivermouth, which lacks a large upstream lake, but had sediment nutrient fluxes that were generally similar in magnitude and direction to those we measured in the Fox rivermouth. This offers some evidence that the sediment flux rates measured in the Fox rivermouth are not dominated by some unique Lake Winnebago effect, but overall these two watersheds are relatively similar. All of these sites are within the zone where Lake Michigan has a heavy influence on hydrology.
Is sediment flux a major contributor to overall annual nutrient flux?
Klump et al. (1997) suggested 700 metric tons of P were delivered to Green Bay each year, and newer data (Robertson et al. 2018) indicates between 154 and 316 metric tons of orthophosphate P are delivered to Green Bay annually (estimated for data from 2014 to 2016 using models from Robertson et al. 2018). The total SRP released by the sediments by our estimates over the 153 days of this study was ~ 1.5 metric tons, or between 0.2 and 1% of the annual orthophosphate P load. For NOX, about 4.2 metric tons were removed by the rivermouth during the study period, compared to ~ 657 metric tons passing through the rivermouth and into Green Bay (estimated for data from 2016). Similar load estimates are not available for DOC and NH4, but it seems likely sediment fluxes will be a small portion of the annual load for these solutes as well.
Are sediment flux rates likely to be ecologically important?
The timing of sediment nutrient release indicates that, at times, sediment nutrient flux may have a large effect on local ecological processes. For example, during our August sampling event, we visually observed cyanobacterial surface scums, bright green surface waters, and other anecdotal evidence of phytoplankton blooms. At this time, surface-water nutrient concentrations are very low, and nutrient inputs from sediment would probably be rapidly assimilated by benthic and pelagic primary producers. In a recent review, Glibert (2017) emphasized the importance of nutrient ratios in dictating the phytoplankton community composition and toxin production. During late summer in the Fox rivermouth, when SRP and DIN are low, we observed nutrients being released from sediments at ratios that would imply P limitation and more NH4 than NOX. High N:P ratios and high NH4:NOX ratios are hypothesized to be favorable for cyanobacterial dominance and microcystin production (Glibert 2017). Production of phytoplankton during these late summer periods, when discharge and nutrient concentrations are low, may be sustained by sediment nutrient flux into the water column.
References
Aguilar L, Thibodeaux LJ (2005) Kinetics of peat soil dissolved organic carbon release from bed sediment to water. Part 1. Laboratory simulation. Chemosphere 58:1309–1318
Alkhatib M, del Giorgio PA, Gelinas Y, Lehmann MF (2013) Benthic fluxes of dissolved organic nitrogen in the lower St. Lawrence estuary and implications for selective organic matter degradation. Biogeosciences 10:7609–7622. https://doi.org/10.5194/bg-10-7609-2013
Annex 4 Task Team (2015) Recommended phosphorus loading targets for Lake Erie. United States Environmental Protection Agency. https://www.epa.gov/glwqa/report-recommended-phosphorus-loading-targets-lake-erie. Accessed 17 Dec 2019
American Public Health Association, APHA (2011) Standard methods for the examination of water and wastewater, 21st edn. APHA, Washington, DC
Avnimelech Y, Ritvo G, Meijer LE, Kochba M (2001) Water content, organic carbon and dry bulk density in flooded sediments. Aquacult Eng 25:25–33. https://doi.org/10.1016/S0144-8609(01)00068-1
Bertani I, Obenour DR, Steger CE et al (2016) Probabilistically assessing the role of nutrient loading in harmful algal bloom formation in western Lake Erie. J Gt Lakes Res.https://doi.org/10.1016/j.jglr.2016.04.002
Carrascal LM, Galván I, Gordo O (2009) Partial least squares regression as an alternative to current regression methods used in ecology. Oikos 118:681–690. https://doi.org/10.1111/j.1600-0706.2008.16881.x
Cory R, Miller M, McKnight DM et al (2010) Effect of instrument-specific response on the analysis of fulvic acid fluorescence spectra. Limnol Oceanogr Methods 8:67–78. https://doi.org/10.4319/lom.2010.8.67
Davis T, Harke M, Marcoval M et al (2010) Effects of nitrogenous compounds and phosphorus on the growth of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Aquat Microb Ecol 61:149–162. https://doi.org/10.3354/ame01445
Dila DK, Biddanda BA (2015) From land to lake: contrasting microbial processes across a Great Lakes gradient of organic carbon and inorganic nutrient inventories. J Gt Lakes Res 41:75–85. https://doi.org/10.1016/j.jglr.2015.04.014
