Abstract
Environmental stability and oligotrophy are considered the main drivers of species distribution within caves due to physiological and nutritional requirements presented by many cave dwellers. However, such patterns are poorly evaluated in tropical caves, especially with regard to habitat selection and interspecific competition between invertebrate groups. Considering that troglobitic species are usually highly specialized, presenting specific requirements for environmental conditions, we hypothesize that troglobitic species will be preferentially associated with deeper areas inside the cave. These areas are stable and present trophic and physical constraints, which may favors the troglobites in competitive interactions with non-troglobitic species. The study carried out in the Águas Claras Cave System revealed a new hotspot of subterranean biodiversity, represented by 30 cave-restricted species (29 invertebrates and 1 fish species), being 73.3% terrestrial, 16.7% amphibian, and 10% aquatic. The richness of troglobitic species did not respond to physical attributes or resources availability as postulated, but increased with temperature, humidity content and with non-troglobitic species richness. The similarity of the troglobitic species along the cave was determined by the moisture content. Furthermore, the richness of troglobites was higher in those areas with greatest taxonomic distinctness of non-troglobitic species and higher values of the TB/nTB species richness ratio. The habitats requirements of the troglobitic species were not coincident, thus indicating that such species avoid niche overlapping. We highlighted the studied cave system as a singular subterranean habitat that contributes to both local and regional biodiversity. Additionally, the condition of high temperature and humidity seems to be key factors that are favoring the existence of a high number of endemic species. Unfortunately, this cave system is devoid of any official protection, thus deserving urgent actions to ensure its conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding how the local and regional species pool is related to the physical, trophic and microclimatic traits of a given habitat is one of the most important paradigms in community ecology (Hutchinson 1959; Rosenzweig 1981; Benedetti-Cecchi et al. 1997; Amarasekare 2003; Bregović and Zagmajster 2016; Foster et al. 2019). Based on the theory of niche overlap, many authors have used habitat structure or heterogeneity to predict species richness in a given area (Amarasekare and Nisbet 2001; Cornell 2010; Yang et al. 2015; Stein et al. 2015; Vargas-Mena et al. 2020). In those cases, the differential use of microhabitats is one of the main determinants of the coexistence of many species (MacArthur and Levins 1967; Tilman 1982; Chesson 2000a; Mehrabi et al. 2014). However, the differential use of habitats/microhabitats and resources by different species depends not only on their availability but also on the presence of competing species (Chesson 2000b; Amarasekare 2003; Amarasekare et al. 2004). Although habitat heterogeneity is a good predictor of abundance and diversity, the relationship between these synecological components is dependent on the spatial and temporal scale under analysis (González-Megías et al. 2007; Mehrabi et al. 2014).
Despite being considered as simplified and stable environments when compared to the surface, caves may present many types of microhabitats and organic resources, such as small cracks and interstices, rocks of different sizes, speleothems, gravel, sand, clay, lentic and lotic water bodies, biofilms, trunks, leaves, fine vegetable debris, roots, guano and carcasses (Moseley 2008; Souza-Silva et al. 2011b; Du Preez et al. 2015; Lunghi et al. 2017; Mammola and Isaia 2017; Mammola 2019; Lunghi and Manenti 2020; Mammola et al. 2020). Furthermore, environmental changes from the entrance to deeper cave locations promote a gradient of conditions and resources that provide distinct microhabitats for fauna (Moseley 2009; Tobin et al. 2013; Lunghi et al. 2014; Prous et al. 2015; Mammola and Isaia 2018; Lunghi and Manenti 2020). The occurrence of zonation in the conditions of light, temperature, humidity, and organic resources availability allows a species exchange that can give rise to distinct areas or zones with singular composition and richness (Tobin et al. 2013; Prous et al. 2004; Kozel et al. 2019; Mammola et al. 2017; Lunghi and Manenti 2020).
Species inhabiting zones close to the entrances may experience most pronounced daily and seasonal fluctuations in environmental conditions, being able to tolerate microclimatic changes to survive (Prous et al. 2004; Lunghi et al. 2014; Prous et al. 2015; Mammola et al. 2017; Mammola and Isaia 2017). On the other hand, higher stability in temperature and humidity and scarcity of organic resources occurs in the deepest cave locations (Tobin et al. 2013; Moseley 2008; Ficetola et al. 2018; Mammola 2019). Exceptionally, such traits typically observed in subterranean environments can be altered because of disturbances, caused both by human activities, such as tourism (Pellegrini and Ferreira 2016), and naturally, such as bat colonies that can alter the temperature and humidity conditions of a given cave (Ladle et al. 2012) and/or promote trophic enrichment by guano deposition (Ferreira 2019). Furthermore, rivers can transport organic resources and change trophic and microclimatic conditions (Souza-Silva et al. 2011b; Lobo et al. 2015; Simões et al. 2015; Souza-Silva et al. 2020), as well as roots of external vegetation that can access the caves (Du Preez et al. 2015; Souza-Silva et al. 2011b).
Organisms that live in caves are classified according to its ecological-evolutionary characteristics in three main categories: troglobites, troglophiles and trogloxens, proposed first by Schiner in 1854 and modified by Racovitza in 1907. Troglobites are unable to establish viable populations in external environments. The remaining groups alternatively use caves, as a shelter or as a residence (Gibert and Deharveng 2002; Sket 2008; Culver and Pipan 2013). The troglophiles are frequently found in caves and can complete their life cycle in both external and subterranean environments. The trogloxens are also frequently found in caves, but must periodically come out to the external environment to complete their life cycle. The dynamics of the spatial and temporal distribution of these categories of animals in caves are usually determined by variations in physical, microclimatic and trophic conditions, which can occur seasonally (Tobin et al. 2013; Ferreira et al. 2015; Bento et al. 2016; Lunghi et al. 2017; Kozel et al. 2019; Lunghi and Manenti 2020) or discreetly from the entrance to deeper regions (Novak et al. 2012).
