Abstract
Confronting a sustained coral reef conservation crisis, we need new opportunities to rethink how to protect areas successfully and efficiently in the face of a changing world. We studied the benthic community, including foraminifera, fish community, and genetic connectivity (SSRs and SNPs) of main reef-building corals, Orbicella faveolata and Agaricia undata, along a Non-Protected Area (NPA) reef tract in Barú peninsula, including some isolated banks, near Cartagena and the National Natural Park Corales del Rosario y San Bernando (NNP CRySB), Colombia. The fringing reef track is homogeneous in benthic components, including algae, sponges, and foraminifera between all the studied sites, while corals exhibited differences between sites and depth ranges. Many reef sites sustain between 42.8 and 53% coral cover, which are among the highest recorded in this region, even higher than the nearby NNP. A total of 82 fish species were found, and the Foram Index-FI varies between 2 and 2.5, showing environmental conditions marginal for reef growth. The Barú NPA reef system can be considered spatial refugia under climate change and Anthropocene conditions, including resilient reefs at the mouth of Cartagena Bay (Magdalena River), a place of increased stressing factors. The admixture between NPA and NNP populations, the high coral cover in the NPA, the fish density and composition, the uniqueness of the diapiric banks, and the disturbance resistance are major arguments to protect this reef tract. We suggest designing a co-management scheme to ensure species connectivity, avoid further degradation, and involve different stakeholders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first marine protected areas (MPAs) were chosen decades ago on an ad hoc basis by a combination of aesthetics and opportunism, e.g., untouched ecosystems, which usually included little scientific input (Knight and Cowling 2007; UNEP-WCMC 2008; Grorud-Colvert et al. 2014). In any case, fully protected areas, i.e., closed to fishing, achieve good results despite their selection methods (Roberts 2000), such as the increase in the abundance of species, length of individuals, community diversity and restoration of trophic cascades (Sala and Giakoumi 2018; Ferreira et al. 2022). However, many MPAs worldwide have been degraded so much that they no longer serve the purpose they were created; hence, we need to spatially re-design the MPA network in many countries.
Marine ecosystems face threats due to global warming and ocean acidification, where the traditional management tools in coral reefs do not seem to buffer deterioration (Jackson et al. 2014; Hoegh-Guldberg et al. 2017). The steady decline of coral reefs, greatly exacerbated in the Caribbean, introduces urgent challenges to MPAs. Even the remotest reefs, hundreds of kilometers away from direct continental influence, have lost coral cover and bottom tridimensionality (Sánchez et al. 2005, 2019a; Bruno and Valdivia 2016). Such a level of deterioration can lead to reef bioerosion and further loss of ecological integrity, or even the functional nature of reefs as water motion regulators, which will intensify in synergy with water level rise; the declining of reef accretion will increase sediment mobility, shoreline change, and island overtopping due to a higher wave energy (Perry et al. 2014, 2018). Scientists and stakeholders are reaching a consensus on the causes leading to coral reef deterioration, which point to global issues related to warming temperatures producing coral bleaching and disease (Hughes et al. 2017a, b, 2018; Sully et al. 2019), together with local stressors such as nutrient input and overfishing (Carpenter et al. 2008; Camargo et al. 2009; Pendleton et al. 2016; Thiault et al. 2019). MPAs should confront this situation, focusing on adaptive management and accounting for changing or deteriorating conditions (McCook et al. 2010; López-Angarita et al. 2014; Kroeker et al. 2019; Nickols et al. 2019).
MPAs are essential conservation tools that promote connectivity between populations, support trophic webs under disturbances, and offset the impacts of climate change (Magris et al. 2014; Fung et al. 2017). The role of connectivity of larvae or gametes is a broadly recognized process of recovery but is not often included in building resilient reserves (Botsford et al. 2009; Beger et al. 2010; Mumby et al. 2014; Kininmonth et al. 2011), despite spillover being the most desirable ecosystem service from marine reserves (Brown et al. 2016; Bryan-Brown et al. 2017; White et al. 2019). The decline of ecosystems and wildlife can sometimes occur at the same pace in both protected and non-protected areas (NPAs) (Western et al. 2009). Although protected areas usually provide better ecosystem services, NPAs are usually more extensive and worth managing or integrating for the same conservation purposes (Avigliano et al. 2019; Benez-Secanho and Dwivedi 2020). In marine ecosystems, MPAs are known to be largely ineffective for global conservation purposes (Mora et al. 2006), which prompted the need for a larger target (Kunming COP 15) under the protection of at least 30% of the sea areas (CBD 2022). This target, still far from being achieved, also stands for efficiently managed MPAs. Consequently, MPAs need local communities' adaptive capacity and opportunities for co-management to improve governance and effectiveness and become an efficient management tool for conservation (Moreno-Sánchez and Maldonado 2010; López-Angarita et al. 2014; Kockel et al. 2019; Muhl et al. 2020).
New opportunities to find key conservation areas and resilient or resistant reef ecosystems to protect nowadays successfully and efficiently are greatly needed. Despite the steady deterioration of natural ecosystems, there are emerging natural conservation cases for reasons not entirely obvious. One such case is the Varadero reef, located in one of the entries to Cartagena Bay (López-Victoria et al. 2015), one of the busiest and most contaminated ports in the Caribbean sea, with conditions opposed to coral growth and health (Restrepo et al. 2006b), good conditions by Caribbean standards (Pizarro et al. 2017). Varadero reef is located at the end of a 22 km fringing reef system along the Barú peninsula (Cendales et al. 2002), Cartagena, Colombia. Varadero and Barú reefs are affected directly by the Magdalena River, the longest in Colombia (≅ 1500 km), through the Dique channel with 144 mill tons yr−1 of sediment input to the Caribbean Sea (Restrepo et al. 2006a). Over half of the fringing reef system from the Barú peninsula is part of the National Natural Park Corales del Rosario and San Bernardo (NNP CRySB), representing the highest protection category in the Colombian protected areas system (Camargo et al. 2009). The track towards Varadero reef is threatened by a dredging license for opening a new channel that will serve the Cartagena Port (Pizarro et al. 2017). The continental platform in this area comprises an extensive uninterrupted tectonic arc that is part of the prism of the San Jacinto and Sinú belts, where the Pleistocene cover of the platform is disrupted by older unconsolidated shale forming large dome-like mud diapirs that are capped by coral formations (Vernette et al. 1992). These banks, in the proximity of the Barú fringing reef, provide mesophotic coral ecosystems with well-developed mixed coral zones and unique octocoral and black coral animal forests (Sánchez 1999a, 2017). In this work, we performed a realistic ecological assessment of this NPA reef system integrating the fringing reefs and the diapiric banks to evaluate a management strategy and conservation figure for this area.
Materials and methods
Benthic components and Scleractinia community
The study was conducted southwest of Cartagena, the Colombian Caribbean, in the Barú peninsula. The reef system consists of fringing reefs and diapiric domes along the coastal zone. The fringing reefs are characterized by a high coral cover of lettuce coral (Agaricia tenuifolia) on the fore-reef with large coral heads towards valley-like sand channels (Cáceres and Sánchez 2015; Grajales and Sánchez 2016). The diapiric domes are located between 0.5 and 1.5 km from the coast with a depth between ~ 14 and 70 m. The diapiric domes present a narrow plateau at the top zone with coral reefs extending through the slopes beyond 50 m. For a detailed description of the study site, see Sánchez (1999a, b), Cáceres and Sánchez (2015) and Pizarro et al. (2017).
In 2013, using SCUBA and Closed-Circuit Rebreather-CCR diving, 14 coral reef sites (Fig. 1) were surveyed for main benthic components (coral, coralline algae, and fleshy/turf algae cover) through photo-quadrants. Three belt transects were placed parallel to the coast at three depth ranges: 0–8 m, 9–15 m, and 16–22 m, to measure the cover percentage of benthic organisms in 10 coastal reefs at the shallow zone (0–30 m; V: Varadero, V2: Varadero, L: Lincon, N: Niko, LC: La Caida, CV: Ciénaga de los Vásquez, LQ: La Quebrada, PB; Playa Bonita, LM: Lado Montañíta, PG: Punta Gigante). Ten quadrants of 1 × 1 m were zigzagged (placing the frame on alternate sides, moving diagonally from left to right) along each transect, sampling 30 m2 per depth (90 m2 per site). At four diapiric domes (I: Imelda, M: Montañita, OR: Octubre Rojo, TR: Trompadas; Fig. 1), one transect was located at each depth range at shallow zone (0–30 m; 16–22 m and 24–30 m) and mesophotic zone (30–70 m; 16–22 m, 24–30 m, 34–40 m, 44–50 m, 54–60 m and 64–70 m). A variable number of 0.5 × 0.5 m quadrants at each range depth and site (16–20 m: 28 ± 8 quadrants, 24–30 m: 14 ± 5 quadrants, 34–40 m: 10 ± 5 quadrants, 44–50 m: 8 ± 2 quadrants, 54–60 m: 11 quadrants, 64–70 m: 4 quadrants) were zigzagged due to the air(gas)/time restrictions; TR was the only site with depth ranges below 54 m. The total sampled area was 870 m2 at the shallowest zone and 62.75 m2 at the mesophotic zone. Photo-quadrants were analyzed using CPCe software (Kohler and Gill 2006) for the identification and determination of the percentage cover of the broad benthic categories (Coral, Turf/Fleshy algae, Calcareous algae, Octocorals, Sponges, Sand/Hard substratum/Rubble, Cyanobacteria, Black coral) and Scleractinia corals at the species level.