Evans MA, Fahnenstiel G, Scavia D (2011) Incidental oligotrophication of North American Great Lakes. Environ Sci Technol 45:3297–3303. https://doi.org/10.1021/es103892w
Great Lakes Interagency Task Force (2014) Great Lakes Restoration Initiative Action Plan II
Glibert PM (2017) Eutrophication, harmful algae and biodiversity—challenging paradigms in a world of complex nutrient changes. Mar Pollut Bull 124:591–606. https://doi.org/10.1016/j.marpolbul.2017.04.027
Håkanson L, Jansson M (2002) Principles of lake sedimentology, 2nd edn. The Blackburn Press, Caldwell
Helms JR, Stubbins A, Ritchie JD et al (2008) Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr 53:955–969
Helsel DR (2005) Nondetects and data analysis. Wiley, Hoboken
Hilton J, O’Hare M, Bowes MJ, Jones JI (2006) How green is my river? A new paradigm of eutrophication in rivers. Sci Total Environ 365:66–83. https://doi.org/10.1016/j.scitotenv.2006.02.055
Hupfer M, Lewandowski J (2008) Oxygen controls the phosphorus release from lake sediments—a long-lasting paradigm in limnology. Int Rev Hydrobiol 93:415–432. https://doi.org/10.1002/iroh.200711054
James WF (2017) Internal phosphorus loading contributions from deposited and resuspended sediment to the Lake of the Woods. Lake Reserve Manag 33:347–359. https://doi.org/10.1080/10402381.2017.1312647
Juckers M, Williams CJ, Xenopoulos MA (2013) Land-use effects on resource net flux rates and oxygen demand in stream sediments. Freshw Biol 58:1405–1415. https://doi.org/10.1111/fwb.12136
Klump JV, Edgington DN, Sager PE, Robertson DM (1997) Sedimentary phosphorus cycling and a phosphorus mass balance for the Green Bay (Lake Michigan) ecosystem. Can J Fish Aquat Sci 54:10–26. https://doi.org/10.1139/f96-247
Krieger K (2003) Effectiveness of a coastal wetland in reducing pollution of a Laurentian Great Lake: hydrology, sediment, and nutrients. Wetlands 23:778–791
Kruschke JK (2011) Doing Bayesian data analysis. Elsevier, Oxford
Larson JH, Frost PC, Vallazza JM et al (2016) Do rivermouths alter nutrient and seston delivery to the nearshore? Freshw Biol 61:1935–1949. https://doi.org/10.1111/fwb.12827
Larson JH, Frost PC, Xenopoulos MA et al (2014) Relationships between land cover and dissolved organic matter change along the river to lake transition. Ecosystems 17:1413–1425. https://doi.org/10.1007/s10021-014-9804-2
Larson JH, Trebitz AS, Steinman AD et al (2013) Great Lakes rivermouth ecosystems: scientific synthesis and management implications. J Gt Lakes Res 39:513–524. https://doi.org/10.1016/j.jglr.2013.06.002
Maher DT, Eyre BD (2010) Benthic fluxes of dissolved organic carbon in three temperate Australian estuaries: implications for global estimates of benthic DOC fluxes. J Geophys Res 115:G04039. https://doi.org/10.1029/2010JG001433
Makowski D, Ben-Shachar MS, Ludecke D (2019) Understand and describe Bayesian models and posterior distributions using bayestestR. J Open Source Softw.https://doi.org/10.21105/joss.01541
Marko KM, Rutherford ES, Eadie BJ et al (2013) Delivery of nutrients and seston from the Muskegon River Watershed to near shore Lake Michigan. J Gt Lakes Res 39:672–681. https://doi.org/10.1016/j.jglr.2013.08.002
Matisoff G, Kaltenberg EM, Steely RL et al (2016) Internal loading of phosphorus in western Lake Erie. J Gt Lakes Res 42:775–788. https://doi.org/10.1016/j.jglr.2016.04.004
McKnight DM, Boyer EW, Westerhoff PK et al (2001) Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol Oceanogr 46:38–48
Mevik B, Wehrens R (2007) The pls package: principal component and partial least squares regression in R. J Stat Softw 18
Moody AT, Neeson TM, Wangen S et al (2017) Pet project or best project? Online decision support tools for prioritizing barrier removals in the Great Lakes and beyond. Fisheries 42:57–65. https://doi.org/10.1080/03632415.2016.1263195
Morrice JA, Kelly JR, Trebitz AS et al (2004) Temporal dynamics of nutrients (N and P) and hydrology in a Lake Superior Coastal Wetland. J Gt Lakes Res 30:82–96. https://doi.org/10.1016/S0380-1330(04)70379-2
Murphy KR, Butler KD, Spencer RGM et al (2010) Measurement of dissolved organic matter fluorescence in aquatic environments: an interlaboratory comparison. Environ Sci Technol 44:9405–9412. https://doi.org/10.1021/es102362t
Newell SE, Davis TW, Johengen TH et al (2019) Reduced forms of nitrogen are a driver of non-nitrogen-fixing harmful cyanobacterial blooms and toxicity in Lake Erie. Harmful Algae 81:86–93. https://doi.org/10.1016/j.hal.2018.11.003
Ohno T (2002) Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ Sci Technol 36:742–746
Orihel DM, Baulch HM, Casson NJ et al (2017) Internal phosphorus loading in Canadian fresh waters: a critical review and data analysis. Can J Fish Aquat Sci 25:1–25. https://doi.org/10.1139/cjfas-2016-0500
