Abstract
The removal of polycyclic aromatic hydrocarbons by membrane bioreactor (MBR) under aerobic conditions had been studied using naphthalene (NAP) and phenanthrene (PHE) as model compounds. Three MBRs with submerged ultra-filtration hollow fiber membranes were operated applying different operational conditions during 6.5 months. Complete NAP and PHE removal was obtained applying loads of 7 gNAP kgTSS−1 day−1 and 0.5 gPHE kgTSS−1 day−1, while the organic loading rate was adjusted to 0.26 kgCOD kgTSS−1 day−1, with the biomass concentration being 6000 mgTSS L−1, the hydraulic retention time (HRT) 8 h and the solids retention time (SRT) 30 days. Load increases, as well as HRT and SRT reductions, affected the NAP and PHE removals. Biodegradation was found to be the major NAP and PHE removal mechanism. There was no NAP accumulation in the biomass. Low PHE quantities remain sorbed in the biomass and the contribution of the sorption in the removal of this compound was estimated to be less than 0.01 %. The volatilization does not contribute to the PHE removal in MBRs, but the contribution of NAP volatilization can reach up to 0.6 % when HRT of 8 h is applied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds made up of two or more fused benzene rings, arranged in linear, angular or clustered structures. They are hydrophobic non-polar compounds which do not ionize and as a result they have low water solubility which decreases as the molecular weight increases. PAHs are lipophilic compounds because of their high octanol–water partition coefficient and consequently they tend to adsorb on organic particulate matter. The PAHs of low molecular weight are semi volatile compounds. PAHs are widespread environmental contaminants in nature due to their characteristics, molecular stability, persistence and tendency to accumulate in different matrices (Kanaly and Harayama 2000; Haritash and Kaushik 2009). It has being demonstrated that they can cause adverse negative impacts on ecosystems and human health, even at low concentrations (Samanta et al. 2002; Ball and Truskewycz 2013). Many of them are toxic, genotoxic, endocrine disrupting as well as tumorigenic substances (Lofrano et al. 2009; Payne et al. 2003). In addition, PAHs with four or more rings are carcinogenic and mutagenic as a result of their ability to suffer metabolic transformations (Akcha et al. 2003). That is why sixteen PAHs had been classified as priority pollutants by the United States Environmental Protection Agency (US EPA), Water Framework Directive (WFD) 2000/60/EC and European Union Decision 2455/2001/EC with the objective of reducing the release of these compounds to the environment. The main sources of PAHs in the environment are anthropogenic as they are by-products of incomplete combustion of fossil fuels and organic matter, coal gasification and liquefaction processes, waste incineration, fuel-burning kitchen stove, petroleum cracking, petrochemical processing and automobile exhausts, and in the production of coke, coal tar pitch, black carbon and asphalt (Kochany 2015). PAHs may also be released directly to the receiving water bodies via sewage, industrial wastewaters, road runoff, street dust, and through accidental spilling of processed hydrocarbons and oils, leaching from creosote-impregnated wood, discharges of non-combusted fossil fuel residual products and ship traffic due to their presence in un-combusted petroleum (US EPA 1990; Jiries et al. 2000; Blanchard et al. 2004; Katsoyiannis and Samara 2004; Busetti et al. 2006; Fatone et al. 2011; Zhang et al. 2013). Industrial outfalls are the major point sources of PAHs in water bodies such as rivers, lakes, estuaries and seawater; therefore the implementation of proper wastewater treatment technology is an important issue to avoid PAH inputs to the environment. A water treatment alternative using aerobic membrane bioreactors was assessed in this study. Naphthalene (NAP) and phenanthrene (PHE) were selected as model compounds as they are two of the most abundant PAHs found in nature, in wastewater treatment plants and frequently used as indicators for monitoring PAH-contaminated wastes (Cai et al. 2007). Both compounds are classified as priority pollutants. NAP and PHE have different physical–chemical properties; both of them are relatively soluble in water (31.7 and 1.3 mg L−1 for NAP and PHE respectively, at 25 °C), however they have different octanol–water partitioning; the values of Log Kow are 3.46 and 4.52 for NAP and PHE respectively (Kochany 2015). The values of the Henry’s constants are also significantly different, 48.9 and 4.29 Pa m3 mol−1 for NAP and PHE respectively (Torretta 2015; Goodson et al. 2015). Therefore the fate of these pollutions and the contribution of the biotic and non-biotic processes