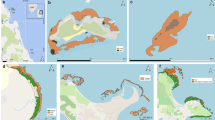
Sampling locations with the type of analysis performed: Benthic community, foraminifera assemblages, and molecular. At the top map, the green color represents the National Natural Park Corales del Rosario y San Bernando; any sampling site that falls outside the green color belongs to the Non-Protected Area (NPA). At the bottom 3D map with topography and elevation profile (coastal line in brown). Orange dots are the sampled sites. Model-based on multibeam data from Universidad Pontificia Bolivariana-CIOH-DIMAR, Armada Nacional de Colombia (Octubre Rojo) and Ecoral-Argos (coastal zone, Imelda, Montañita and Trompadas). V Varadero, V2 Varadero2, VL Varadero Lado, L Lincon, N Niko, LC La Caída, CV Ciénaga de los Vásquez, Q La Quebrada, PB Playa Bonita, LM Lado Montañita, I Imelda, M Montañita, PG Punta Gigante, CU as Cuevas, P Péndales, SD Santo Domingo, OR Octubre Rojo and TR: Trompadas
Benthic components data were analyzed to establish the differences between sites and depths using the Kruskal–Wallis test. A multidimensional scaling (NMDS) was made using Bray–Curtis dissimilarity measures. Percentage cover data and coral species were analyzed to establish the variations in the coral community between sites and depths through the Kruskal–Wallis and Mood's median tests using the R packages tydiverse (Wickham et al. 2019) and dunn.test (Dinno 2017). Pairwise comparisons using Dunn's test were applied to evaluate differences between pairs of sites and depths. We carried out an NMDS analysis to observe the grouping of the samples. A pairwise permutational multivariate analysis of variance (PERMANOVA) was made using the Bray–Curtis method to determine differentiation between sites and depth ranges in the composition and cover of coral species. We analyzed similarity percentages (SIMPER) to identify the main species of corals contributing more to the differences between pairwise sites and pairwise depth. All this analysis was performed with the R Package Vegan (Oksanen 2007).
Fish community
In 2013, the reef fish community was assessed by visual census using linear transects of 50 m at 14 sites, ten coastal reefs (V, V2, VL, L, N, CV, LQ, PB, LM, and PG; Fig. 1), and four diapiric domes (M, I, OR and TR; Fig. 1). For the shallow depths (0–8 m, 9–15 m, 16–22 m, 24–30 m), in all the sites, the transects with 50 m of length were extended in four directions (north, south, east, and west) from the center of the sample area at each depth range, for a plot area near 200 m2. Data were registered at two meters from each side of the transect, where counts of each fish individual and length (L) estimates were recorded. The deepest transects (34–40 m, 44–50 m, 54–60 m, 64–70 m) were made in three sites (I, OR and TR) and were extended in one direction with 1 m wide and an area of 50 m2 due to the domes’ geomorphology and dive time restrictions. Data were standardized using density per m2 due to the differences in the total areas sampled between shallow and deepest zones (See Supplementary Table S2). The herbivorous fish length was converted to weight using the allometric length–weight conversion (Froese et al. 2014): W = aLb; constants a and b were obtained from Fishbase (Froese and Pauly 2022).
Fish community density differentiation was evaluated with the non-parametric Kruskal–Wallis’s test between sites and depths. Pairwise comparisons using Dunn's and Wilcoxon’s tests were used for both factors, depth and site. An NMDS graphically represented samples to visualize the fish community grouping by depth and site, and ANOSIM was applied to verify differences between the groups. PERMANOVA with Bonferroni corrections and square root transformation was made using the Bray–Curtis method to determine differences between the depth and sites. Pairwise PERMANOVAs were used to determine differences between groups. A SIMPER analysis was made to identify the fish species contributing most to the dissimilarity between depths and sites.
FORAM Index—Foraminifera assemblages
Foraminifera assemblages were surveyed at nine sites (V, V2, L, N, LC, CV, LQ, PB, PG; Fig. 1), taking three samples of 10 g of superficial undisturbed sandy sediment at each site and depths between 5 and 12 m. The 27 samples were stored with absolute ethanol, dried (> 24 h, 60 ºC) in a laboratory oven, and thoroughly mixed. A portion of 1 g was washed with freshwater over a 63-µm mesh sieve to remove mid-size sediments and dried on filter paper. The dried sample was gently disaggregated, thoroughly mixed, and poured on a clean, smooth surface. A sub-sample (approximately 0.1 g) was taken, weighed, and placed in a 90 mm-diameter Petri dish to examine.
All foraminifera were removed and counted until a minimum of 150 individuals were found in each sample for each site, excluding heavily worn and reworked specimens (Hallock et al. 2003). The foraminifers were counted, identified by genus, and sorted into three functional groups: symbiont-bearing, opportunistic, and other small heterotrophic taxa (Hallock et al. 2003). The proportion (P) of specimens for each functional group was calculated by summing the specimens of each genus of that group (N) and dividing by the total number of specimens counted (T) to calculate the original Foram Index (FI) (Hallock et al. 2003). The FI ranges from 1 to 10, where FI < 2 indicates unfavorable conditions for coral growth, 2 < FI < 4 represents the limit for coral growth and is unsuitable for recovery of coral communities after a mortality event, and FI > 4 allows coral growth and recovery (Hallock et al. 2003).
Genetic diversity and population genetic structure
Orbicella faveolata
A total of 231 individuals of O. faveolata were sampled at eight sites (V, V2, LC, M, LM, PG, CU, PE, and SD; Fig. 1), including six sites in the NPA and two sites in the NNP CRySB (research permit DTC‐CR‐T‐036-03-09). Coral fragments were collected haphazardly, maintaining a distance of 5 m between samples to minimize sampling clones. Each sample consisted of a 1–2 cm fragment from the basal portion of the colony. Samples were preserved in dimethyl sulfoxide (DMSO), snapped frozen in liquid nitrogen, and transferred to a – 80 ºC ultra-freezer. The DNA extraction was carried out following the modified Cetrimonium bromide (CTAB) protocol (Coffroth et al. 1992). RNAse A and Proteinase K were added during the extraction to eliminate contaminants.
Samples were genotyped using five microsatellite primers pairs designed for Orbicella annularis (Severance et al. 2004). After screening seven loci, five were retained in our study, excluding two microsatellites: MS2-17 because they amplified less efficiently (Severance et al. 2006) and MaMS12, which had a high frequency of null alleles and heterozygotes deficit across all populations (See Supplementary Method S1 for PCR protocol details). Samples of clones were removed from six sets of clones in Varadero (V), La Caida (LC) and Punta Gigante (PG), one set in each location, and three in Varadero2 (V2). Therefore, seven individuals were excluded from the analysis. Colonies that had missing data were also excluded (n = 16). After adjusting the data set, 201 individuals were analyzed.
Genetic diversity indices, the number of alleles per locus, effective number of alleles (Ne), expected (He), observed heterozygosity (Ho) fixation index (F) for each population, each microsatellite per population and the probability of identity (PID) were calculated in the GenAlEx version 6.5 (Peakall and Smouse 2012). To confirm the genetic identity of all collected samples of O. faveolata, we ran STRUCTURE 2.3.4 (Pritchard et al. 2000) with the samples collected in this study and additional 295 samples of O. annularis (Foster et al. 2012), keeping samples with a > 0.9 posterior probability of belonging to the O. faveolata cluster. Deviations from Hardy–Weinberg equilibrium and linkage equilibrium were performed using ARLEQUIN v. 2.00 (Excoffier and Lischer 2010).
Pairwise genetic differentiation (FST values) between eight populations was estimated in ARLEQUIN v. 2.00 (Excoffier and Lischer 2010). An analysis of molecular variance (AMOVA) was conducted with GenAlEx, testing population structure within and among populations. Distinct genetic clusters of the eight populations of O. faveolata were identified in STRUCTURE. Ten runs were performed for each K value (K = 1 to 8) with a burn-in period of 100,000 iterations and 50,000 MCMC after burning under the admixture model. The best-fit number of clusters was determined based on the ΔK method (Evanno et al. 2005) and ln Pr(X|K) summarized by Structure Harvester (Earl and vonHoldt 2012) and Clumpp (Jakobsson and Rosenberg 2007). A Discriminant Analysis of Principal Components (DAPC) was implemented in the R package adegenet (Jombart 2008). Based on migration probability, gene flow was estimated with the divMigrate function of the diveRsity package (Sundqvist et al. 2016), using Nei's genetic distance Gst (Nei 1973), with 1,000 bootstrap repetitions and an arbitrary filter threshold of 0.30. Gene flow patterns were visualized using network graphics in qgraph (Epskamp et al. 2012) and represented in a map (ArcGIS Pro 2.8).
Agaricia undata
Tissues samples of Twenty-one A. undata were collected pooling six sites and samples from shallow (0–30 m) and mesophotic (30–45 m) zones (7 vs. 14 respectively) in five diapirs: Montañita (M), Imelda (I), Octubre Rojo (OR), Trompadas (TR), Isla Tesoro (IT) and one coastal reef: La caida (LC) (Research permit No. 1177/2014 - IBD 0359). All collected samples were ID-labelled (location and depth), and sample preservation and DNA extraction followed the same method for O. faveolata.
Library preparation was carried out using the nextRAD method (Nextera-fragmented-reductively-amplified-DNA) following the manufacturer's protocol (Baird et al. 2008; Etter et al. 2012; Bongaerts et al. 2017; Gonzalez-Zapata et al. 2018a). Quality control, clustering, SNP variant calling, parsing and analyses are detailed in Supplementary Method S2. The alignment-based clustering recovered 27,012 nextRAD sequence loci (“RAD loci”); 200,321 SNPs. An 8% of the dataset was removed for contaminants from Symbiodinacea. One of the 23 samples was removed because only 9.18% was genotyped (IM-M5), and three clones were removed. We identified two clonal sets with a 94.5–99% match, the first with two samples (MO-S1 and MO-M4) and the second with three samples (TR-S2, TR-S3, and TR-M1). A total of 6039 bi-allelic SNPs were retained after data filtering. For the STRUCTURE analysis, 3803 loci were used for the overall dataset and 3294 for the neutral dataset. After the filtering, 17 coral samples were used from the six sites (M, I, OR, TR, IT, and LC) and grouped in shallow and mesophotic samples (6 vs. 11, respectively) to run the genetic analysis.