Parlanti E, Wörz K, Geoffroy L, Lamotte M (2000) Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org Geochem 31:1765–1781
Peter S, Isidorova A, Sobek S (2016) Enhanced carbon loss from anoxic lake sediment through diffusion of dissolved organic carbon. J Geophys Res Biogeosciences 121:1959–1977. https://doi.org/10.1002/2016JG003425
Plumb RH Jr (1981) Procedures for handling and chemical analysis of sediment and water samples. U.S. Army Engineer Waterways Experiment Station, Vicksburg
Psenner R, Pucsko R (1988) Phosphorus fractionation: advantages and limits of the method for the study of sediment P origins and interactions. Arch Hydrobiol Beih Ergebn Limnol 30:43–59
Richardson WB, Strauss EA, Bartsch LA et al (2004) Denitrification in the Upper Mississippi River: rates, controls, and contribution to nitrate flux. Can J Fish Aquat Sci 61:1102–1112
Robertson DM, Hubbard LE, Lorenz DL, Sullivan DJ (2018) A surrogate regression approach for computing continuous loads for the tributary nutrient and sediment monitoring program on the Great Lakes. J Gt Lakes Res 44:26–42. https://doi.org/10.1016/j.jglr.2017.10.003
Schade JD, Espeleta JF, Klausmeier CA et al (2005) A conceptual framework for ecosystem stoichiometry: balancing resource supply and demand. Oikos 109:40–51
Sharpley A, Jarvie HP, Buda A et al (2013) Phosphorus Legacy: overcoming the effects of past management practices to mitigate future water quality impairment. J Environ Qual 42:1308. https://doi.org/10.2134/jeq2013.03.0098
Soballe DM, Fischer JR (2004) Long term resource monitoring program procedures: water quality monitoring. Technical Report LTRMP 2004-T02-1 (Ref. 95-POO2-5). La Crosse
Steinman A, Chu X, Ogdahl M (2009) Spatial and temporal variability of internal and external phosphorus loads in Mona Lake, Michigan. Aquat Ecol 43:1–18. https://doi.org/10.1007/s10452-007-9147-6
Steinman AD, Ogdahl ME (2012) Macroinvertebrate response and internal phosphorus loading in a Michigan Lake after alum treatment. J Environ Qual 41:1540. https://doi.org/10.2134/jeq2011.0476
Steinman AD, Ogdahl ME, Weinert M et al (2012) Water level fluctuation and sediment–water nutrient exchange in Great Lakes coastal wetlands. J Gt Lakes Res 38:766–775. https://doi.org/10.1016/j.jglr.2012.09.020
Steinman AD, Ogdahl ME, Weinert M, Uzarski DG (2014) Influence of water-level fluctuation duration and magnitude on sediment–water nutrient exchange in coastal wetlands. Aquat Ecol 48:143–159. https://doi.org/10.1007/s10452-014-9472-5
Sterner RW (2008) On the phosphorus limitation paradigm for lakes. Int Rev Hydrobiol 93:433–445. https://doi.org/10.1002/iroh.200811068
Stutter MI, Graeber D, Evans CD et al (2018) Balancing macronutrient stoichiometry to alleviate eutrophication. Sci Total Environ 634:439–447. https://doi.org/10.1016/j.scitotenv.2018.03.298
Weishaar J, Aiken G, Bergamaschi B et al (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708
Wetzel RG (2001) Limnology, 3rd edn. Academic, Amsterdam
Williams CJ, Yamashita Y, Wilson HF et al (2010) Unraveling the role of land use and microbial activity in shaping dissolved organic matter characteristics in stream ecosystems. Limnol Oceanogr 55:1159–1171. https://doi.org/10.4319/lo.2010.55.3.1159
Williamson CE, Morris DP, Pace ML, Olson OG (1999) Dissolved organic carbon and nutrients as regulators of lake ecosystems: resurrection of a more integrated paradigm. Limnol Oceanogr 44:795–803
Wilson HF, Xenopoulos MA (2009) Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat Geosci 2:37–41. https://doi.org/10.1038/ngeo391
Withers PJA, Jarvie HP (2008) Delivery and cycling of phosphorus in rivers: a review. Sci Total Environ 400:379–395. https://doi.org/10.1016/j.scitotenv.2008.08.002
Zsolnay A, Baigar E, Jimenez M et al (1999) Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 38:45–50
Acknowledgements
Thanks to Enrika Hlavacek for assistance in creating Fig. 1. Thanks to Laura Hubbard for assistance in applying statistical models from Robertson et al. 2018 to the 2016 water year. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This research was funded by the Great Lakes Restoration Initiative and the U.S. Geological Survey Ecosystem Mission Area.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Amy M. Marcarelli
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Larson, J.H., James, W.F., Fitzpatrick, F.A. et al. Phosphorus, nitrogen and dissolved organic carbon fluxes from sediments in freshwater rivermouths entering Green Bay (Lake Michigan; USA). Biogeochemistry 147, 179–197 (2020). https://doi.org/10.1007/s10533-020-00635-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00635-0