can be substantially different in the studied biological treatment systems. As the present study focuses a technology for industrial wastewater treatment, it is important to know the typical NAP and PHE concentrations in the effluents. The range of naphthalene concentration in wastewaters is from 5 ng L−1 to more than 30 mg L−1 (US EPA 1990). Concentrations of 56–220 ng L−1 had been reported in municipal wastewater (Jiries et al. 2000), almost 2 mg L−1 in wastewater samples from the radioisotope manufacturing facilities (Tikilili and Chirwa 2011), 0.1–2.1 mg L−1 in dyeing and textile wastewater (Storm et al. 1999), up to 45 mg L−1 in wood processing effluents, 285 mg L−1 in effluents from ion-exchange resin towers (Panizza et al. 2000). Phenanthrene concentrations reported for wastewaters are as follows: Auto and other laundries, 10–66 µg L−1; coal mining, 3–35 µg L−1; leather tanning and finishing, 1.4–7.3 µg L−1; aluminum forming, 1–1100 µg L−1; battery manufacturing, 10 µg L−1; coil coating, 7–25 µg L−1; electrical/electronic components, 10 µg L−1; foundries, 4–3200 µg L−1; photographic equipment/supplies, 10 µg L−1; nonferrous metals manufacturing, 11 µg L−1; organic chemicals manufacturing/plastics, 7.2 µg L−1; paint and ink formulation, 10 µg L−1; timber products processing, 10–1700 µg L−1 (US EPA 1990; Irwin et al. 1997). On the other hand, the World Health Organization and EPA limit for wastewater discharges is 0.059 mg L−1 for NAP (US EPA 2003) and it is indicated the necessity of a complete removal for PHE. This means that a high removal efficiency of the wastewater treatment is required before the final disposal into the environment (Tikilili and Chirwa 2011).
Biological and chemical methods are usually applied in wastewater treatment to achieve the maximum possible removal of target pollutants (Byrns 2001; Katsoyiannis et al. 2006). The activated sludge process, in any of its variants, is the most used biological process for industrial wastewater treatment (Buitron et al. 2006). Biodegradation, sorption and volatilization are the main routes that control the outcome of soluble pollutants in this process (Byrns 2001). Therefore, it is possible for a fraction of the pollutant to be released into the environment as part of the final effluent discharge, as a component of the wasted sludge or in the off-gas emitted to the atmosphere (Fatone et al. 2011). In addition, there is a risk of poor biomass settlement, especially when the activated sludge systems are used for the treatment of industrial effluents with hazardous and hardly degradable compounds (Zhang et al. 2013). Consequently, the removal efficiency decreases and many micro pollutants are released with the turbid effluent. On the other hand, the membrane bioreactor (MBR) technology, which combines biological activated sludge process and membrane filtration, provides an excellent solid–liquid separation and offers better effluent quality. Subsequently, MBR has become a well-established technology for the treatment of industrial wastewaters and has demonstrated a capability to remove effectively organics and toxic compounds from wastewater (Melin et al. 2006; González et al. 2012). Due to the complete retention of biomass MBR can be operated at much higher sludge concentrations (MLSS 10–30 g L−1); sludge retention time (SRT) and hydraulic retention time (HRT) can be selected independently from each other, enabling MBR operation at high SRT and low HRT (Stephenson et al. 2000; Rosenberger et al. 2002). Concerning the concentration of PAHs in the MBR effluent, it is reasonable to think that the membrane gives an advantage by retaining the small particles containing adsorbed PAHs, which would pass through a conventional settling tank. The solids retention time (SRT) is considered as the most suitable operating parameter to evaluate micro-pollutant removal in activated sludge and MBR (Clara et al. 2005; Sponza and Gok 2011). As micro-pollutant degradation is generally considered to increase with longer SRT, MBRs may have an advantage because they can work at higher SRT than activated sludge for a similar footprint. The sludge sorption concepts developed for activated sludge systems can be transposed to MBR only to some extent, because of the modification of bio-floc properties due to shear stress and membrane retention, which could modify the transport and partitioning phenomena. Since the contribution of this phenomenon in conjunction with the biodegradation had not been quantified in MBRs yet, the aim of this study is to evaluate the effect of different operational parameters on naphthalene and phenanthrene removals in aerobic membrane bioreactors, as well as to determine the contribution of the biodegradation, sorption and volatilization processes on the removal.