Observed heterozygosity (Ho) and Goudet’s G-statistic Monte Carlo test (Goudet 2005) to test population structure (99 permutations) were calculated in the R package hierfstat. Genetic structure analysis was assessed through principal component analysis PCA and a DAPC with the adegenet R package. To determine the number of PCs to retain in the DAPC, we used the α-score (See Supplementary Figure S4a). Pairwise FST value was calculated between shallow and mesophotic populations, between sites, and within the depths zone at each site to determine the differentiation among the six populations.
The genetic STRUCTURE analysis was run using an admixture model with burn-in 100,000, MCMC reps 50,000, and 10 runs for each K = 1–5. It was visualized via a plot of posterior probabilities for each individual and population with Distruct (Rosenberg 2003), and the Evanno method (Evanno et al. 2005) was tested to identify the single k value (See Supplementary Figure S4b). All diversity and population differentiation analyses mentioned above were calculated for the all-inclusive and neutral loci. The relative migration levels (Nm) between 4 populations (MO, TR, IT, and I) were estimated to elucidate migration patterns based on 5,516 neutral SNPs, using the divMigrate function, with 1,000 bootstrap repetitions and without filter threshold. Gene flow patterns were represented using ArcGIS Pro 2.8.
Assessment of management strategies
To evaluate the management strategies for the study zone, we compiled the ecological properties in different layers that were represented on a map. Among these ecological attributes, we included coral cover, fleshy algae cover, herbivorous fish biomass, sink and source sites of genetic material for the two coral species studied, and the Foram Index obtained. Additionally, we reviewed documents such as decrees (AMP-ARS; Resolution number 0679 of May 31, 2005; Decree 1076/2015; Resolution number 0551 of May 25, 2022) and the Management Plan for Corales del Rosario, San Bernardo e Isla Fuerte 2013–2023 to understand the actual state of the "AMP CRSBeIF". Therefore, by compiling the ecological information, the bathymetric data, bathymetric distribution of coral and fish species and the regulations and management information for the zone, we proposed the area in a map that should be integrated into the National Inventory of Coral Reefs and a recommended management strategy.
Raw data availability
Project
Orbicella faveolata genetic data: https://doi.org/10.6084/m9.figshare.16786243
Agaricia undata genetic data: https://doi.org/10.6084/m9.figshare.21547128
Foraminifera: https://doi.org/10.6084/m9.figshare.16786126
Fish Data: https://doi.org/10.6084/m9.figshare.16786102
Benthic cover data: https://doi.org/10.6084/m9.figshare.16640341
Statistics results from Scleractinian corals: https://doi.org/10.6084/m9.figshare.19189289
Statistics results from the fish community: https://doi.org/10.6084/m9.figshare.19189367
Results
Benthic cover
Fleshy algae exhibited the highest bottom cover at NPA sites, with a mean of 34.91 ± 21.0%, followed by 24.50 ± 16.7% from 47 hard coral species and calcareous algae with 14.67 ± 16.1% (Fig. 2). The coral species with the highest mean cover values were Agaricia agaricites (3.7 ± 4.7%), Orbicella faveolata (3.5 ± 5.6%), Agaricia tenuifolia (3.1 ± 4.9%), Orbicella annularis (3.0 ± 6.9%), Agaricia lamarcki (1.8 ± 2.6%), Porites astreoides (1.9 ± 2.4%) and Colpophyllia natans (1.7 ± 2.3%), A. undata exhibited a mean cover value of 0.48 ± 1.86 (See Supplementary Table S1). The other species represented 0.7% and 0.003% of the coral cover, the least frequent Porites colonensis and Porites branneri (See Supplementary Figure S1a). Percent cover comparisons of the major benthic categories evaluated did not show significant differences between all the sites (Kruskal–Wallis H = 6.94, p = 0.9) and all the depths ranges (Kruskal–Wallis H = 3.49, p = 0.83), including the coastal reef-tract and the diapirs.
Box-plot graph for benthic organisms (calcareous algae, coral, and fleshy algae) cover along with the NPAs sites in Barú peninsula. Coastal reefs from V to LM. Diapiric domes from M to TR. The black point/crossbar represents the media for each category at each site. The plot shows the three dominant groups with a mean percentage cover calculated for all the benthic categories and places with a minimum value of 10%
However, the mean coral cover values (Fig. 3a) between NPA sites showed significant differences (Kruskal–Wallis H = 93.69, p < 0.001; Mood’s Median = 92.46, p < 0.001) and between depth ranges (Kruskal–Wallis H = 105.79, p < 0.001; Mood’s Median = 104.17, p < 0.001). Additionally, coral community structure (composition and cover) showed significant differences between sites (PERMANOVA, F = 1.59, p = 0.001) and depths (PERMANOVA, F = 3.25, p = 0). Nevertheless, pairwise comparisons using the Dunn test indicated that the shallow ranges (0–8 m, 9–15 m, 16–22 m, 24–30 m) did not exhibit significant differences between them (Bonferroni-corrected p > 0.05); cover percentages were similar along the shallow coastal zone (See Supplementary Table S3). Coral covers at mesophotic ranges (34–40 m, 44–50 m, 54–60 m; p < 0.001) located at the diapirs showed a lower coral cover than shallow corals. Differences between pairwise sites (Dunn test) were found between the reefs located in the diapiric domes (OR, M, I, and TR; p < 0.001) and some coastal sites (CV, L, LC, PB, PG, and N; p < 0.001, Supplementary Table S3). Thus, the diapiric domes are the sites and depths with coral cover explaining the differentiation displayed.
Coral cover, coral richness, fish density and fish richness in the coastal reefs and diapiric domes in the Peninsula of Barú. A Percentage cover of coral species by the site. B Percentage cover of coral species by depth range. The Y-axis in A and B are represented in the log10 scale. C species richness of coral at each site. D species richness of coral at depth ranges. E density in 100 m2 of fish by the site. F density in 100 m2 of fish by depth range. The Y-axis in A and B are represented in the log10 scale. G species richness of fish on each site. H species richness of fish depth range
Pairwise PERMANOVA comparisons within the shallow depths (0–30 m) indicated significant differences, and also between shallow and mesophotic ranges (PERMANOVA, p < 0.05), while within the deeper ranges below 30 m showed no differences between them (PERMANOVA, p > 0.05, see Supplementary Table S3). These results were confirmed through an NMDS (Fig. 4a), where coral species located in the shallow zone (0–30 m) exhibit differentiation between depth ranges, while it was not observed in the mesophotic zone (30–60 m).
NMDS plots. A NMDS for coral cover and species richness at the seven different depth ranges (colors) and sites represented with the abbreviation for each site. NMDS plot of the reef fish species and density at the different depth ranges (colors) and sites represented with the abbreviation for each site. V Varadero, V2 Varadero2, VL Varadero Lado, L Lincon, N Niko, LC La Caída, CV Ciénaga de los Vásquez, Q La Quebrada, PB Playa Bonita, PG Punta Gigante, LM Lado Montañita, I Imelda, M: Montañita, OR Octubre Rojo, TR Trompadas
The differences between sites may be explained by the coral species A. tenuifolia, O. faveolata, P. astreoides, and A. agaricites, where the former two species have a cumulative contribution of around the 34–35% to the differences, and the last two of 10–13% (SIMPER, see Supplementary Table S3). The differentiation found between the shallower depths against the mesophotic sites is mainly due to the presence-dominance of certain coral species, O. annularis at 0–8 m, A. tenuifolia at 9–15 m, A. agaricites at 16–22 m, and A. lamarcki at 24–30 m (SIMPER, see Supplementary Table S3). Additionally, it was evident that shallow reefs exhibit important reef-building species such as O. annularis, O. faveolata, Montastraea cavernosa, Siderastrea siderea, Pseudodiploria strigosa, P. astreoides, Porites porites. In contrast, fast-growing species dominate deeper reefs, such as Agaricia spp., Helioseris cucullata, and Madracis spp.
Fish communities
We found 82 fish species in all the sites and depths sampled, with the highest richness between 9 and 15 m with 60 species and the lowest at 64 and 70 m with only six species (Fig. 3h). The highest mean density values for the fish species were Clepticus parrae 71.52 ind/100 m2, Chromis cyanea 27.24 ind/100 m2, Scarus iseri 24.32 ind/100 m2, Chromis multilineata 21.05 ind/100 m2, Thalassoma bifasciatum 11.09 ind/100 m2, and Stegastes partitus 9.38 ind/100 m2. The least frequent species were Caranx bartholomaei, Diodon hystrix, Gymnothorax moringa, Halichoeres poeyi, Ripticus saponaceus, Sphyraena barracuda, Urobates jamaicensis with a mean density of 0.0036 ind/100 m2 for each species and Haemulon chrysargyreum, Hypoplectrus guttavarius, Sargocentron coruscum with 0.0073 ind/100 m2 (See Supplementary Figure S1b). The 18.3% of the fish species were herbivorous, exhibiting a mean biomass of 26.1 ± 19.9 g m−2 (ranging from 4.18 to 79.81 g m−2).
The mean fish density values between sites (Fig. 3d) showed significant differences (Kruskal–Wallis H = 69.26, p < 0.001), as well as between depth ranges (Kruskal–Wallis H = 67.28, p < 0.001). Moreover, the fish community composition exhibited significant differences between sites (PERMANOVA, F = 1.92, p = 0.001) and depths (PERMANOVA, F = 2.66, p = 0.001). Pairwise comparisons to determine which sites were different showed that CV, N, LM, OR, and TR are the sites with differences related to other sites (Wilcoxon signed-ranks, p < 0.05; Dunn’s test, p < 0.05, See Supplementary Table S4). The comparisons by depth range indicated fish density differences between 0–8 m with all the ranges from 16–70 m, 9–15 m with 24–30 m and 64–70 m, and 16–22 m with 64–70 m (Wilcoxon signed-ranks, p < 0.05; Dunn’s test, p < 0.05, See Supplementary Table S4). Therefore, the fish density was heterogenous along the reef tract due to the differentiation found in CV, N, OR, and TR bank, the last being the most highly differentiated from the rest of the sites. Pairwise PERMANOVA comparisons by sites showed differences between OR and several coastal reef sites; V, V2, CV, L, and N. Additionally, fish community comparisons between pairwise depth range indicated differences between shallower ranges (0–8 m and 9–15 m) with the rest of the depths (PERMANOVA, p < 0.05, see Supplementary Table S4). Furthermore, these results agree with the NMDS, indicating that fish species located within the shallow zone (0–30 m) exhibit differentiation between depth ranges, while the mesophotic zone (30–60 m) seems to be homogeneous.