The main drivers of species distributional patterns include abiotic traits (e.g. temperature, moisture and altitude), quality and availability of trophic resources and the presence of superior competitors (Cisneros et al. 2014). In subterranean habitats, interspecific competition patterns are influenced by the ecological-evolutionary category of the species with the cave. The low organic supply may favors troglobitic species in competitive interactions with non-troglobitic species, since the former demand low energy cost to survive (Sket 1999). Some studies carried out in temperate regions have shown that troglobites are mainly distributed in more stable areas inside caves, although some species can also occur in areas closer to entrances (Novak et al. 2012; Tobin et al. 2013; Manenti et al. 2015; Mammola and Isaia 2018; Kozel et al. 2019). Studies carried out in tropical caves have shown that several factors can act in structuring communities of troglobitic invertebrates in different scales. Therefore, such communities can be influenced by the cave lithology, the presence of water bodies, the cave size, the number and size of entrances, by the structure and availability of microhabitats and by trophic attributes (Souza-Silva et al. 2011a; Jaffé et al. 2016; Pellegrini et al. 2016; Jaffé et al. 2018). Different patterns for niche size are expected for temperate and tropical regions, in which it is predicted a more specialized niche use at low latitudes (Klopfer and MacArthur 1960). However, specific studies aiming to understand the role of habitat structure (e.g. temperature, humidity, substrate diversity), available resources (guano, vegetable debris, carcasses) and interespecific competition in structuring assemblages of troglobitic species throughout a cave or cave system have not yet been carried out in the tropics.
Thus, the main goal of this study was to identify the variables determining the spatial distribution of troglobites along with a new hotspot of subterranean biodiversity in the Neotropical region. Considering that troglobites are highly specialized fauna with specific requirements for environmental conditions, we used variables describing the physical, trophic, and microclimatic attributes on the cave and the richness of non-troglobitic species to test three hypothesis: (i) the troglobitic species will be preferably associated with deepest areas of the cave, which presents higher temperature and humidity stability; (ii) variations in habitat components and habitat heterogeneity on the cave floor should be the main drivers of troglobites distribution, instead of competition with non-troglobitic species and (iii) the competitive exclusion would not allow a long-term coexistence between troglobitic species, thus resulting in low niche overlapping among those species.
Methods
Study area
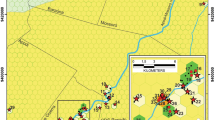
To identify the main variables driving the spatial distribution of troglobitic invertebrates in the cave substrates, we investigated the Água Clara cave system (ACCS), located in the karst region of Serra do Ramalho, municipality of Carinhanha, Bahia state, Brazil (Fig. 1). The ACCS has approximately 24 km, and is composed of four limestone caves (Table 1) trespassed by an intermittent stream, active during the austral summer (October until March). The local climate is “Aw”, according to Köppen’s climate classification system, with dry winter and an average annual rainfall of 640 mm3 (Alvares et al. 2013). The Serra do Ramalho region is inserted in the Caatinga domain (the only Brazilian semiarid biome), with transitional areas to the Cerrado (Brazilian Savanna) (Cole 1960). Due to the heavy tropical rains that occur in the region during the summer, safe access to the system is only possible in dry periods of the year (March to October).
Location of the study area in the municipality of Carinhanha, Bahia state, Brazil (A and B). Spatial distribution of the caves that form the Água Clara cave system (ACCS) system (C). Bambui Speleological group provides the cave maps (https://bambuiespeleo.wordpress.com/)
Sampling design
Habitat structure parameters, as well as the richness and composition of cave invertebrates, were determined along 59 transects (10 × 3 m2 each) equally distributed on the caves' floor, from the entrances to the deeper regions of the system. Quadrats (1 × 1 m2) were inserted in triplicates at the limits of each transects (Fig. 2), totalizing 177 quadrats. Table 1 shows the number of transects and quadrats distributed in the system’s caves. The distance between the sample units was about 150 m (Supplementary Material I). Each sample unit was collected only once in October 2017.
Measuring habitat structure
The survey of the habitat structure parameters in the transects was performed using the methodology modified from Pellegrini et al. (2016). Each transect was subdivided into 10 1 × 3 m sections (Fig. 2). In each section, the surface area occupied by different organic and inorganic substrates (Supplementary Material I) was visually quantified. After the measurement of all 10 sections, a sum was made to obtain the area occupied by each substrate throughout the entire transect. Temperature and humidity values were obtained from a digital thermo-hygrometer positioned inside the median portion of the transects, at ground level.
Invertebrate sampling
Invertebrate sampling was carried out by visual search and exhaustive manual collection with the aid of tweezers and brushes along the transects and quadrats (Sharratt et al. 2000; Wynne et al. 2019). The additional use of quadrats, which comprises small-scale sampling, allows the detection of small-size and low mobility species, which can then be thoroughly searched in the remaining transect if detected. The invertebrates sampling was firstly performed in the quadrats and later in the respective transect, always by two collectors, and was only completed when all the invertebrates had been sampled and/or accounted. Given the structural distinction between the different sampling areas along with the caves (due to the presence/absence of crevices, rocks, and ledges) the searching time varied among each sampling unit (harratt et al. 2000). Furthermore, to maximize the detection of cave-restricted species, direct intuitive search techniques were also applied outside the mentioned sample units for better coverage of all microhabitats inside the caves (Wynne et al. 2019).
Invertebrate identification
All sampled invertebrates (troglobites—TB and non-troglobites—nTB) were identified to the lowest accessible taxonomic level and grouped into morphotypes (Oliver and Beattie 1996). The determination of potentially troglobitic species was carried out based on the presence of troglomorphic traits (for instance eyes and pigmentation reduction, appendages elongation) (Christiansen 1962). Specialists in different taxa were also consulted to assist in the detection of specific troglomorphisms (specialists are acknowledged further on). Voucher specimens were deposited in the Collection of Subterranean Invertebrates of Lavras (ISLA), linked to the Center for Studies in Subterranean Biology from the Federal University of Lavras (www.biologiasubterranea.com.br).
Data analysis
We performed a data analysis sequence as recommended by Shmueli (2010), firstly conducting an exploratory data analysis and secondly an explanatory data analysis. To analyze the eventual existence of spatial autocorrelated data we used generalized linear models (glms), with the troglobitic species richness as a response variable and the distance among the transects as an explanatory variable. This distance was obtained from an Euclidean distance matrix between the transects built through the ‘dist’ function of the ‘stats’ package using the geographical coordinates of each sample unit, which were obtained from the plotting of the transects on the map of each cave, and subsequently projected in the surface. Then, the first column of the matrix was used as the distance vector, which corresponds to the distances in relation to the AC-1 point, which was used as an explanatory variable in the glm. We performed ‘shapiro.test’ function from the ‘stats’ package, which revealed non-normal distribution of the data. We used quasi-poisson error distribution, since the ‘dispersiontest’ function from the ‘aer’ package revealed overdispersion for the poisson family. The glm was constructed using the ‘glm’ function from the ‘stats’ package. We assumed that there is no spatial auto-correlation in the data since there was no significant relationship between the variables.