Materials and methods
Feed wastewater composition
The experiments were performed using synthetic wastewater. Methanol was used as a major carbon source and the synthetic wastewater was complemented with nitrogen, phosphorus and micronutrients (Table 1). Methanol was selected because it is biodegradable substance which can support the normal biomass growth and functioning, in addition it is frequently present in the industrial effluents. Naphthalene and phenanthrene (97 % purity, purchased from Sigma-Aldrich) were added at concentrations of 15 and 1 mg L−1 respectively, similar to their solubility. These concentrations were chosen considering the worst scenario which could be observed for the selected compounds in real industry wastewaters. The wastewater pH value was adjusted to 7 ± 0.14. The synthetic water had a chemical oxygen demand (COD) and NH4–N of 522 ± 5 and 22 ± 2 mg L−1 respectively.
Experimental set up and procedure
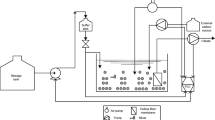
Three MBRs with submerged membranes (6 L effective volume) were continuously fed with synthetic wastewater. The schematic diagram of the experimental setup is presented in Fig. 1. The MBRs were equipped with ultra-filtration hollow fiber membranes made of polysulfone (General Electric). The membrane had a molecular weight cut-off of 100 kDa and total surface area of 0.042 m2. Permeate was withdrawn from outside-to-inside hollow membrane fibers; fluxes and trans-membrane pressure were 28–35 L m−2 h−1 and 5–11 kPa respectively. To control fouling, the reactors were operated under 3 min suction and 15 s backwashing sequential cycles which were controlled using a timer and solenoid valves (V1 and V2), suction and backwashing pumps. Continuous aeration was provided using a blower. The air flow entered the reactor through a stone diffuser placed in the bottom of each reactor, the dissolved oxygen concentration was kept between 1 and 2 mg L−1. Membranes were washed with water once a week as a membrane maintenance procedure.
The inoculation of the reactors was performed using thickened activated sludge from a municipal wastewater treatment plant with effective NH4–N and TN removal. The biomass was acclimated to the substrates in the synthetic wastewater by feeding continuously the reactors during 35 days applying organic loads of 0.26 kgCOD kgTSS−1 day−1 in all the reactors. The MLTSS concentrations were almost 6000 mg L−1 in the reactors during the start up. These biomass concentrations had been maintained during all the experimentation. The process was considered stabilized when COD and NH4–N removals reached more than 90 %, as well as the biomass growth and the specific oxygen uptake rate became relatively constant. The operational parameters are presented in Table 2 for each experimental phase. The organic load was increased in MBR2 and MBR3 starting the second experimental phase. The third increase was practiced only in MBR3 from the beginning of the third phase. The hydraulic retention times (HRT) were varied between 4 and 8 h, the sludge retention times (SRT) between 12 and 30 days. Daily biomass extractions were performed in the reactors to keep the desired MLTSS and SRT in each experimental phase.
The contribution of sorption on the biomass was evaluated following the NAP and PHE specific content in samples of the biomass in each MBR reactor during all the experimentation period. Mass balances were then performed for each reactor and experimental phase and the removals due to the extractions of biomass were calculated. The other non-biotic process which can take place in the MBR reactors is the NAP and PHE volatilization. Both compounds are semi-volatile, however the aeration increases their volatilization. The rate of volatilization from a water–air interface depends on many factors: the volatility of the individual compounds related with the value of Henry’s constant, the composition of the water and the concentration of the NAP and PHE, the temperature, the surface area of the water exposed to the gas phase during aeration, the degree of agitation of the water surface and the residence time of the water in the tank. In order to consider the effects of the factors described above, the volatilization tests were performed with the synthetic water used as a model for this experimentation, under abiotic conditions in the reactors, at the same effective volume and air flow rate as the ones applied for the evaluation with biomass. The membrane modules were removed from the reactor in order to avoid the interference of adsorption to their surface during these tests. Target pollutant concentrations were determined in the reactors during 48 h and NAP and PHE removals were calculated. Other tests were performed without aeration to determine the removal due to adsorption to the reactor walls and stripping through the water surface.
Analytical methods
In order to assess the process performance in the bioreactors, COD and NH4–N were determined twice a week in the influent and in the MBR effluents, as well as NO2–N, NO3–N in the effluents. TSS and VSS were determined once a week in the reactors. The analyses of these parameters were performed according to the standard methods (APHA 2012). Dissolved oxygen, pH and temperature were measured daily using Hatch multi-parameter model SensION 156.