The differences found between OR and V, V2, L, and N in the SIMPER analysis were observed by the presence and density of two fish species, C. cyanea, with a cumulative contribution of around 22–27%, and C. multilineata with 21–24% cumulative contribution for all the pairwise comparisons (See Supplementary Table S4). Differentiation between OR and CV by the species S. iseri and C. parrae, with a cumulative contribution of around 22% and 18%, respectively. The differences in the depth range of 0–8 m with the other depths by the cumulative contribution of C. parrae, S. iseri, T. bifasciatum, and C. cyanea. The differences from the 9–15 m with the rest of the depth ranges by the presence of C. parrae and C. cyanea (0–40 m, Supplementary Table S4).
FORAM Index—Foraminifera assemblages
We examined 27 sediment samples in 9 sites, counted 1483 individuals, and identified 43 genera: 7 symbiont-bearing, 6 opportunistic, and 30 “other small heterotrophic” (Fig. 5a). The most abundant genera for each functional group were Amphistegina, Bolivina, and Discorbis, respectively (see Supplementary Table S5). The 92% of all individuals from the foraminifera assemblage belong to the small heterotrophic genera group, followed by 5% opportunistic and 3% symbiont-bearing. All sites sampled (CV, L, LC, N, PB, PG, Q, V, and V2) had an average Foram Index-FI between 2 and 2.5 (Fig. 5b). Additionally, a negligible variation among sites was observed (Kruskal–Wallis chi-squared = 8, df = 8, p = 0.43). Indicating conditions are marginal to unsuitable for recovery of coral communities after a mortality event but not unsuitable for reef growth in normal environmental conditions.
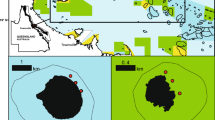
Foraminifera information at the reef track in the Peninsula of Barú. A Boxplot of the individuals' number categorized in the three functional groups (Symbiont-bearing, opportunistic, and other small heterotrophic taxa) at each site. B FORAM Index along the NPAs sites, FI > 4 indicates environment conducive to reef growth (CRG), 2 < FI < 4 indicates environment marginal for reef growth (MRG), and FI < 2 indicates stressed conditions unsuitable for reef growth (UGR). V Varadero, V2 Varadero2, VL Varadero Lado, L Lincon, N Nico, LC La Caída, CV Ciénaga de los Vásquez, Q La Quebrada, PB Playa Bonita, PG Punta Gigante
Genetic diversity and population genetic structure
Orbicella faveolata
The number of alleles (Na) ranges from 6 to 10.4, and the number of effective alleles (Ne) ranges from 3.272 to 5.647. Observed heterozygosity (Ho) per site was between 0.540 and 0.669, while expected heterozygosity (He) ranged between 0.576 and 0.789. V2, M, P, and SD showed positive F-values, indicating heterozygote deficits ranging between 0.072 and 0.151. The other sites (V, LC, PG, and CU) showed negative F-values, ranging between − 0.007 and − 0.090 (Table 1). A significant heterozygote deficit was observed in five populations for the locus STR4 (V2, LC, PG, CU, SD), one population for STR8 (LC), and three populations for STR11 (MO, CU, P) (See Supplementary Table S6).
Significant genetic structuring was found among all localities, with a small but significant overall FST-value of 0.037 (p = 0.001); F-statistics from the AMOVA analysis showed that the largest variation percentage corresponds to within-individual variation (90%) followed by variation among individuals (6%), and variation among locations (4%). MO significantly differed from other localities with moderate genetic differentiation (Table 2). The Bayesian clustering from STRUCTURE was inconclusive (Supplementary Figure S5b). Delta K and Ln Pr(X|K) resulted in different best K values. Evanno method (Delta K) resulted in K = 2 and Ln Pr(X|K) in K = 3 (Supplementary Figure S3). DAPC analysis revealed a pattern consistent with K = 2 where MO differs from the other populations (Supplementary Figure S5a).
The directional relative migration (Fig. 6a) shows that CU and PG are probably sinks from almost all the sites evaluated. PG with 100% relative immigration from V2, 82% from V, 89% from LC, 58% from PE, and 30% from SD. At the same time, CU has immigration from 35 to 52% from V, V2, LC, and PE. V, V2, and SD are larvae sources contributing migrants to all the sites. M exhibited a relative migration rate of under 10%, which supports the result observed in the STRUCTURE. Thus, Varadero Reef is connected with populations inside the NNP, and PG could be a sink.
Relative migration networks for O. faveolata and A. undata. A O. faveolata migration based on 5 microsatellites among 8 sample sites obtained with Nei's Gst based. The threshold filter > 0.3, circles represent localities, and arrows indicate the direction and magnitude of relative migration levels between populations described by decimal values. Darker arrows indicate a stronger migration relationship compared to lighter arrows. V Varadero, V2 Varadero2, LC La Caída, M Montañita, PG Punta Gigante, CU: as Cuevas, P Péndales, SD Santo Domingo. B A. undata migration based on 5,516 neutral SNPs among 4 sample sites obtained with the Nei’s Gst. Circles represent localities, while arrows indicate the direction and magnitude of relative migration levels between populations described by decimal values. Darker arrows indicate a stronger migration relationship compared to lighter arrows. TR Trompadas, MO Montañita, IM Imelda, LC La Caida, IT Isla Tesoro, OR Octubre Rojo
Agaricia undata
No genetic structuring across populations was identified for A. undata (G-statistic Monte Carlo test for all SNPs: Gobs = 9613.4, P = 0.85). The lack of genetic structure was further confirmed by assessing pairwise genetic distances between individuals at bathymetric comparison with an FST of 0.0055 (FST over all loci = − 0.009 ± 0.13), also geographically resulting in all the possible pairwise comparisons between sites and depths with negative values, interpreted as 0 (FST = 0, P < 0.05; Table 3), and the lack of clustering identified by Principal Component Analysis (PCA; Supplementary Figure S5c) and the Discriminant Analysis of Principal Components (DAPC; Supplementary Figure S5d).
In the admixture analysis with STRUCTURE (Supplementary Figure S5d), the Evanno method (Delta K) identified the uppermost level of structure of 2 K (this method did not evaluate K = 1) and Ln Pr(X|K) in K = 1 (Supplementary Fig. S5), where two samples (TR_S1 and MO_M2) showed a differentiation, as the PCA results. However, no introgression was found from one community to another, confirmed with the PCA, DAPC, and FST values; hence it may be interpreted as one population. As inferred by STRUCTURE, genetic clustering for the “neutral” data set was very similar to the overall data set. Genetic diversity was similar in mesophotic populations (0.143) and shallow (0.132).
The directional relative migration for A. undata (Fig. 6b) shows that a genetic sink could be possible for the IT population (Next to the NNP-CRySB), with 100% relative immigration from I, 80% from M, and 62% from TR. In turn, the relative migration from IT to the coastal zone was 89% to I and 62% to M. The diapiric domes TR, I, and M have a relative migration turnover rate between 30 and 67% (Table 4). This analysis showed the high connectivity between the A. undata population at the diapirs and the NNP zones. Vertical migration exhibited a turnover between the shallow and mesophotic zones, resulting in 100% of relative migration from the shallow to the mesophotic zone and 73% from the mesophotic to the shallow.
Assessment of management strategies
The ecological attributes we found in this NPA reef system were a high coral cover, a moderate cover of fleshy algae, and an important cover of calcareous algae (mostly crustose coralline algae). The coral communities were heterogeneous between sites and depth zones; differences were found between shallow and mesophotic zones and between the diapiric domes with some coastal zones. The fish communities were heterogeneous along the reef tract, exhibiting differences in sites near the coastal lagoon (CV and N; higher values of richness and density) with the farthest diapirs (TR and OR) and within the shallow zone (0–30 m), and herbivorous fish biomass was higher at the diapirs (Fig. 7b). The population genetics from O. faveolata and A. undata supports the dispersal between sites, extending well into the nearby NNP CRySB. Regarding source-sink dynamics for O. faveolata, V and SD are displayed as possible sources of larvae, and the PG sector as a sink. However, O. faveolata in MO exhibits remarkable genetic differentiation and a low migration with the remaining zones (Fig. 7a). Additionally, a turnover in the gene flow was found between the mesophotic and shallow zones for A. undata.
Maps with compiled information on ecological attributes found and the assessment of management strategies. A Coral cover, Foram Index, and Source and sink Dynamics found for **Orbicella faveolata and ***Agaricia undata. B Fleshy algae cover and biomass of herbivorous fish at each site. C Management strategies informed in regulation documents to date and zoning classification. D Proposed Area for co-management with the summary of ecological attributes found in the study
The misnamed “MPA Corales del Rosario, San Bernardo, and Isla Fuerte” is not a constituted MPA and is considered an in-situ conservation strategy in the resolutions and decrees reviewed. Additionally, the NNPs are the zones with financial support, defined management strategies, and monitoring activities. In contrast, the remaining zones do not possess these tools, although being in a Management Plan. Additionally, we found only a small part of the reef was included in the Colombian Coral Areas and the Management Plan; however, it was categorized as a restoration zone without fundaments because there was no information on cover reductions or impacts.