Abiotic attributes on the cave floor
All the physical, trophic and microclimatic characteristics of the transects were evaluated and classified in the following classes: retraction cracks—GRE, hardpan—HRP, silt—SIL (0.2 < diameter ≤ 0.05 mm), fine gravel—GRAF (2−16 mm), coarse gravel—GRAC (17−63 mm), rock blocks—BLO (64−250 mm), matrix rock—ROC, water ponds—PON, temperature—TEMP, humidity—MOT, actinomycetes biofilms—ACT, wood—WOO, guano—GUA, fine particulate organic matter—FOM, roots—ROO. Based on such classes we obtained the physical features of the caves that included the distance from the entrance, the substrate diversity (calculated considering all the classes aforementioned) using Shannon-Weaver index (Buttigieg and Ramette 2014), and the availability of shelter for the invertebrates (calculated by the sum of GRE, GRAC, BLO, and WOO in each transect). We considered as trophic resources GUA, OM (comprising FOM and ROO) and WOO. Finally, the microclimatic variables, TEMP and MOT were also considered separately.
To explore the differences in trophic, microclimatic, and physical attributes of the four caves, we evaluated the existence of differences in the averages of temperature, humidity, substrate diversity, availability of shelter, and trophic resources of each transect among caves using Wilcoxon-Mann-Whitney U test (Sprent and Smeeton 2000). In sequence, we checked if such trophic, microclimatic, and physical attributes varied depending on the distance from the nearest entrance using a distance-based linear model (DistLM) with Euclidean distance. We used the stepwise procedure and the AICc model selection criterion, in which models with smaller values of AICc are the better ones, after 999 permutations (Anderson et al. 2008).
Biotic attributes on the cave floor
The abundance and richness of cave-restricted species for each transect were accounted. Exploratory data analysis was performed using species composition similarities among the four caves from ACCS with a Bray-Curtis distance index after the sqroot transformation, using the transects as the sampling units (Buttigieg and Ramette 2014; Clarke et al. 2014). To access differences on average in cave-restricted species richness among caves and transects it was performed a Wilcoxon−Mann−Whitney U test. All the significance was regarded at p < 0.05. To account for the number of undetected troglobitic species, it was calculated the extrapolated richness for the transects dataset using non-parametric richness estimators (Jackknife 1 and 2) (Buttigieg and Ramette 2014). The level of ‘completeness’ of the sampling effort was achieved by dividing the observed number of taxa by the estimated values calculated by Jacknife 1 estimator (Àvila et al. 2019).
The average taxonomic distinctness, the richness of non-troglobitic species and the ratio between troglobitic with non-troglobitic species were used as a proxy of interspecific competition with the troglobitic species. The average taxonomic distinctness (∆+) analysis was conducted using non-troglobitic species, being Class (weight 100), Order (weight 75), Family (weight 50), and morphotypes (weight 25) as info variables for a matrix of morphotypes distribution among transects (Anderson et al. 2008).
Finally, we presented a visual representation of the biodata matrix according to the spatial distribution of the transects along the ACCS (after it has been sqroot transformed), in a shaded plot. The species composition and sample distribution have been re-ordered in a cluster analysis utilizing Whittaker’s Index of Association (Whittaker 1952; Clarke et al. 2014).
Relationship between habitat structure and interspecific competition with cave-restricted fauna
To analyze the effects of physical, trophic, microclimatic features and interspecific competition influence on cave-restricted species richness, we performed four independent glm's. In the glm's, the error distribution with the best fit was the Quasi-Poisson family. Then we used the ‘anova’ function from ‘vegan’ package to compare the glm's results with the null model. For those significantly different from the null model, we used an information-theoretic approach based on the AICc to rank the models, which indicates the most parsimonious model (Burnham et al. 2011). Further, we used the ‘dredge’ function from the ‘mumin’ package to test all possible combinations of the variables included in the full model and ranked them by the AICc-based model weight. Since the best fit was for the quasi-poisson family, we used the steps proposed by Bolker (2020) for extracting the AICc or quasi-models. Finally, all possible models were ranked, and we considered only those models that had ΔAICc lower than two to be strongly supported. To obtain the adjusted r2 values we used the ‘rsq’ function from the ‘rsq’ package.
Before running the glm's analysis, we tested them for multicollinearity among explanatory variables to prevent variance inflation factors using the ‘chart.correlation’ function from the ‘performanceanalytics’ package. Since none of the explanatory variables from physical, trophic and microclimate features showed correlation values higher than 65%, all variables were included in the models. For interspecific competition variables, we found a strong correlation between non-troglobitic species richness with the troglobitic and non-troglobitic species ratio. In this case, we run two glm analyses alternating the insertion of the correlated variables. After obtaining the AIC values following Bolker`s method (2020), we applied the ‘model.sel’ function found in the ‘mumin’ package to obtain the best model.
In order to evaluate if the distance from entrance affects the ratio between troglobitic and non-troglobitic species richness, we also performed glm analyses. We checked troglobitic/non-troglobitic ratio data normality using the shapiro.test from stats package. Since the response variable had a non-normal distribution, we also built the model using the Quasi-Poisson family, which showed the best fit for the error distribution. We performed the glm's analyses in the r software, version 3.6.2 (R Core Team 2019).
To explain the eventual relationships between troglobitic species composition with physical, trophic, and microclimatic variables, we applied a Distance-based linear model (DistLM) with Bray-Curtis index for faunal composition, excluding singletons and doubletons. We used the stepwise procedure and the AICc model selection criterion, in which models with smaller values of AICc are the better ones, after 999 permutations (Anderson et al. 2008). We performed the distance-based redundancy analysis (dbRDA) to ordinate and visualize the strength of all predictor variables on species composition from the DistLm result (Buttigieg and Ramette 2014; Clarke et al. 2014).
Habitat selection
We evaluated the habitat selection using a multivariate approach based on the niche concept, considering the presence of the ten most frequent troglobitic species—which occurred in at least five transects. We performed Outlying Marginality Index (omi) analyses, which measures the distance between the mean used for each species and the mean available values for each environmental condition—including the physical, trophic and microclimatic variables—of the total sampled area (Dolédec et al. 2000). Then the omi analysis plot each species niche in relation to one reference species, that is the most tolerant to the general habitat condition. omi analysis fits well for strong driving forces such as gradients; in the present study the gradient is represented by the environmental factors changing from entrance to deep cave. The given results provide the variability of each species decomposed into three components: (i) omi—index of marginality (distance of each species from an uniform distribution); (ii) tol—index of tolerance or niche breadth; and (iii) rtol—residual tolerance (determines the confiability of the determined niche).