The NAP and PHE concentrations were determined according EPA Method 8270D for semivolatile organic compounds using gas chromatography/mass spectrometry (GC/MS) and the sampling frequency was once a week for the influent and effluents and once each two weeks for biomass samples. The chemical analyses were carried out by GC–MS (Varian CP-3800) with ion-trap tandem mass spectrometer (Varian Saturn 2200). Standards and samples concentrates were injected using a Varian CP-8400 (Switzerland) automatic sample injector. A 30 m × 0.25 mm ID column coated with 0.25 µm chemically bonded phase VF-5 ms, 5 % phenyl + 95 % dimethylpolysiloxane was used (Variant Technologies). The oven temperature was set at 90 °C for the first 2 min and then increased at 10 °C min−1 up to 140 °C; next the temperature was increased at 20 °C min−1 up to 250 °C (held for 1 min), and finally at a rate of 20 °C min−1 up to 300 ° C. The injection temperature was maintained at 260 °C. The carrier gas was nitrogen and the injection flow was 1 mL min−1. The analytical quantification limits were of 0.0001 mg/L and 0.012 mg/kg for liquid and biomass samples respectively. All the target compounds as well as the internal standards were purchased from Sigma-Aldrich with a >97 % purity grade. The solvents and water used were HPLC grade, provided by Burdik & Jackson.
Results and discussions
Process performance
During the acclimatization period COD and NH4 –N removals of 92 % were reached in all the reactors after 35 days of operation. The MLTSS in the reactors was of 5950 ± 180 mg L−1. Figure 2a shows the COD removal obtained during the three experimental phases. The average COD removal was 97.9 ± 1.4 % in the three reactors during the first experimental phase. The organic loading rate increases, practiced in MBR2 and MBR3 at day 66, caused a 15 % decay of the removal. Almost 20 days were required to reach 98 % COD removal again. The average removals in MBR2 and MBR3 were 98.2 ± 1.3 % and 98.2 ± 1.2 % respectively (days 91–122), while 99.2 ± 0.5 % was determined in MBR1. The second organic load increase was practiced only in MBR3 at day 123 and caused a 5 % removal decay which was overcome in 14 days. The average removals in MBR3, at organic load of 0.51 kgCOD kgTSS−1 day−1 was 99.0 ± 0.7 % (days 143-189). During the third experimental phase MBR1 and MBR2 were operated with loads of 0.26 and 0.34 kgCOD kgTSS−1 day−1 respectively and average COD removal of 99.4 ± 0.4 % were obtained in both reactors.
The ammonia nitrogen removal varied between 93 and 100 % and the removal averages were very similar in the three MBR reactors during the three experimental stages. The load increases did not affect the efficiency of NH4–N removal. As it can be seen on Fig. 2b, NO3–N concentrations increased slightly in the MBR reactors over time, the averages were 9.7 ± 0.5, 10.0 ± 0.4 and 10.7 ± 0.5 mg L−1 in MBR1, MBR2 and MBR3 respectively during Phase 3. Nitrogen mass balance indicated that nitrification and denitrification took place in all the reactors. The average temperature, pH and dissolved oxygen were 23.4 ± 1.1 °C, 7.0 ± 0.3 and 1.8 ± 0.1 mg L−1 respectively. The obtained results showed a good process performance in all reactors during the experimentation period.
Naphthalene and phenanthrene removals
The variations of the NAP and PHE concentrations are presented on Fig. 3 for all the experimental period. As it can be seen the concentrations of both compounds decreased gradually over time and removals of 86–97 % and 85–95 % were obtained for NAP and PHE respectively during the first experimental stage (days 36–65). The first step of the possible degradation pathways during the aerobic biological treatment is the oxidation of the aromatic ring, incorporating atoms of molecular oxygen into the aromatic nucleus (Singh et al. 2009). These hydroxylated intermediates are then oxidized to aromatic dihydroxy compounds which may further be broken down to simpler molecules through ortho- or metacleavage pathways (Bamforth and Singleton 2005). The products of these pathways can finally be mineralized to carbon dioxide via tricarboxylic acid cycle (Chauhan et al. 2008). During biodegradation of PAH, toxic intermediate metabolites such as oxy-PAHs may be formed which are more resistant to microbial biodegradation than their parent PAH compound (Lundstedt et al. 2003; Bamforth and Singleton 2005). Nonetheless, formation of less/nontoxic intermediate metabolites during PAHs biodegradation was also reported (Andersson et al. 2003; Lau et al. 2003).
The organic load increase to 0.34 kgCOD kgTSS−1 day−1 caused increases of the concentrations in the effluents from MBR2 and MBR3, with removals of 49–68 % and 54–68 % for NAP and PHE respectively during the second experimental phase (days 66–122). The organic load of 0.26 kgCOD kgTSS−1 day−1 was conserved for MBR1 and concentration decrease was observed for both compounds during Phase 2; the average removals were 98.04 ± 0.01 and 94.76 ± 0.04 % for NAP and PHE respectively.