Discussion
The NPA reef-tract system from Barú peninsula, Cartagena (Colombia), located near but outside the NNP Corales del Rosario and San Bernando, without any practical or formal regulation and control, comprises a unique opportunity for coral reef conservation and stakeholder management approach. Our study used an integrative approach; the results exhibit at least five biological attributes that sustain a well-connected and diverse reef community beyond shallow coral reef environments. Additionally, this geographical area involves mesophotic coral ecosystems and urban coral reefs persisting in unlikely conditions. Among the biological attributes found, some are included as indicators of healthy coral reefs and others are used in MPA's delimitation decisions; these attributes were: (1) high live coral cover and moderate cover of fleshy algae, (2) Heterogeneity of coral community, (3) Heterogeneity of fish community, (4) Genetic connectivity and gene flow of coral species A. undata and O. faveolata, and (5) Resistant corals to high sedimentation and turbidity.
Despite the worldwide decline of coral reefs due to bleaching and diseases, this fringing reef remains resilient with high coral cover. In the last two decades, the coral cover in the Southwestern and Western Caribbean has declined from 22.6% in 1999 to 21.6% in 2019 (relative loss of 1%; Souter et al. 2020). In the Colombian Caribbean, in 2004, coral cover fluctuated between 5.4 and 67.2% (Rodríguez-Ramírez et al. 2010), presenting a steady decline in the last three decades by an average of ~ 38% (Garzon-Ferreira and Kielman 1993; Sánchez et al. 2005; Navas-Camacho et al. 2011; Jackson et al. 2014; Sánchez et al. 2019b). Our results show that the NPA reefs in the Barú area had a mean coral cover at the shallow zone of 30.51 ± 13.5% between 0 and 30 m and 34.1 ± 13.3% between 0 and 15 m, with many reefs sites sustaining between 42.8 and 53%. Which are among the highest recorded in this region, even higher than the coral cover values in the nearby MPA (35.0% in 2001, 23.2% in 2009 and 23.88% in 2012; Camargo et al. 2009; López-Angarita et al. 2014). Moreover, in 2015, Pizarro et al. (2017) found a coral cover from 3 to 15 m in Varadero of 45.1 ± 3.9% and the rest of the Barú reef of 38.1 ± 3.2%.
In addition to this, the fleshy algae cover in the Barú NPA does not exhibit the same pattern as the Caribbean, presenting mean values of 34.91 ± 21.01 (0–60 m) and 39.89 ± 19.66 (0–30 m), below the 45% estimated in 2019 for the Southwestern and Western Caribbean (Souter et al. 2020). In the Caribbean, fleshy algae cover has increased by 9.7% in 16 years, from 42.7% in 2003 to 52.4% in 2019 (Souter et al. 2020). Fleshy algae are dominant competitors, replacing corals when their cover declines, nutrient input levels increase, and herbivores are reduced, impoverishing coral reef health (Done 1992; Hughes 1994; Pandolfi 2003). Thus, besides this zone having high nutrients and contaminant input, the moderate cover of fleshy algae could be associated with herbivorous organisms and fast-growing coral species. The algae-dominated reefs prevent coral reefs from recovering; however, the presence of corals such as P. astreoides and A. tenuifolia (fast-growing) could ameliorate the conditions for more vulnerable slow-growing corals such as O. faveolata. Mainly, A. tenuifolia, a species with a greater surface-to-volume ratio, can endure highly sedimented reefs (Cáceres and Sánchez 2015), conferring this Baru reef an advantage in competing with fleshy algae.
The results of the mean herbivory fish biomass of 26.1 ± 19.9 g m−2, a value near the reported for non-fishing areas in the Caribbean (29.19 ± 6.17 g m−2; Edwards et al. 2014), may are related to the fleshy algae moderate cover found (Diaz-Pulido et al. 2010; Fong and Paul 2011). Additionally, the diapirs showed a high biomass of herbivorous fish (49.2 ± 20.42 g m−2), highlighting the importance of the mesophotic ecosystems maintaining a key functional group in coral reefs. We found that the coral and fish communities show heterogeneity along the coastal reefs and in the diapiric domes. This spatial heterogeneity may offer an opportunity to design an appropriate management figure that protects diverse functional traits in communities (Nyström et al. 2008). Moreover, the higher fish richness and density values found at sites near the coastal lagoon could indicate that the Ciénaga de Los Vásquez Lagoon may function as nursery grounds/or shelter during different life stages cycles, as has been reported in other coastal lagoons (Verdiell-Cubedo et al. 2013; Tournois et al. 2017). The fish community could migrate (functional connectivity) to complete their life cycles using mangroves, coral reefs, seagrass meadows, and soft bottoms around the study NNP sites (Franco et al. 2006).
Along with these ecological components, genetic connectivity is an essential criterion to be included for increasing the effectiveness of conservation and management strategies (Balbar and Metaxas 2019). The complete genetic homogeneity between NPA and NNP sectors of the corals in the Barú peninsula, as seen with admixture analyses of O. faveolata and A. undata, is paramount to protecting this reef tract. Likewise, Alegría‐Ortega et al. (2021), in a study with O. faveolata in the MPA, with samples from Varadero (VR), the NNP CRySB, found no genetic structure and relatively high migration from VR to CR and SB. Although A. undata is a brooding species, no local-scale genetic differentiation between shallow and mesophotic zone was observed, which was not expected for this reproductive strategy as has been reported for other species (e.g., Flavia fragum, Seriatopora hystrix, Stylophora pistillata; Ayre and Hughes 2000; Underwood et al. 2006; Goodbody-Gringley et al. 2010; Warner et al. 2016). This result may be attributed to planulae features and reproduction traits in agariciid corals conferring the potential dispersal process at considerable distances (capacity to swim and to stay in the water column for several days; Van Moorsel 1983; Raimondi and Morse 2000; Petersen et al. 2007).
The pattern where geographically distant populations are genetically more differentiated than closer populations appears not to apply to coral species (Gorospe and Karl 2013). This coral population genetic paradox could be explained by local oceanographic conditions, such as the larvae and gamete exchange during spawning events could be facilitated by sea surface currents. In the Colombian Caribbean, the Panama–Colombia Countercurrent (PCC) is the dominant superficial water current (southwest to northeast direction) throughout the year and mainly during the rainy season (August–November; Gordon 1967; Pujos et al. 1986), when most of the spawning events of these species occur. However, larval and gamete migration during spawning events may be affected during the dry season (June–September) due to winds frequently changing direction (north to south direction; Pineda et al. 2006), facilitating the gene flow between different locations. Varadero, Punta Gigante, and the diapirs are key zones with sink/source dynamics that need to be in consideration for their role in management plans.
Conditions of the NPA reef tract are typical of the Anthropocene, which besides local stressors like overfishing and pollution, includes global climate change (Hughes et al. 2017a, b). This environmental situation was corroborated according to the Foram Index-FI range; the values observed at the NPA reef tract, between 2 and 4, correspond to reefs with water conditions from marginal to marginal unsuitable for recovery of coral communities after a mortality event (Hallock et al. 2003). The results were high in smaller foraminifera tests, indicating eutrophication and pollution. This situation along Colombia's Caribbean coast was already noted with the FI Index, where the water quality was related to the vicinity of freshwater inputs rather than management or protection status (Velásquez et al. 2011). This reef tract has been under the strong continental siliciclastic influence, probably before opening artificial freshwater canals from the Magdalena River to Barbacoas and Cartagena bays. This river contributes approximately 9% of the total sediment load discharged from the eastern basins of South America (Restrepo and Kjerfve 2000), which has a tremendous environmental impact on the adjacent coastal ecosystems and may influence the light penetration, exacerbating the decline of corals at the other end of Barú peninsula and the Rosario and San Bernardo archipelago (Restrepo et al. 2006b).
The exclusion of the NPA Barú reef tract as part of the NNP CRySB, which includes most of Barú’s fringing reef, was a major MPA design failure that apparently did not affect the conservation of this NPA reef. The NNP CRySB was established in 1977 when some of the reefs dominated by Acropora spp. were the central objective for conservation (Sánchez et al. 2004). In 2005, Colombia issued a management plan document for the Corales del Rosario, San Bernando e Isla Fuerte region, recommended conservation strategies, and proposed a delimitation of an MPA. The document included the Barú reef tract without monitoring, evaluation instruments, strategies, and management programs; additionally, it was classified as a recovery/restoration zone without scientific support. The Barú NPA reef tract and diapiric banks can be considered spatial refugia under global climate change and Anthropocene conditions (Hoegh-Guldberg et al. 2008; Bongaerts et al. 2010; Smith et al. 2017; Bates et al. 2019). The overlapping of 34% of the hard coral species at mesophotic and shallow zones and 42% of the fish species (See Supplementary Figure S2), and the genetic connectivity of the two coral species observed in this study, are conditions that could make this area be considered as a refuge (Bongaerts et al. 2010).
The uniqueness of the associated reefs along the Barú NPAs sums up its conservation value. The independent diapiric banks of the Barú reef system, such as Montañita, Imelda, Octubre Rojo, and Trompadas, may comprise isolated reefs with different communities and population structures as seen with O. faveolata genetics for Montañita bank. However, it also may facilitate the connectivity between the coastal zone and the NNP, as was observed with A. undata. In addition, these diapirs include dense octocoral, black coral and sponge communities (Granados et al. 2008; Escobar et al. 2012; Velásquez and Sánchez 2015; Grajales and Sánchez 2016), together with mesophotic coral ecosystems (MCEs) containing unique coral symbionts (Gonzalez-Zapata et al. 2018a, b). This distinct coral reef comprises a living laboratory for studying several aspects of marine biodiversity and MCEs. Likewise, the paradoxical Varadero reef located at the mouth of Cartagena Bay, as suggested before (Pizarro et al. 2017), is living proof of coral reef resilience maintaining the same conditions as less stressed portions of the reef: the result of a robust and connected reef system that should be protected in its integrity.