To run the omi analyses, first, we summarized the patterns of covariation among physical, trophic, and microclimatic variables by performing a principal component analysis (pca). Then the niche from each of the ten selected species was calculated and plotted in the environmental niche. The analyses were performed in the R program (Development Core Team 2019) utilizing the ‘ade4’ package (Dray and Dufour 2007). The Monte Carlo test with 999 permutations was used to evaluate the significance of niche marginality and the average marginality of each species (Dolédec et al. 2000).
Results
Habitat structure
The values for temperature, humidity, substrate diversity, availability of shelter, and trophic resources are shown in Spplementary Material I. Only temperature and humidity showed significant differences between the caves. The substrates diversity presented a negative relationship with the distance from the nearest entrance (AICc = – 109.11, R2 = 0.14, p = 0.005), while the humidity showed a positive relationship with the nearest entrance (AICc = 247.03, R2 = 0.21, p = 0.001).
Richness and composition of cave-restricted fauna
Considering the whole ACCS (including the sampling in the transects, quadrats, and other habitats), a total of 621 specimens with troglomorphic traits were recorded, comprising 30 troglobitic species distributed in Hexapoda (9 spp.), Arachnida (7 spp.), Crustacea (6 spp.), Myriapoda (4 spp.), Gastropoda (2 spp.), Turbellaria (1 sp.) and Siluriformes (1 sp.) (Fig. 3). Terrestrial species were predominant (22 spp.), followed by amphibious (5 spp.), and aquatic species (3 spp.) (Table 2). Illustrative pictures of some troglobitic species found in the ACCS are shown in Fig. 4. The species composition and richness distribution of troglobitic species are shown in Supplementary Materials II and III.
Some of the species restricted to the Água Clara cave system (ACCS), Brazil. Xangoniscus aganju (A), Styloniscidae sp.2 (B), Pectenoniscus carinhanhensis (C), Trichorhina sp.1 (D), Blattodea sp.1 (E), Mesodiplatys falcifer (F), Nylanderia sp.1 (G), Endecous sp (H), Sminthuridae sp.2 (I), Ochyroceratidae sp.1 (J), Eukoenenia sp.1 (K), Chthoniidae sp.1 (L), Giupponia chagasi (M), Charinus troglobius (N), Chelodesmidae sp.1 (O), Pyrgodesmidae sp.1 (P), Trichopolydesmidae sp. (Q), Geophilomorpha sp.1 (R), Spiripockia punctata (S), Trichomycterus rubbioli (T)
In both the quadrats and transects, 25 species of terrestrial troglobites were registered, distributed in Hexapoda (8 spp.), Arachnida (7 spp.), Crustacea (5 spp.), Myriapoda (4 spp.), and Gastropoda (1 sp.). Hence, amphibian and aquatic troglobites/stygobites were only registered in habitats not incorporated within transects. In the transects, 22 troglobitic species were registered (5 spp., exclusively found in such units), while in the quadrats 18 species were found (2 spp., exclusively found in such units) (Table 3) (Figs. 5 and 6).
Shade plot showing the distribution of the 25 trogobites species at Gruna da Água Clara (AC), Gruna dos Índos (IN), Lapa dos Peixes I (LPI) e Lapa dos Peixes II (LPII). Numbers from 70 to 3300 are the distances of the transects concerning the entrance (meters). The species list and sample units were grouped with a cluster analysis utilizing Whittaker’s Index of Association (displayed left and up). Abundance was sqroot transformed
Spots of the richness of the cave-restricted species from the entrance to deeper parts in the Água Clara cave system (ACCS). The distance among sample units (colored bubbles) is approximately 150 m (except Gruna dos Índios; 75 m). The bubble size represent troglobitic species richness and the color gradient represent the proportion between troglobitic and non-troglobitic species in each site (log transformed)
Considering the whole ACCS (including the sampling in the transects, quadrats, and other habitats), a total of 6783 specimens with non-troglomorphic traits were recorded, comprising 142 species distributed in Hexapoda (85 spp.), Arachnida (43 spp.), Myriapoda (5 spp.), Annelida (3 spp.), Mollusca (3 spp.), Turbellaria (2 spp.), and Nematoda (1 sp.). The number of non-troglobitic species by transect is shown in Supplementary Material II.
Considering the whole ACCS (including the sampling in the transects, quadrats, and other habitats), Gruna da Água Clara cave presented the highest richness of troglobitic species (23 spp.), with four species exclusively observed in this cave (Symphypleona sp2, Rhagidiidae sp1, Caponiidae sp.1, Trichorhina sp.1). Lapa dos Peixes I cave had 19 species, while Lapa dos Peixes II cave had 17 species and Gruna dos Índios cave only four species (Table 1). Caves with the largest number of shared species were Gruna da Água Clara and Lapa dos Peixes I cave (16 spp.), both located at the “extremes” of the ACCS (presenting approximately 3 km of the linear distance between the nearest entrances). Only two species (Chelodesmidae sp.1 and Endecous sp.1) were distributed along all caves in the system (Table 2).
The estimated troglobitic species richness suggests that the sampling effort achieved adequate levels of completeness, as the observed richness (25 spp.) corresponded to over 78% of the estimated richness. No significant differences were observed in the average troglobitic richness (per square meter) between the caves of the ACCS. The average richness was 3 spp./30 m2 (sd = 2) in the Gruna da Água Clara cave, 2 spp./30m2 (sd = 2) in the Gruna dos Índios cave, 3 spp./30 m2 (sd = 1) in the Lapa dos Peixes I cave and 3 spp./30m2 (sd = 1) in the Lapa dos Peixes II cave.
Relationship between habitat structure and interspecific competition with cave-restricted fauna
The models selected for physical and trophic variables showed no significant difference from the null model to explain troglobitic species richness. Only the microclimate and interspecific competition variables presented models that differed from the null model. The most parsimonious model to explain the cave-restricted fauna richness among microclimate variables showed a positive effect of temperature and humidity (Table 3; Fig. 7 A and B). For the models selected including interspecific competition variables, troglobitic species richness also increased with the TB/nTB species richness ratio and non-troglobitic taxonomic distinctness (∆+) (Tables 3 and 4; Fig. 7 C and D). Models including the non-troglobitic richness were not different from the null model. The TB/nTB species richness ratio increased from the regions close to entrances to deeper portions of the caves (r2 = 0.172, p = 0.001, Figs. 6 and 8). Thus, troglobitic species tend to be more frequent than non-troglobitic species in deeper zones of the ACCS.
The variable that explained the variation in troglobitic composition throughout the ACCS was humidity (AICc = 490.71; R2= 0.06, p = 0.001). The first two axes of the distance-based redundancy analysis (dbRDA) using all the substrate variables sampled in transects explained 12.7% of the total variation of the biodata cloud (Fig. 9).