The third organic load increase to 0.51 kgCOD kgTSS−1 day−1 was performed only in MBR3 at the beginning of the Phase 3. The NAP concentrations increased again and more than 25 days were required to reach a relatively constant concentration of 6.2 mg L−1 in the effluent. The average NAP removal was 58.62 ± 0.01 % (days 157–196). The load increase did not cause a significant rise of PHE concentration, however the removal did not increase and the calculated average for this experimental phase was 53.33 ± 0.02 %. During phase 3 the organic load of 0.26 kgCOD kgTSS−1 day−1 was maintained in MBR1. Complete NAP and PHE removals were obtained in the period days 165–196. The organic load of 0.34 kgCOD kgTSS−1 day−1 was maintained in MBR2 during phase 3. The NAP concentrations decreased over time and the average removal was 72.17 ± 0.04 %, while the PHE concentration remained as in the previous experimental phase and the average removal was 60.63 ± 0.04 %.
The effects of the operation parameters on the NAP and PHE removals are summarized in Table 3. The obtained results indicate that the organic load, SRT and HRT are important factors for NAP and PHE removals. A complete removal of both compounds could be obtained only with the lowest organic load of 0.26 kgCOD kgTSS−1 day−1, a HRT of 8 h and a SRT of 30 days. The period required to reach a complete removal of the studied compounds was very long, almost 165 days of operation. The obtained NAP and PHE removal rates were 7 and 0.5 g kgTSS−1 day−1 respectively. Each organic load increase required a period for the acclimatization to the new operating conditions. The NAP and PHE removals decreased significantly with the organic load increase to values in the range of 0.34–0.51 kgCOD kgTSS−1 day−1, as well as with the HRT and SRT decreases to 4–6 h and 12–21 days respectively.
The NAP and PHE removals obtained in the MBR operated with a load of 0.26 kgCOD kgTSS−1 day−1 were consistent with Mozo et al. (2011), as they reported NAP and PHE removals of 99.16-99.99 % for two types of MBR, based on cross-flow and semi dead-end filtration, applying HRT and SRT of 18 h and 20 d respectively. The removal rates calculated by Mozo et al. (2011) were lower than those obtained in this study because of the lower initial NAP and PHE concentrations used in their study, 6.14 and 0.31 mg L−1 respectively. NAP and PHE removals of 52–94 % and 77–88 % respectively were reported for real scale MBR treating municipal wastewater with NAP and PHE effluent concentrations of 0.03–0.04 and 0.01-0.02 µg L−1 respectively (Fantone et al. 2011). The evaluation of pre-denitrifying MBR treatment, performed by González et al. (2012) for municipal wastewater with PAHs concentration of 0.48 µg L−1, indicated a complete removal of PAHs applying HRT of 35 h and SRT of 25 days. The effect of the operational parameters had not been evaluated in the mentioned studies.
Phenantrene at relatively high concentration (2 mg L−1) was used as a model compound for the evaluation of PAHs removal using MBR with external flat-sheet membrane filtration module (ultrafiltration or microfiltration membranes), combining powdered activated carbon (PAC) adsorption with bacterial degradation applying a bacterial consortium known for its ability to mineralize PHE (Dosoretz and Böddeker 2004). The reported PHE removals, 76–96 % for the 250 kDa membranes and 80–95 % for the 1500 kDa membranes, were slightly lower than those obtained with HRT of 8 h and PHE load of 3 mg L−1 day−1 in this study. The authors indicated that there was not a significant effect of the membrane type, HRT (5–12 h) and PHE loads (4–9.6 mg L−1 day−1) on the removal efficiency in the MBR-PAC reactors. However, the PHE retained within the system (biosorbed and adsorbed by the PAC) was 5–25 %, degradation was not measured and the outcome of the removed PHE remained unknown.
Naphthalene and phenanthrene specific content in biomass samples
The determinations of the NAP and PHE specific content in biomass of the three MBRs, performed to elucidate the sorption contribution to the removal, are presented in Fig. 4. As it can be seen the NAP content in the biomass decreased over the time reaching values lower than the detection limit at day 59 after the start up. The naphthalene concentrations remained below the detection limits in the biomass samples from the three MBR until the end of the experimental period. The load increases practiced in the reactors MBR2 and MBR3 did not cause NAP sorption and accumulation in the biomass, not even during the operation with the highest organic load. This indicated that after the acclimatization the major mechanism of NAP removal was the biodegradation.