Conservation status of the NPA reef tract and future recommendations
The Barú area is within a national conservation strategy declared in 2005, at the time called the Rosario and San Bernardo Archipelago MPA (AMP-ARS; Resolution number 0679 of May 31, 2005), which was driven by local communities demanding the need to conserve marine ecosystems through the articulation of various institutions. However, it was not until 2018 that this area became legitimate for the local communities through a widespread consultation process introducing a Management Plan and the Sustainable Development Model (Ministry of Environment and Sustainable Development-MADS of Colombia), documents that to date have not been legally adopted. The AMP-CRSBeIF is not a protected figure registered in the Single National Registry of Protected Areas from Colombia or the World Database of Protected Areas (IUCN). It is not considered a protected zone, but an “in-situ conservation strategy” (Decree 1076/2015), and the management plan has been focused on the NNP CRySB, letting the other zones out of the scope.
In conclusion, the coral reefs of Barú do not currently have a constituted conservation figure that rules or suggests concrete conservation actions in the area. Additionally, the reefs of Barú outside the limits of the National Park were not included in the Official Atlas of Coral Areas of Colombia (Díaz et al. 2000), a significant setback because the laws protecting coral reefs in Colombia, whether or not under protection, only act on the areas laid out in the Atlas. Despite the above, various environmental planning documents for this region (Invemar and Cardique, 2014) and NNP CRySB and NNP Corales de Profundidad management plans highlight the need to study the composition and structure of these reefs. These documents acknowledge that connectivity can be essential for conserving other reefs in the region or improving the marine protected areas network. Furthermore, these reefs, depending on the live coral cover, are considered to be in "acceptable" (31%) or "good" (52%) conditions (Gomez and Yap 1988; Gomez et al. 1994; Tun et al. 2005). Therefore, this study provides the necessary scientific basis for the corresponding environmental authorities (Cardique and MADS) to propose strategies that allow these reefs to be established as protection zones, avoiding further alteration or degradation, and maintaining their connectivity and ecosystem function. Albeit co-management schemes (e.g., Integrated Managed Districts: "Distrito de Manejo Integrado") should be preferred over more restrictive options, particularly given the multiplicity of stakeholders in this pivotal place Cartagena and Barú areas. This co-management should be part of a broader, institutionalized management scheme to be successful and receive external support from NGOs or other nested governance institutions, as demonstrated in some studies in marine zones (Gurney et al. 2015; Voorberg and Van der Veer 2020). Likewise, co-management zones give place to the recovery of degraded sectors and establish areas of sustainable use that allow local communities to use fishery resources through participatory conservation.
Data availability
All the raw data is published as supplementary material.
Change history
13 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10531-023-02601-2
References
Alegría‐Ortega A, Sanín‐Pérez MJ, Quan‐Young LI, Londoño‐Mesa MH (2021) Genetic structure of Orbicella faveolata population reveals high connectivity among a marine protected area and Varadero Reef in the Colombian Caribbean. Aquat Conserv 14
Avigliano E, Rosso JJ, Lijtmaer D et al (2019) Biodiversity and threats in non-protected areas: a multidisciplinary and multi-taxa approach focused on the Atlantic Forest. Heliyon 5:e02292. https://doi.org/10.1016/j.heliyon.2019.e02292
Ayre DJ, Hughes TP (2000) Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution 54:1590–1605. https://doi.org/10.1111/j.0014-3820.2000.tb00704.x
Baird NA, Etter PD, Atwood TS et al (2008) Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE 3:e3376. https://doi.org/10.1371/journal.pone.0003376
Balbar AC, Metaxas A (2019) The current application of ecological connectivity in the design of marine protected areas. Glob Ecol Conserv 17:e00569. https://doi.org/10.1016/j.gecco.2019.e00569
Bates AE, Cooke RSC, Duncan MI et al (2019) Climate resilience in marine protected areas and the ‘Protection Paradox.’ Biol Conserv 236:305–314. https://doi.org/10.1016/j.biocon.2019.05.005
Beger M, Grantham HS, Pressey RL, Wilson KA, Peterson EL, Dorfman D, Mumby PJ, Lourival R, Brumbaugh DR, Possingham HP (2010) Conservation planning for connectivity across marine, freshwater, and terrestrial realms. Biol Conserv 143(3):565–575. https://doi.org/10.1016/j.biocon.2009.11.006
Benez-Secanho FJ, Dwivedi P (2020) Analyzing the provision of ecosystem services by conservation easements and other protected and non-protected areas in the Upper Chattahoochee Watershed. Sci Total Environ 717:137218. https://doi.org/10.1016/j.scitotenv.2020.137218
Bongaerts P, Ridgway T, Sampayo EM, Hoegh-Guldberg O (2010) Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29:309–327. https://doi.org/10.1007/s00338-009-0581-x
Bongaerts P, Riginos C, Brunner R et al (2017) Deep reefs are not universal refuges: reseeding potential varies among coral species. Sci Adv 3:e1602373. https://doi.org/10.1126/sciadv.1602373
Botsford LW, White JW, Coffroth M-A, Paris CB, Planes S, Shearer TL, Thorrold SR, Jones GP (2009) Connectivity and resilience of coral reef metapopulations in marine protected areas: matching empirical efforts to predictive needs. Coral Reefs 28(2):327–337. https://doi.org/10.1007/s00338-009-0466-z
Brown CJ, Harborne AR, Paris CB, Mumby PJ (2016) Uniting paradigms of connectivity in marine ecology. Ecology 97:2447–2457. https://doi.org/10.1002/ecy.1463
Bruno JF, Valdivia A (2016) Coral reef degradation is not correlated with local human population density. Sci Rep 6:29778. https://doi.org/10.1038/srep29778
Bryan-Brown DN, Brown CJ, Hughes JM, Connolly RM (2017) Patterns and trends in marine population connectivity research. Mar Ecol Prog Ser 585:243–256
Cáceres SM, Sánchez JA (2015) Growth strategies of an abundant reef-building coral in the southern Caribbean (Undaria tenuifolia). Rev Acad Colomb Cienc Exactas Físicas Nat 39:348. https://doi.org/10.18257/raccefyn.195
Camargo C, Maldonado JH, Alvarado E et al (2009) Community involvement in management for maintaining coral reef resilience and biodiversity in southern Caribbean marine protected areas. Biodivers Conserv 18:935–956
Carpenter KE, Abrar M, Aeby G et al (2008) One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Sci New Ser 321:560–563
CBD (2022) Report of the Open-ended Working Group on the Post-2020 Global Biodiversity Framework on its third meeting (Part II). Convention of Biological Diversity, Geneva, Switzerland
Cendales MH, Zea S, Díaz JM (2002) Geomorfología y unidades ecológicas del complejo de arrecifes de las Islas del Rosario e Isla Barú (Mar Caribe, Colombia). Rev Acad Colomb Cienc 26:497–510
Coffroth MA, Lasker HR, Diamond ME et al (1992) DNA fingerprints of a gorgonian coral: a method for detecting clonal structure in a vegetative species. Mar Biol 114:317–325
Díaz JM, Barrios LM, Cendales MH et al (2000) Áreas coralinas de Colombia. INVEMAR Ser Publicaciones Espec 5:176
Diaz-Pulido G, Harii S, McCook LJ, Hoegh-Guldberg O (2010) The impact of benthic algae on the settlement of a reef-building coral. Coral Reefs 29:203–208. https://doi.org/10.1007/s00338-009-0573-x
Dinno A (2017) dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums
Done TJ (1992) Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121–132
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361. https://doi.org/10.1007/s12686-011-9548-7
Edwards CB, Friedlander AM, Green AG et al (2014) Global assessment of the status of coral reef herbivorous fishes: evidence for fishing effects. Proc R Soc B Biol Sci 281:20131835. https://doi.org/10.1098/rspb.2013.1835
Epskamp S, Cramer AOJ, Waldorp LJ, et al (2012) qgraph: network visualizations of relationships in psychometric data. J Stat Softw. https://doi.org/10.18637/jss.v048.i04
Escobar D, Zea S, Sánchez JA (2012) Phylogenetic relationships among the Caribbean members of the Cliona viridis complex (Porifera, Demospongiae, Hadromerida) using nuclear and mitochondrial DNA sequences. Mol Phylogenet Evol 64:271–284. https://doi.org/10.1016/j.ympev.2012.03.021
Etter PD, Bassham S, Hohenlohe PA et al (2012) SNP discovery and genotyping for evolutionary genetics using RAD sequencing. In: Orgogozo V, Rockman MV (eds) Molecular methods for evolutionary genetics. Humana Press, Totowa, pp 157–178
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567. https://doi.org/10.1111/j.1755-0998.2010.02847.x
Ferreira HM, Magris RA, Floeter SR, Ferreira CEL (2022) Drivers of ecological effectiveness of marine protected areas: a meta-analytic approach from the Southwestern Atlantic Ocean (Brazil). J Environ Manag 301:113889. https://doi.org/10.1016/j.jenvman.2021.113889
Fong P, Paul VJ (2011) Coral reef algae. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Netherlands, pp 241–272
Foster NL, Paris CB, Kool JT et al (2012) Connectivity of Caribbean coral populations: complementary insights from empirical and modelled gene flow. Mol Ecol 21:1143–1157. https://doi.org/10.1111/j.1365-294X.2012.05455.x
Franco A, Franzoi P, Malavasi S et al (2006) Use of shallow water habitats by fish assemblages in a Mediterranean coastal lagoon. Estuar Coast Shelf Sci 66:67–83. https://doi.org/10.1016/j.ecss.2005.07.020
Froese R, Pauly D (2022) Fishbase World Wide Web electronic publication. http://www.fishbase.org.