Explanatory analysis using dbRDA with physical, microclimatic, and trophic variables at the cave floor against troglofauna composition. The biggest Bray-Curtis similarity values are circled. Actinomycetes (ACT), wood (WOO), guano (GUA), fine particulate organic matter (FOM), roots (ROO), substrate diversity (DIVS), availability of shelter (DIVSH), temperature (TEMP), humidity (MOT), distance from the entrance (DIST)
The ten troglobitic species included in the OMI analysis were three isopods (Trichorhina sp.1, Pectenoniscus carinhanhensis and Styloniscidae sp.2), the two springtails (Sminthuridae sp.2 and Entomobryomorpha sp.1), the harvestman Giupponia chagasi Pérez & Kury, 2002; the spider Ochyroceratidae sp.1; the microwhip scorpion Eukoenenia sp.1; the millipede Chelodesmidae sp.1; and finally, the most widespread troglobitic species found in the system, the cricket Endecous sp.1 (Table 2; Fig. 10). From those species, the most specialized, with higher values of niche marginality were Trichorhina sp.1, which was more associated with the presence of wood, and Sminthuridae sp.2, preferably associated with the deeper regions of the caves. Among the most generalist species, with lower values of niche marginality and consequently higher environmental tolerance, stood out Chelodesmidae sp.1, Ochyroceratidae sp.1, Entomobryomorpha sp.4 and Eukoenenia sp.1. The other species represented a subtle deviation from the overall average habitat. Styloniscidae sp.2 was preferably associated with warmer regions; Giupponia chagasi with deeper regions of the caves; Pectenoniscus carinhanhensis and Endecous sp.1 with drier regions with wood. The overall omi analysis was able to explain significantly (p = 0.05) the distances between the habitat conditions and the habitat used by the evaluated species (Fig. 10; Table 4).
Discussion
The results showed that among the tested cave features, only the microclimatic and interspecific competition affected cave-restricted species richness. More specifically, troglobitic species richness was positively related with temperature, humidity and with non-troglobitic species richness and taxonomic distinctness. This finding suggests that organic resources and the habitat physical structure are not the main drivers of cave-restricted species richness distribution in this cave system. Troglobitic species tend to be relatively more frequent than non-troglobitic species in deeper zones of the ACCS. Similarly, the microclimatic conditions are the most determinant attribute for the variation in troglobitic species composition. Species-habitat preferences were different for each troglobitic species analyzed (10 species).
Responses of troglobitic species to environmental conditions are still poorly explored around the world. Although the temperate and tropical regions are quite distinct in many environmental attributes, caves share some physical, microclimatic, and trophic characteristics that drive similar patterns for troglobitic communities. The high environmental stability has shaped similar environmental requirements and ecological traits of terrestrial invertebrate species highly specialized to moist conditions and to oligotrophic environments. However, troglobitic species can be specialized to distinct habitats, as already shown for the species composition among shallow and deep subterranean habitats, which can be considerably distinct (Novak et al. 2012; Kozel et al. 2019).
The cuticle thinning (which in turn leads to greater intolerance to desiccation) and reduced metabolic rates are among the most observed adaptations in cave-restricted species. However, such traits often limit the distribution of these species to highly humid places that also tend to present narrow temperature ranges (Tobin et al. 2013; Lunghi et al. 2014 and 2017; Kozel et al. 2019). Thus, troglobites tend to occupy more stable areas inside caves, even if these conditions also occur closer to the entrances (Novak et al. 2012; Tobin et al. 2013; Lunghi et al. 2017; Mammola and Isaia 2018). Furthermore, we observed a gradual reduction in the richness of non-troglobitic species from the entrance to deeper cave sites, suggesting the existence of ecotones between the entrance regions and the innermost areas, which are climatically more stable. Thus, in the regions closest to the entrances, troglobitic and non-troglobitic species can coexist, while in the inner portions of the caves TB/nTB rate favors the troglobites (Novak et al. 2012; Tobin et al. 2013; Kozel et al. 2019).
The increase in troglobitic species richness related to the rise of temperature and humidity is probably related to their high degree of specialization, which makes them select more stable areas in caves, which are generally located in deeper areas, and are usually oligotrophic (Novak et al. 2012; Tobin et al. 2013). Corroborating this hypothesis, Deharveng and Bedos (2000) observed that troglobitic invertebrates become more frequent in areas far from trophic resources since these organisms avoid the coexistence with non-troglobitic competitors. Moreover, Sket (1999) hypothesized that the comparatively high number of stygobitic crustaceans in limited and oligotrophic subterranean habitats in the Dinaric karst, seems to be related to the lack of competitors (mainly insects), spatial and ecological habitat partition, and favorable temperatures.
The reduced metabolic rates and/or changes in life history towards the K strategy allows the troglobites to survive during long periods of starvation (Hüppop 2005). However, an increase in the organic resources input can be harmful to these organisms, which in such circumstances tend to be excluded by more energetically demanding and competitively superior non-troglobitic species (Sket 1999). Despite this, the presumably high competition for scarce resources in deep subterranean environments leads to specializations in specific habitats, preventing a high niche overlapping. Thus, the usually limited occurrence range observed for troglobites must be caused by their high ecological specialization concerning specific habitat traits. Both resource scarcity and reduced substrate diversity decrease the potential number of competing species capable of establishing populations in subterranean environments (Sket 1999). Thus, the contrasting distribution of non-troglobitic and the richness of troglobites may indicate a tendency towards a reduction in the interspecific spatial overlap between these two ecological-evolutionary categories.
The analysis performed in ACCS showed that the most widespread troglobitic species can use areas with different microhabitat traits, thus avoiding niche overlapping, what allows their coexistence. Furthermore, the different microhabitat preferences showed by the troglobites may be the reason why no negative significant relationship among their richness and the richness of non-troglobitic species was observed, as initially hypothesized. Thus, since each troglobitic species tends to respond differently to distinct microhabitats, the richness, in this case, ends up not being a good predictor variable. In addition, even though it has not been evaluated, it is likely that microhabitat preferences also occurs in non-troglobitic species, so that simply comparing the number of species between these categories can be innocuous when addressing this kind of hypothesis.