Phenanthrene was determined in the biomass of the three reactors during all the experimental period. The concentrations of this compound decreased slightly during the acclimatization phase which can be explained with an adjustment of the biomass consortia and metabolism to the PHE biodegradation which is limited by its relatively low concentration and difficult biodegradability.
During the first experimental phase the average PHE concentration in the biomass of the three reactors was 0.36 mg kg−1. During the next phases, the PHE concentrations decreased in the biomass of MBR1 which was operated with the lowest organic load of 0.26 kgCOD kgTSS−1 day−1 all the time. The mass balance performed for PHE indicated that the removal due to the extractions of biomass with sorbed PHE was only 0.001–0.002 %, being the total removal of 100 %. The contribution of the sorption onto the biomass was 0.006–0.009 % when loads of 0.34 and 0.51 kgCOD kgTSS−1 day−1 were applied and average removals of 61 and 53 % respectively were obtained. This means that the major mechanism of the phenantrene removal was also the biodegradation. The PHE was however harder to biodegrade compared with the NAP, and PHE quantities of 0.19 mg kg−1 remained sorbed onto the biomass even operating with 0.26 kgCOD kgTSS−1 day−1.
Volatilization tests
The results of the non-biotic tests performed are presented in Fig. 5. The removal during the tests without aeration can be attributed to the adsorption on the reactor walls and striping through the water surface. The NAP removal was 0.46 % in 4 h and after that increased slightly reaching 0.49 % removal in 48 h. The difference of 0.03 % can be attributed to striping with a rate of 1 × 10−4 mg L−1 h−1. The PHE removal was 0.82 % in 4 h and reached 0.83 % in 48 h, and the striping rate can be estimated of 2 × 10−6 mg L−1 h−1. The higher PHE removal compared with the NAP one can be attributed to the higher Log Kow of PHE and the lower striping to the lower Henry’s law constant. Comparing the removals due to striping with the total removals, it can be concluded that adsorption was the main removal mechanism during the test without aeration.
The NAP removal during the test with aeration showed a gradual increase over the time. The NAP removal determined in 4 h was 0.34 %, lower than the one obtained without aeration which can be attributed to a partial desorption from the walls due to the higher water agitation in the test with aeration. The NAP removal at 6 h was of 0.49 % and after that, the removals in the aerated water were higher than those obtained without aeration. The removal determined at 8 h, the highest HRT used in this study, was 0.56 %. The PHE removals obtained with aeration were lower than those obtained without aeration during the 48 h of the experiments. This may be attributed to less PHE adsorption to the walls when applying aeration. In addition, there was a variation of the PHE concentrations and removals over the time which can be explained with the adsorption–desorption phenomenon in the reactor. There was no difference between the removals determined in 6 and 48 h, which indicates that volatilization of PHE is practically inexistent during the aeration. The results obtained are in agreement with those reported by Sponza and Gok (2011) for activated sludge systems, as they indicated that the volatilization of PAHs was very poor during the aerobic treatment process. Some studies, such as Mozo et al. (2011, 2012), had reported higher volatilization for NAP than the ones obtained in this study, however the volatilizations depends on many factors (air flow/surface area, HRT, temperature, composition of the water, NAP concentration), that is why it was important to determine the NAP volatilization at the same experimental conditions as the ones being used during the process evaluation.
Conclusions
The membrane bioreactors with submerged ultrafiltration membranes are able to remove naphthalene and phenanthrene present in wastewater. SRT of 30 days and HRT of 8 h would be sufficient for the complete removal of both compounds using MLTSS of 6000 mg L−1 with loads of 0.26 kgCOD kgTSS−1 day−1, 7 and 0.5 g kgTSS−1 day−1 for naphthalene and phenanthrene respectively. The SRT and HRT decreases and load increases affect the process performance negatively. The removal of both compounds can be attributed mainly to biodegradation. There is no naphthalene accumulation in the biomass. Low phenanthrene quantities remain sorbed in the biomass and the contribution of the sorption in the removal of this compound was estimated to be less than 0.01 %. The volatilization does not contribute to the PHE removal in MBR reactors, but the contribution of NAP volatilization can reach up to 0.6 % when HRT of 8 h is applied. Hence this work provides important information about the operation of MBR with submerged ultra-filtration hollow fiber membranes for industrial wastewater treatment. It improves our understanding of the final outcome of hazardous PAHs in membrane bioreactors, suggesting possible ways of MBR performance enhancement.