Froese R, Thorson JT, Reyes RB Jr (2014) A Bayesian approach for estimating length-weight relationships in fishes. J Appl Ichthyol 30:78–85. https://doi.org/10.1111/jai.12299
Fung E, Imbach P, Corrales L et al (2017) Mapping conservation priorities and connectivity pathways under climate change for tropical ecosystems. Clim Change 141:77–92. https://doi.org/10.1007/s10584-016-1789-8
Garzon-Ferreira J, Kielman M (1993) Extensive mortality of corals in the Colombian caribbean during the last two decades. In: Proceedings of the colloquium on global aspects of coral reefs: health, hazards and history. MiamiRSMAS, Univ. of Miami, pp 247–253
Gomez ED, Yap HT (1988) Monitoring reef condition. P: 187–195 dalam RA Kenchington dan BET Hudson. Coral Reef Manag Handb UNESCO Reg Off Sci Technol South East Asia Jkt ID
Gomez ED, Aliño PM, Yap HT, Licuanan WY (1994) A review of the status of Philippine reefs. Mar Pollut Bull 29:62–68. https://doi.org/10.1016/0025-326X(94)90427-8
Gonzalez-Zapata FL, Bongaerts P, Ramírez-Portilla C et al (2018a) Holobiont diversity in a reef-building coral over its entire depth range in the mesophotic zone. Front Mar Sci 5:29. https://doi.org/10.3389/fmars.2018.00029
Gonzalez-Zapata FL, Gómez-Osorio S, Sánchez JA (2018b) Conspicuous endolithic algal associations in a mesophotic reef-building coral. Coral Reefs 37:705–709
Goodbody-Gringley G, Vollmer SV, Woollacott RM, Giribet G (2010) Limited gene flow in the brooding coral Favia fragum (Esper, 1797). Mar Biol 157:2591–2602. https://doi.org/10.1007/s00227-010-1521-6
Gordon AL (1967) Circulation of the Caribbean Sea. J Geophys Res 1896–1977(72):6207–6223. https://doi.org/10.1029/JZ072i024p06207
Gorospe KD, Karl SA (2013) Genetic relatedness does not retain spatial pattern across multiple spatial scales: dispersal and colonization in the coral, Pocillopora damicornis. Mol Ecol 22:3721–3736. https://doi.org/10.1111/mec.12335
Goudet J (2005) hierfstat, a package for r to compute and test hierarchical F-statistics. Mol Ecol Notes 5:184–186. https://doi.org/10.1111/j.1471-8286.2004.00828.x
Grajales A, Sánchez JA (2016) Holobiont assemblages of dominant coral species (Symbiodinium types and coral species) shape Caribbean reef community structure. Rev Acad Colomb Cienc Exactas Físicas Nat 40:300–311. https://doi.org/10.18257/raccefyn.294
Granados C, Camargo C, Zea S, Sánchez JA (2008) Phylogenetic relationships among zooxanthellae (Symbiodinium) associated to excavating sponges (Cliona spp.) reveal an unexpected lineage in the Caribbean. Mol Phylogenet Evol 49:554–560
Grorud-Colvert K, Claudet J, Tissot BN et al (2014) Marine protected area networks: assessing whether the whole is greater than the sum of its parts. PLoS ONE 9:e102298. https://doi.org/10.1371/journal.pone.0102298
Gurney GG, Pressey RL, Cinner JE et al (2015) Integrated conservation and development: evaluating a community-based marine protected area project for equality of socioeconomic impacts. Philos Trans R Soc B Biol Sci 370:20140277. https://doi.org/10.1098/rstb.2014.0277
Hallock P, Lidz BH, Cockey-Burkhard EM, Donnelly KB (2003) Foraminifera as bioindicators in coral reef assessment and monitoring: the FORAM index. In: Coastal monitoring through partnerships. Springer, pp 221–238
Hoegh-Guldberg O, Hughes L, McIntyre S et al (2008) Assisted colonization and rapid climate change. Science 321:345–346. https://doi.org/10.1126/science.1157897
Hoegh-Guldberg O, Poloczanska ES, Skirving W, Dove S (2017) Coral reef ecosystems under climate change and ocean acidification. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00158
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a caribbean coral reef. Science 265:1547–1551. https://doi.org/10.1126/science.265.5178.1547
Hughes TP, Barnes ML, Bellwood DR et al (2017a) Coral reefs in the Anthropocene. Nature 546:82
Hughes TP, Kerry JT, Álvarez-Noriega M et al (2017b) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hughes TP, Anderson KD, Connolly SR et al (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80. https://doi.org/10.1126/science.aan8048
Jackson EJ, Donovan M, Cramer K, Lam V (2014) Status and trends of Caribbean coral reefs. 1970–2012. 306
Jakobsson M, Rosenberg NA (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806. https://doi.org/10.1093/bioinformatics/btm233
Jombart T (2008) Adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405. https://doi.org/10.1093/bioinformatics/btn129
Kininmonth S, Beger M, Bode M, Peterson E, Adams VM, Dorfman D, Brumbaugh DR, Possingham HP (2011) Dispersal connectivity and reserve selection for marine conservation. Ecol Modell 222(7):1272–1282. https://doi.org/10.1016/j.ecolmodel.2011.01.012
Knight AT, Cowling RM (2007) Embracing opportunism in the selection of priority conservation areas. Conserv Biol 21:1124–1126. https://doi.org/10.1111/j.1523-1739.2007.00690.x
Kockel A, Ban NC, Costa M, Dearden P (2019) Evaluating approaches for scaling-up community-based marine-protected areas into socially equitable and ecologically representative networks. Conserv Biol 34:137–147. https://doi.org/10.1111/cobi.13368
Kohler KE, Gill SM (2006) Coral point count with excel extensions (CPCe): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269. https://doi.org/10.1016/j.cageo.2005.11.009
Kroeker KJ, Carr MH, Raimondi PT et al (2019) Assessing the potential role of marine protected areas and fisheries management approaches for resilience management in a changing ocean. Oceanography 32:117–125
López-Angarita J, Moreno-Sánchez R, Maldonado JH, Sánchez JA (2014) Evaluating linked social-ecological systems in marine protected areas. Conserv Lett 7:241–252. https://doi.org/10.1111/conl.12063
López-Victoria M, Rodríguez-Moreno M, Zapata FA (2015) A paradoxical reef from Varadero, Cartagena Bay, Colombia. Coral Reefs 34:231–231. https://doi.org/10.1007/s00338-014-1246-y
Magris RA, Pressey RL, Weeks R, Ban NC (2014) Integrating connectivity and climate change into marine conservation planning. Biol Conserv 170:207–221. https://doi.org/10.1016/j.biocon.2013.12.032
McCook LJ, Ayling T, Cappo M et al (2010) Adaptive management of the Great Barrier Reef: a globally significant demonstration of the benefits of networks of marine reserves. Proc Natl Acad Sci 107:18278–18285. https://doi.org/10.1073/pnas.0909335107
Mora C, Andréfouët S, Costello J et al (2006) Coral reefs and the global network of marine protected areas. Science 312:1750–1751
Moreno-Sánchez RDP, Maldonado JH (2010) Evaluating the role of co-management in improving governance of marine protected areas: an experimental approach in the Colombian Caribbean. Ecol Econ 69:2557–2567. https://doi.org/10.1016/j.ecolecon.2010.07.032
Muhl E-K, Esteves Dias AC, Armitage D (2020) Experiences with governance in three marine conservation zoning initiatives: parameters for assessment and pathways forward. Front Mar Sci 7:629. https://doi.org/10.3389/fmars.2020.00629
Mumby PJ, Wolff NH, Bozec Y-M, Chollett I, Halloran P (2014) Operationalizing the resilience of coral reefs in an era of climate change: mapping resilience. Conserv Lett 7(3):176–187. https://doi.org/10.1111/conl.12047
Navas-Camacho R, Vega Sequeda J, Gómez-Campo K, et al (2011) El “Sistema Nacional de Monitoreo de Arrecifes Coralinos en Colombia – SIMAC” en el Parque Nacional Natural Corales del Rosario y de San Bernardo: 1998 – 2009. En Zarza-Gonzalez, E (ed.). El Entorno Ambiental del Parque Nacional Natural Corales del Rosario y de San Bernardo. pp 330–350
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 3
Nickols KJ, White JW, Malone D et al (2019) Setting ecological expectations for adaptive management of marine protected areas. J Appl Ecol 56:2376–2385. https://doi.org/10.1111/1365-2664.13463
Nyström M, Graham NAJ, Lokrantz J, Norström AV (2008) Capturing the cornerstones of coral reef resilience: linking theory to practice. Coral Reefs 27:795–809. https://doi.org/10.1007/s00338-008-0426-z
Oksanen J (2007) Vegan: community ecology package. R package version 1.8–5. Httpwww Cran R-Proj Org
Pandolfi JM (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958. https://doi.org/10.1126/science.1085706
Peakall R, Smouse P (2012) GenAlEx 6. 5: genetic analysis in excel. Population genetic soft- ware for teaching and research—an update. Bioinformatics 1:6–8
Pendleton L, Comte A, Langdon C et al (2016) Coral reefs and people in a high-CO2 world: where can science make a difference to people? PLoS ONE 11:e0164699. https://doi.org/10.1371/journal.pone.0164699
Perry CT, Murphy GN, Kench PS et al (2014) Changing dynamics of Caribbean reef carbonate budgets: emergence of reef bioeroders as critical controls on present and future reef growth potential. Proc R Soc B Biol Sci 281:20142018. https://doi.org/10.1098/rspb.2014.2018
Perry CT, Alvarez-Filip L, Graham NAJ et al (2018) Loss of coral reef growth capacity to track future increases in sea level. Nature 558:396–400. https://doi.org/10.1038/s41586-018-0194-z
Petersen D, Laterveer M, Visser G (2007) Sexual recruitment of the coralsFavia fragum andAgaricia humilis in a 30–m3 exhibit aquarium: species-specific limitations and implications on reproductive ecology. Zoo Biol 26:75–91. https://doi.org/10.1002/zoo.20120