The niche determined for the most frequent species revealed some patterns. Although it was possible to observe that predator species represented by Ochyroceratidae sp 1, Eukoenenia sp 1 and Giupponia chagassi showed a tendency towards occupying deeper portions of the caves, these species showed a very low level of niche specialization. Predators are known to be more related to prey distribution than to environmental conditions (Bogan and Lytle 2007), which explains their occurrence associated with the average habitat conditions available in the studied system. This pattern can be expected to be even more pronounced in the case of oligotrophic systems as caves. The lack of prey availability makes the active foraging strategy more advantageous for predators, instead of sit-and-wait strategies. The generalist behavior was also observed for Chelodesmidae sp 1 and Endecous sp 1, two generalist scavenger species (Barker 2004; Paixão et al. 2017) for which high levels of habitat tolerance are expected.
Another interesting pattern found is that Trichorrina sp 1 was by far the most specialized species evaluated, and together with the other isopods species, showed preference for habitats with wood availability. This finding brings conservation insights, evidencing the need of preserving the surrounding environment for the continuous inputs of wood resource and the consequent maintenance of such isopod populations. The springtails showed distinct patterns: while the Entomobryomorpha sp 1 was more generalist, the Sminthuridae showed a strong preference for the deep parts of the cave. The more generalist habit exhibited by the Entomobryomorpha sp 1 is compatible with its detritivorous habit. This characteristic allows a small part of the troglobitic springtails to be able to reach very wide geographic distributions, including most of Europe (Dányi 2011). In the other hand, more sensitive species, such as Sminthuridae, can be closely related to conditions only found in deep caves, with more stable environmental conditions and high humidity as observed in the present study. Springtails are among the few animals able to reach major depths in caves, as the case of Schaefferia profundissima Jordana & Baquero 2012, recorded at -1600 m and Plutomurus ortobalaganensis Jordana & Baquero 2012, recorded at -1980 m, both at the Krubera-Voronja cave (Sendra & Reboleira 2012). This ability of springtails to colonize very deep regions of caves can be advantageous in a cave system colonized by so many troglobitic species, allowing them to avoid interspecific competition.
The Água Clara cave system as a hotspot of subterranean biodiversity
The high richness of troglobites (30 cave-restricted species), makes the Água Clara cave system a new hotspot of subterranean biodiversity in South America (Souza-Silva and Ferreira 2016), being currently the richest system in troglobitic/stygobitic species in Brazil. In the world, there are 38 hotspots of subterranean biodiversity, which occur on all continents, except for Antarctica and Africa (Pipan et al. 2020), although the Wynberg cave system, in South Africa, with 19 cave-restricted species, has a huge potential to soon become the first hotspot of subterranean biodiversity in Africa (Ferreira et al. 2020).
Most hotspots are located in Europe and North America and tropical hotspots of subterranean biodiversity are restricted to Australia, Brazil and Indonesia. It may be speculated that this pattern stems from the fact that most karst areas of the world are located in temperate regions, thus a higher richness of cave-restricted species can be expected, since such habitats served as a refuge during past climate changes (Bar 1968; Romero 2009). However, the high number of new troglomorphic species recently discovered in Brazilian caves (Souza-Silva et al. 2011a; Souza-Silva and Ferreira 2016; Souza-Silva et al. 2020; Cardoso et al. 2021) indicates that events of climatic changes in the Neotropics, could also have led to the isolation of subterranean lineages (Souza-Silva and Ferreira 2016). Furthermore, other mechanisms of isolation (for instance, parapatric speciation, oceanic introgressions and regressions) might have led to the evolution of many lineages of subterranean fauna in Brazil (Fišer et al. 2013; Leal-Zanchet et al. 2014; Benítez-Álvarez et al. 2020; Castro-Souza et al. 2020). Moreover, most of the caves, particularly in tropical areas, have not been thoroughly explored (Deharveng and Bedos 2012). Currently, only few caves in Brazil present more than 10 troglomorphic species (Souza-Silva et al. 2011a; Rabelo et al. 2018; Cardoso et al. 2021) and only two are considered hotspots (Souza-Silva and Ferreira 2016).
High richness of cave-restricted species is generally associated with oversized caves, high productivity and/or the presence of water bodies isolated from the surface (Culver and Pipan 2009). In addition to those previously mentioned conditions, the high richness observed in the ACCS may be related to the Brazilian Tropical Dry Forest (more specifically the Caatinga domain) in which it is located. Many caves within the Caatinga, especially those with the presence of perennial water, present an outstanding diversity and endemism of cave-restricted fauna (Prevorčnik et al. 2012; Bento et al. 2016; Souza-Silva and Ferreira 2016; Cardoso et al. 2021). This fact may be related to the current xeric conditions of the region associated to the historical changes undergone by this biome. Many areas currently covered by the Caatinga formation presented a moist tropical forest in the Last Glacial Maximum (LGM) (Collevatti et al. 2013), which may have sheltered the ancestors of many troglobitic species, which may have been posteriorly “trapped” inside caves after the retraction of the moist forests (Wang et al. 2004; Polhemus and Ferreira 2018).
Preserving the biodiversity of the Água Clara cave system
An important action for preserving the ACCS would be the description of the troglomorphic species that this system shelters since non-described species are often ignored in conservation actions (Cardoso et al. 2011). Most cave-restricted species in tropical and subtropical areas are not formally described (Souza-Silva and Ferreira 2016; Pipan et al. 2020). Furthermore, ignoring undescribed species can artificially indicate that caves are poor in such species (Pipan et al. 2020). However, consultation with specialists associated with the use of troglomorphic traits increases the chances of more accurate diagnoses about the status of these species.
As an example, among the 30 cave-restricted species observed in the ACCS, only 8 (~26%) are formally described: Girardia spelaea (Tricladida), Spiripockia punctata (Gastropoda: Pomatiopsidae), Giupponia chagasi (Opiliones, Gonyleptidae), Charinus troglobius (Amblypygi: Charinidae), Xangoniscus aganju (Isopoda, Styloniscidae), Pectenoniscus carinhanhensis (Isopoda, Styloniscidae), Mesodiplatys falcifer (Dermaptera: Diplatyidae) and Trichomycterus rubbioli (Siluriformes: Trichomycteridae). Most of those species are already inserted in the Brazilian list of threatened species, categorized as critically endangered (ICMBio 2018). A similar proportion of described species was observed for the Toca do Gonçalo cave (22 troglobitic species, with only 22% of them described) (Souza-Silva and Ferreira 2016). This fact unveils the lack of Brazilian taxonomists interested in cave fauna and the difficulties faced by foreign taxonomists to study these organisms (especially due to legal hindrances). Financial support calls focused in the description of subterranean taxa in Brazil resulted in relatively few species descriptions in the last years, indicating urgent needs of rethinking strategies for describing and protecting this singular, endemic and threatened fauna. It is important to highlight that only described species are evaluated according to the IUCN criteria. Hence, around 74% of the cave-restricted species form the ACCS are legally unprotected. This is an extremely disturbing fact since anthropic changes as deforestation and soil disturbance persist around the caves that make up the system.