References
Akcha F, Burgeot T, Narbonne JF, Garrigues P (2003) Metabolic Activation of PAHs: Role of DNA Adduct Formation in Induced Carcinogenesis. In: Douben PET (ed) PAHs: an ecotoxicological perspective., Ecological and environmental toxicology seriesWiley, New York, pp 65–80
Andersson BE, Lundstedt S, Tornberg K, Schnürer Y, Öberg LG, Mattiasson B (2003) Incomplete degradation of polycyclic aromatic hydrocarbons in soil inoculated with wood-rotting fungi and their effect on the indigenous soil bacteria. Environ Toxicol Chem 22:1238–1243
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association (APHA-AWWA-WEF), Washington, DC
Ball A, Truskewycz A (2013) Polyaromatic hydrocarbon exposure: an ecological impact ambiguity. Environ Sci Pollut Res 20:4311–4326
Bamforth SM, Singleton I (2005) Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Technol Biotechnol 80:723–736
Blanchard M, Teil MJ, Ollivon D, Legenti L, Chevreuil M (2004) Polycyclic aromatic hydrocarbons and polychlorobiphenyls in wastewaters and sewage sludges from the Paris area (France). Environ Res 95:184–197
Buitron G, Razo-Flores E, Meraz M, Alatriste Mondragon F (2006) Biological treatment systems. In: Cervantes F, Pavlostathis SG, van Haandel AC (eds) Advanced Biological treatment processes for industrial wastewater. Principles and applications. IWA Publishing, London, pp 141–185
Busetti F, Heitz A, Cuomo M, Badoer S, Traverso P (2006) Determination of sixteen polycyclic aromatic hydrocarbons in aqueous and solid samples from an Italian wastewater treatment plant. J Chromatogr A 1102:104–115
Byrns G (2001) The fate of xenobiotic organic compounds in wastewater treatment plants. Water Res 35:2523–2533
Cai QY, Mo CH, WU QT, Zeng QY, Katsoyiannis A (2007) Occurrence of organic contaminants in sewage sludges from eleven wastewater treatment plants, China. Chemosphere 68:1751–1762
Chauhan A, Fazlurrahman OJ, Jain R (2008) Bacterial metabolism of polycyclic aromatic hydrocarbons: strategies for bioremediation. Indian J Microbiol 48:95–113
Clara M, Kreuzinger N, Strenn B, Gans O, Kroiss H (2005) The solids retention time a suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res 39:97–106
Dosoretz CG, Böddeker KW (2004) Removal of trace organics from water using a pumped bed-membrane bioreactor with powdered activated carbon. J Membr Sci 239:81–90
European Union Decision 2455/2001/EC (2001) EU Decision of the European Parliament and of the Council, Official Journal of European Union, vol. L331, pp. 1–5
Fatone F, Di Fabio S, Bolzonella D, Cecchi F (2011) Fate of aromatic hydrocarbons in Italian municipal wastewater systems: an overview of wastewater treatment using conventional activated-sludge processes (CASP) and membrane bioreactors (MBRs). Water Res 45:93–104
González D, Ruiz LM, Garralón G, Plazab F, Arévalo J, Parada J, Pérez J, Moreno B, Gómez MA (2012) Wastewater polycyclic aromatic hydrocarbons removal by membrane bioreactor. Desalin Water Treat 42:94–99
Goodson KL, Pit R, Clark S (2015) PAHs in wastewater during dry and wet weather. In: Forsgren AJ (ed) Wastewater Treatment: Occurrence and Fate of Polycyclic Aromatic Hydrocarbons (PAHs)., Advances in water and wastewater transport and treatmentCRC Press, Boca Raton, pp 157–175
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15
Irwin RJ, VanMouwerik M, Stevens L, Seese MD, Basham W (1997) Environmental Contaminants Encyclopedia. National Park Service, Water Resources Division, Fort Collins, Colorado, Phenanthrene entry, p 18
Jiries A, Hussan H, Lintelmann J (2000) Determination of polycyclicaromatic hydrocarbons in wastewater, sediments, sludge and plants in Karak Province, Jordan. Water Air Soil Pollut 121:217–228
Kanaly RA, Harayama S (2000) Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol 182:2059–2067
Katsoyiannis A, Samara C (2004) Persistent organic pollutants (POPs) in the sewage treatment plant of Thessaloniki, northern Greece: occurrence and removal. Water Res 38:2685–2698
Katsoyiannis A, Zouboulis A, Samara C (2006) Persistent organic pollutants (POPs) in the conventional activated sludge treatment process: model predictions against experimental values. Chemosphere 65:1634–1641