Pineda I, Martínez LA, Bedoya DM, et al (2006) Plan de manejo del Parque Nacional Natural Corales del Rosario y San Bernardo. UAESPNN Territ Costa Caribe
Pizarro V, Rodríguez SC, López-Victoria M, et al (2017) Unraveling the structure and composition of Varadero Reef, an improbable and imperiled coral reef in the Colombian Caribbean. PeerJ 5:e4119. https://doi.org/10.7717/peerj.4119
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959. https://doi.org/10.1111/j.1471-8286.2007.01758.x
Pujos M, Pagliardini J-L, Steer R, et al (1986) Influencia de la contra-corriente norte colombiana para la circulación de las aguas en la plataforma continental: su acción sobre la dispersión de los efluentes en suspensión del rio Magdalena. Bol Científico CIOH 3–16
Raimondi PT, Morse ANC (2000) The consequences of complex larval behaviour in a coral. Ecology 81:19
Restrepo JD, Kjerfve B (2000) Magdalena river: interannual variability (1975–1995) and revised water discharge and sediment load estimates. J Hydrol 235:137–149. https://doi.org/10.1016/S0022-1694(00)00269-9
Restrepo J, Zapata P, Diaz J et al (2006a) Fluvial fluxes into the Caribbean Sea and their impact on coastal ecosystems: the Magdalena River, Colombia. Glob Planet Change 50:33–49. https://doi.org/10.1016/j.gloplacha.2005.09.002
Restrepo JD, Zapata P, Díaz JM et al (2006b) Fluvial fluxes into the Caribbean Sea and their impact on coastal ecosystems: the Magdalena River, Colombia. Glob Planet Change 50:33–49
Roberts CM (2000) Selecting marine reserve locations: optimality versus opportunism. Bull Mar Sci 66:12
Rodríguez-Ramírez A, Reyes-Nivia MC, Zea S et al (2010) Recent dynamics and condition of coral reefs in the Colombian Caribbean. Rev Biol Trop 58:107–131
Rosenberg NA (2003) distruct: a program for the graphical display of population structure: PROGRAM NOTE. Mol Ecol Notes 4:137–138. https://doi.org/10.1046/j.1471-8286.2003.00566.x
Sala E, Giakoumi S (2018) No-take marine reserves are the most effective protected areas in the ocean. ICES J Mar Sci 75:1166–1168. https://doi.org/10.1093/icesjms/fsx059
Sánchez JA (1999a) Black coral-octocoral distribution patterns in Imelda bank, a deep-water reef, Colombia, Caribbean sea. Bull Mar Sci 65:215–225
Sánchez JA (1999b) Black coral-octocoral distribution patterns in Imelda bank, a deep-water reef, Colombia, Caribbean sea. Bull Mar Sci 215–225
Sánchez JA (2017) Diversity and evolution of octocoral animal forests at both sides of tropical America. In: Marine animal forests: the ecology of benthic biodiversity hotspots. pp 111–143
Sánchez JA, Gil MF, Chasqui LH, Alvarado EM (2004) Grazing dynamics on a Caribbean reef-building coral. Coral Reefs 23:578–583
Sánchez JA, Pizarro V, Acosta AR et al (2005) Evaluating coral reef benthic communities in remote atolls (Quitasueno, Serrana, and Roncador Banks) to recommend marine-protected areas for the Seaflower Biosphere Reserve. Atoll Res Bull 531:1–66
Sánchez JA, Gómez-Corrales M, Gutierrez-Cala L, et al (2019a) Steady decline of corals and other benthic organisms in the SeaFlower biosphere reserve (Southwestern Caribbean). Front Mar Sci. https://doi.org/10.3389/fmars.2019a.00073
Sánchez JA, Gómez-Corrales M, Gutierrez-Cala LM et al (2019b) Steady decline of corals and other benthic organisms in the SeaFlower biosphere reserve (Southwestern Caribbean). Front Mar Sci 6:73
Severance EG, Szmant AM, Karl SA (2004) Microsatellite loci isolated from the Caribbean coral, Montastraea annularis. Mol Ecol Notes 4:74–76. https://doi.org/10.1111/j.1471-8286.2004.00604.x
Severance EG, Karl ÆSA, Karl SA (2006) Contrasting population genetic structures of sympatric, mass-spawning Caribbean corals. Mar Biol 150:57–68. https://doi.org/10.1007/s00227-006-0332-2
Smith TB, Maté JL, Gyory J (2017) Thermal refuges and refugia for stony corals in the Eastern Tropical Pacific. In: Glynn PW, Manzello DP, Enochs IC (eds) Coral reefs of the Eastern Tropical Pacific: persistence and loss in a dynamic environment. Springer, Netherlands, Dordrecht, pp 501–515
Souter D, Planes S, Wicquart J, et al (2020) Status of coral reefs of the world: 2020. 20
Sully S, Burkepile DE, Donovan MK et al (2019) A global analysis of coral bleaching over the past two decades. Nat Commun 10:1264. https://doi.org/10.1038/s41467-019-09238-2
Sundqvist L, Keenan K, Zackrisson M et al (2016) Directional genetic differentiation and relative migration. Ecol Evol 6:3461–3475. https://doi.org/10.1002/ece3.2096
Thiault L, Mora C, Cinner JE, et al (2019) Escaping the perfect storm of simultaneous climate change impacts on agriculture and marine fisheries. Sci Adv 5:eaaw9976. https://doi.org/10.1126/sciadv.aaw9976
Tournois J, Darnaude AM, Ferraton F et al (2017) Lagoon nurseries make a major contribution to adult populations of a highly prized coastal fish. Limnol Oceanogr 62:1219–1233. https://doi.org/10.1002/lno.10496
Tun K, Chou LM, Cabanban A, et al (2005) Status of coral reefs, coral reef monitoring and management in Southeast Asia 2004
Underwood JN, Smith LD, Van Oppen MJH, Gilmour JP (2006) Multiple scales of genetic connectivity in a brooding coral on isolated reefs following catastrophic bleaching: GENETIC CONNECTIVITY IN A BROODING CORAL. Mol Ecol 16:771–784. https://doi.org/10.1111/j.1365-294X.2006.03187.x
UNEP-WCMC, (2008) National and regional networks of marine protected areas a review of progress. UNEP World Conservation Monitoring Centre, Cambridge
Van Moorsel G (1983) Reproductive strategies in two closely related stony corals (Agaricia, Scleractinia). Mar Ecol Prog Ser 13:273–283. https://doi.org/10.3354/meps013273
Velásquez J, Sánchez JA (2015) Octocoral species assembly and coexistence in caribbean coral reefs. PLoS ONE 10:e0129609
Velásquez J, López-Angarita J, Sánchez JA (2011) Evaluation of the FORAM index in a case of conservation. Biodivers Conserv 20:3591–3603. https://doi.org/10.1007/s10531-011-0152-7
Verdiell-Cubedo D, Oliva-Paterna FJ, Ruiz-Navarro A, Torralva M (2013) Assessing the nursery role for marine fish species in a hypersaline coastal lagoon (Mar Menor, Mediterranean Sea). Mar Biol Res 9:739–748. https://doi.org/10.1080/17451000.2013.765580
Vernette G, Mauffret A, Bobier C et al (1992) Mud diapirism, fan sedimentation and strike-slip faulting, Caribbean Colombian margin. Tectonophysics 202:335–349
Voorberg W, Van der Veer R (2020) Co-management as a successful strategy for marine conservation. J Mar Sci Eng 8:491. https://doi.org/10.3390/jmse8070491
Warner PA, Willis BL, van Oppen MJH (2016) Sperm dispersal distances estimated by parentage analysis in a brooding scleractinian coral. Mol Ecol 25:1398–1415. https://doi.org/10.1111/mec.13553
Western D, Russell S, Cuthill I (2009) The status of wildlife in protected areas compared to non-protected areas of Kenya. PLoS ONE. https://doi.org/10.1371/journal.pone.0006140
White JW, Carr M, Caselle J et al (2019) Connectivity, dispersal, and recruitment: connecting benthic communities and the coastal ocean. Oceanography 32:50–59. https://doi.org/10.5670/oceanog.2019.310
Wickham H, Averick M, Bryan J et al (2019) Welcome to the tidyverse. J Open Source Softw 4:1686
Acknowledgements
We greatly acknowledge the generous support from (Ecoral) and Federico Botero to accomplish this research. Grupo Argos kindly allowed us to use their premises (Finca Polonia), provided boat logistics to explore and study the non-protected marine areas along the Barú peninsula, and coordinated the assembly of scientists with multiple stakeholders of the study area. Alejandro Henao, Milena Marrugo from NNP Corales de Profundidad, Adrian Devia from CEINER, Adriana Rodríguez from BIOMMAR and Javier Torres from Makarela Dive Center for their field support. We express great appreciation to several BIOMMAR members for lab and field assistance.
Funding
Open Access funding provided by Colombia Consortium. This work was supported by an agreement between Argos-Ecoral-Pontificia Universidad Javeriana. Additional information from Pontificia Universidad Bolivariana was used to complete multibeam data. This project was possible with the funds provided by the National Geographic Society from an Early Career Grant, Colombia Biodiversa grant from Fundación Ángel Escobar, the support from Universidad de Cartagena and NNP Corales de Profundidad. Additional funding was possible thanks to the University of Los Andes (Vicerrectoría de Investigaciones and Facultad de Ciencias) and MinCiencias (COLCIENCIAS, Project code 120465944147).
Author information
Authors and Affiliations
Contributions
LR, JAS, AA, LFD and EA-Ch conceived the idea. JAS and LR wrote the paper with inputs from FL-GZ, MG-C, MM, EA-Ch, AA, JA and LFD. All authors collected data, LR, FL-GZ, AA, MG-C, EA-Ch and MM did the analyses. All authors revised and approved the final document.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Cesar Cordeiro.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
About this article
Cite this article
Rodríguez, L., Acosta, A., González-Zapata, F.L. et al. Conservation at the edge: connectivity and opportunities from non-protected coral reefs close to a National Park in the Colombian Caribbean. Biodivers Conserv 32, 1493–1522 (2023). https://doi.org/10.1007/s10531-022-02539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02539-x