The Serra do Ramalho region underwent rural settlement programs by the Brazilian government in the 1970s, which established small agricultural villages in the region (Moura and Bichuette 2008). According to Moura and Bichuette (2008), deforestation in areas close to the ACCS occurs mainly due to charcoal production and the establishment of monocultures. The removal of epigean vegetation in the areas that directly influence the caves can affect the supply of organic and inorganic sediments to the caves (Gilieson 1996; Culver 1982; Souza-Silva et al. 2011b) and, in turn, the food resources available for the hypogean fauna. Cave communities associated with systems with low availability of guano and carcasses, as the ACCS, may be more dependent on allochthonous food resources (Souza-Silva et al. 2011b).
According to Auler et al. (2001), the silting up of the adjacent rivers, as well as some of the cave conducts, has increased in the last years. Despite the stream’s ability to transport organic matter and to keep high humidity conditions in the ACCS, the degradation of surface environments ends up transforming them into vehicles of environmental impacts, thus potentially reducing the cave fauna. In addition, Gonçalves et al. (2018) reported the vulnerability of the Serra do Ramalho´s aquifer due to deficiencies in basic sanitation in the area. Thus, protecting the ACCS requires actions to both encourage taxonomic and ecological research and manage the area considering social, environmental, and economic contexts (Ford 2005; Brinkmann and Garren 2011; Van Beynen and Van Beynen 2011; Culver and Pipan 2013; Osborne 2019).
However, efforts must be directed to ensure a consensus between the multiple environmental and cultural facets involved in the creation of conservation areas. Usually, the communities involved or affected by the delimitation of these areas, depending on the process, can be a source of cooperation or conflicts (Rylands and Brandon 2005). In this sense, the creation of conservation units, with the community involvement, can be an important strategy to protect the Água Clara Cave System. Sugai et al. (2015) found that 11.6% of the 13,816 registered caves in Brazil are within protected areas, whereas almost half of those caves are at risk to be affected by mining expansion. Historically, the main justification for creating protected areas for the conservation of Brazilian speleological heritage has been based on scenic beauty and geological, paleontological, and archaeological attributes (Souza-Silva et al. 2015). However, this scenario has changed, and the cave fauna has been considered when establishing priority areas for the creation of conservation units. The first Brazilian conservation unit that considered cave biodiversity as an important attribute for its creation was the National Park of Furna Feia, located in Rio Grande do Norte state (Brazil 2012). Thus, we hope that the uniqueness of the cave fauna from the ACCS will contribute to the creation of a conservation unit that will ensure its maintenance along time.
Data availability
The data used in this work are available in the Supplementary Material I, II and III.
References
Alvares CA, Stape JL, Sentelhas PC, Moraes-Gonçalves JL, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Amarasekare P (2003) Competitive coexistence in spatially structured environments: a synthesis. Ecol Lett 6(12):1109–1122
Amarasekare P, Nisbet R (2001) Spatial heterogeneity, source-sink dynamics and the local coexistence of competing species. Am Nat 158:572–584. https://doi.org/10.1086/323586
Amarasekare P, Hoopes MF, Mouquet N, Holyoak M (2004) Mechanisms of coexistence in competitive metacommunities. Am Nat 164(3):310–326. https://doi.org/10.1086/422858
Anderson MJ, Gorley RN, Clark KR (2008) PERMANOVA for PRIMER: Guide to software and statistical methods. PRIMER-E, Plymouth
Auler A, Rubbioli E, Brandi R (2001) As grandes cavernas do Brasil. Grupo Bambuí de Pesquisas Espeleológicas, Belo Horizonte
Ávila AC, Pires MM, Rodrigues EN, Costi JA, Stenert C, Maltchik L (2019) Drivers of the beta diversity of spider assemblages in southern Brazilian temporary wetlands. Ecol Entomol 45(3):466–475. https://doi.org/10.1111/een.12816
Bar TC (1968) Cave Ecology and the Evolution of Troglobites. Evol Biol 2:35–102
Barker GM (2004) Millipedes (Diplopoda) and centipedes (Chilopoda) (Myriapoda) as predators of terrestrial gastropods. In: Barker GM (ed) Natural enemies of terrestrial molluscs. CAB International, Wallingford, pp 405–426
Benedetti-Cecchi L, Airoldi L, Abbiati M, Cinelli F (1997) Exploring the causes of spatial variation in an assemblage of benthic invertebrates from a submarine cave with sulphur springs. J Exp Mar Biol Ecol 208(1-2):153–168. https://doi.org/10.1016/S0022-0981(96)02650-0
Benítez-Álvarez L, Leal-Zanchet AM, Oceguera-Figueroa A, Ferreira RL, de Medeiros Bento D, Braccini J, Sluys R, Riutort M (2020) Phylogeny and biogeography of the Cavernicola (Platyhelminthes: Tricladida): relicts of an epigean group sheltering in caves? Mol Phylogenet Evol 145:106709. https://doi.org/10.1016/j.ympev.2019.106709
Bento DDM, Ferreira RL, Prous X, Souza-Silva M, Bellini BC, Vasconcellos A (2016) Seasonal Variations in Cave Invertebrate Communities in the Semiarid Caatinga, Brazil. J Caves Karst Stud 78:61–71. https://doi.org/10.4311/2015LSC0111
Bogan MT, Lytle DA (2007) Seasonal flow variation allows ‘‘time-sharing’’ by dispatate aquatic insects communities in montane desert streams. Freshwater Biol 52:290–304. https://doi.org/10.1111/j.1365-2427.2006.01691.x
Bolker B (2020) Dealing with quasi-models in R. Retrieved from http://creativecommons.org/licenses/by-nc/3.0/. Accessed 27 May 2021
Brasil D (2012) Decreto de 5 de junho de 2012. Dispõe sobre a criação do Parque Nacional da Furna Feia, nos Municípios de Baraúna e Mossoró, Estado do Rio Grande do Norte. Diário Oficial da União, Brasil