Kochany J (2015) PAHs in natural waters: natural and anthropogenic sources, and environmental behavior. In: Forsgren AJ (ed) Wastewater treatment: occurrence and fate of polycyclic aromatic hydrocarbons (PAHs)., Advances in water and wastewater transport and treatmentCRC Press, Boca Raton, pp 11–47
Lau KL, Tsang YY, Chiu SW (2003) Use of spent mushroom compost to bioremediate PAH-contaminated samples. Chemosphere 52:1539–1546
Lofrano G, Nikolaou A, Kostopoulou Pagano G, Belgiorno V, Napoli RMA (2009) Occurrence and measurement of organic xenobiotic compounds in harbour and coastal sediments. In: Kassinos D, Bester K, Kümmerer K (eds) Xenobiotics in the urban water cycle: mass flows, environmental processes, mitigation and treatment strategies. Springer, Dordrecht Heidelberg London New York, pp 129–146
Lundstedt S, Haglund P, Öberg L (2003) Degradation and formation of polycyclic aromatic compounds during bioslurry treatment of an aged gasworks soil. Environ Toxicol Chem 22:1413–1420
Melin T, Jefferson B, Bixio D, Thoeye C, DeWilde W, De Koning J, Van der Graaf J, Wintgens T (2006) Membrane bioreactor technology for wastewater treatment and reuse. Desalination 187:271–282
Mozo I, Stricot M, Lesage N, Spérandio M (2011) Fate of hazardous aromatic substances in membrane bioreactors. Water Res 45:4551–4561
Mozo I, Lesage G, Yin J, Bessiere Y, Barna L, Sperandio M (2012) Dynamic modeling of biodegradation and volatilization of hazardous aromatic substances in aerobic bioreactor. Water Res 46:5327–5342
Panizza M, Bocca C, Cerisola G (2000) Electrochemical treatment of wastewater containing polyaromatic organic pollutants. Water Res 34:2601–2605
Payne JF, Mathieu A, Collier TK (2003) Ecotoxicological Studies Focusing on Marine and Freshwater Fish. In: Douben PET (ed) PAHs: an ecotoxicological perspective., Ecological and environmental toxicology seriesWiley, New York, pp 191–224
Rosenberger S, Kruger U, Witzig R, Manz W, Szewzyk U, Kraume M (2002) Performance of a bioreactor with submerged membranes for aerobic treatment of municipal wastewater. Water Res 36:413–420
Samanta SK, Singh OM, Jain RK (2002) Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol 20:243–248
Singh A, Kuhad RC, Ward OP (2009) Advances in applied bioremediation, vol 17. Springer, Berlin
Sponza DT, Gok O (2011) Effects of sludge retention time and biosurfactant on the treatment of polyaromatic hydrocarbon (PAH) in a petrochemical industry wastewater. Water Sci Technol 64:2282–2292
Stephenson T, Judd S, Jefferson B, Brindle K (2000) Membrane bioreactors for wastewater treatment. IWA Publishing, London
Storm T, Reemtsma T, Jekel M (1999) Use of volatile amines as ion-pairing agents for the high performance liquid chromatographic–tandem mass spectrometric determination of aromatic sulfonates in industrial wastewater. J Chromatogr A 854:175–185
Tikilili PV, Chirwa EMN (2011) Characterization and biodegradation of polycyclic aromatic hydrocarbons in radioactive wastewater. J Hazard Mater 192:1589–1596
Torretta V (2015) PAHs in wastewater and removal efficiency in conventional wastewater treatment plants. In: Forsgren AJ (ed) Wastewater treatment: occurrence and fate of polycyclic aromatic hydrocarbons (PAHs)., Advances in water and wastewater transport and treatmentCRC Press, Series, pp 141–156
US EPA (1990) Treatibility manual. EPA 540/2-90-007, Washington DC 20460
US EPA (2003) US EPA Code of Federal Regulations 2003e 40 CFR 268.48
Water Framework Directive (2000) Directive 2000/60/EC of the European Parliament and of Council of 23 October 2000 establishing a framework for community action in the field of water policy. Off J Eur Communities 327:1–72
Zhang W, Wei C, Yan B, Feng C, Guobao Z, Lin C, Yuan M, Chaofei W, Ren Y, Hu Y (2013) Identification and removal of polycyclic aromatic hydrocarbons in wastewater treatment processes from coke production plants. Environ Sci Pollut Res 20:6418–6432
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mijaylova Nacheva, P., Esquivel Sotelo, A. Removal of naphthalene and phenanthrene using aerobic membrane bioreactor. Biodegradation 27, 83–93 (2016). https://doi.org/10.1007/s10532-016-9757-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-016-9757-6