Bregović P, Zagmajster M (2016) Understanding hotspots within a global hotspot: identifying the drivers of regional species richness patterns in terrestrial subterranean habitats. Insect Conserv Divers 9:268–281. https://doi.org/10.1111/icad.12164
Brinkmann R, Garren SJ (2011) Karst and sustainability. In: Beynen PEV (ed) Karst Management. Springer, Dordrecht, pp 361–378
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Beh Ecol Sociobiol 65(1):23–35. https://doi.org/10.1007/s00265-010-1029-6
Buttigieg PL, Ramette A (2014) A Guide to Statistical Analysis in Microbial Ecology: a community-focused, living review of multivariate data analyses. FEMS Microbiol Ecol 90:543–550. https://doi.org/10.1111/1574-6941.12437
Cardoso P, Erwin TL, Borges PA, New TR (2011) The seven impediments in invertebrate conservation and how to overcome them. Biol Conserv 144(11):2647–2655. https://doi.org/10.1016/j.biocon.2011.07.024
Cardoso RC, Ferreira RL, Souza-Silva M (2021) Priorities for cave fauna conservation in the Iuiú karst landscape, northeastern Brazil: a threatened spot of troglobitic species diversity. Biodivers Conserv 30:1433–1455. https://doi.org/10.1007/s10531-021-02151-5
Castro-Souza RA, Zefa E, Ferreira RL (2020) New troglobitic and troglophilic syntopic species of Endecous (Orthoptera, Grylloidea, Phalangopsidae) from a Brazilian cave: a case of sympatric speciation. Zootaxa 4810(2):271–304. https://doi.org/10.11646/zootaxa.4810.2.3
Chesson P (2000a) General theory of competitive coexistence in spatially varying environments. Theor Popul Biol 58:211–237. https://doi.org/10.1006/tpbi.2000.1486
Chesson P (2000b) Mechanisms of maintenance of species diversity. Ann Rev Ecol Syst 31:343–366. https://doi.org/10.1146/annurev.ecolsys.31.1.343
Christiansen KA (1962) ) Proposition pour la classification des animaux cavernicoles. Spelunca Mem 2:76–78
Cisneros LM, Burgio KR, Dreiss LM, Klingbeil BT, Patterson BD, Presley SJ, Willig MR (2014) Multiple dimensions of bat biodiversity along an extensive tropical elevational gradient. J Anim Ecol 83(5):1124–1136. https://doi.org/10.1111/1365-2656.12201
Clarke KR, Gorley RN, Somerfield PJ, Warwick RM (2014) Change in marine communities: an approach to statistical analysis and interpretation, 3rd edn. PRIMER-E, Plymouth
Cole MM (1960) Cerrado, Caatinga and Pantanal: The Distribution and Origin of the Savanna Vegetation of Brazil. Geog J 126:168–179. https://doi.org/10.2307/1793957
Cornell HW (2010) Niche overlaping. In: Hastings A, Gross LJ (eds) Encyclopedia of theoretical ecology. University of California Press, Oakland
Collevatti RG, Terribile LC, de Oliveira G, Lima-Ribeiro MS, Nabout JC, Rangel TF, Diniz‐Filho JAF (2013) Drawbacks to palaeodistribution modelling: the case of South American seasonally dry forests. J Biogeogr 40(2):345–358. https://doi.org/10.1111/jbi.12005
Culver DC (1982) Cave life: Evolution and Ecology. Harvard University Press, Massachussets
Culver DC, Pipan T (2009) Biology of Caves and Other Subterranean Habitats, 1st edn. Oxford, New York
Culver DC, Pipan T (2013) Subterranean Ecosystems. In: Levin SA (ed) Encyclopedia of Biodiversity, 2nd edn. Academic Press, Waltham, pp 49–62
Dányi L (2011) Cave dwelling springtails (Collembola) of Hungary: a review. Soil Org 83(3):419–432
Deharveng L, Bedos A (2000) The cave fauna of southeast Asia. In: Wilkens H, Culver DC, Humphries WF (eds) Origin, evolution and ecology—subterranean ecosystems. Elsevier, Amsterdam, pp 603–632
Deharveng L, Bedos A (2012) Diversity patterns in the tropics. In: Culver DC, White WB (eds) Encyclopedia of Caves, 2nd edn. American University, Washington, pp 238–251
Dolédec S, Chessel D, Gimaret-Carpentier C (2000) Niche separation in community analysis: a new method. Ecology 81(10):2914–2927. https://doi.org/10.1890/0012-9658(2000)081[2914:NSICAA]2.0.CO;2
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22(4):1–20
Du Preez G, Forti P, Jacobs G, Jordaan A, Tiedt LR (2015) Hairy Stalagmites, a new biogenic root speleothem from Botswana. Int J Speleol 44(1):37–47. https://doi.org/10.5038/1827-806X.44.1.4
Ferreira RL (2019) Guano communities. In: White WB, Culver DC, Pipan T (eds) Encyclopedia of caves, 3rd edn. Academic Press, Cambridge, pp 474–484. https://doi.org/10.1016/C2017-0-01162-X
Ferreira RL, Martins VM, Paixão E, Souza-Silva MS (2015) Spatial and temporal fluctuations of the abundance of Neotropical cave-dwelling moth Hypena sp. (Noctuidae, Lepidoptera) influenced by temperature and humidity. Subterr Biol 16:47–60. https://doi.org/10.3897/subtbiol.16.5137
Ferreira RL, Giribet G, Du Preez G, Ventouras O, Janion C, Silva MS (2020) The Wynberg Cave System, the most important site for cave fauna in South Africa at risk. Subterr Biol 36:73–81. https://doi.org/10.3897/subtbiol.36.60162
Ficetola GF, Lunghi E, Canedoli C, Padoa-Schioppa E, Pennati R, Manenti R (2018) Differences between microhabitat and broad-scale patterns of niche evolution in terrestrial salamanders. Sci Rep 8(1):1–12. https://doi.org/10.1038/s41598-018-28796-x
Fišer C, Zagmajster M, Ferreira RL (2013) Two new Amphipod families recorded in South America shed light on an old biogeographical enigma. Syst Biodiver 11:117–139. https://doi.org/10.1080/14772000.2013.788579
Ford D (2005) Proposed criteria for groundwater protection zoning in karst regions. In: Milanovic PT (ed) Water resources engineering in karst. Library of Congress Cataloging-in-Publication Data, ISBN 1-56670-671-8
Foster CW, Neumann J, Holloway GJ (2019) Linking mesoscale landscape heterogeneity and biodiversity: gardens and tree cover significantly modify flower-visiting beetle communities. Landscape Ecol 34(5):1081–1095. https://doi.org/10.1007/s10980-019-00822-x
Gibert J, Deharveng L (2002) Subterranean ecosystems: a truncated functional biodiversity. BioScience 52:473–481. https://doi.org/10.1641/0006-3568(2002)052[0473:SEATFB]2.0.CO;2
Gillieson D (1996) Caves: processes, development, and management. British Library Cataloguing in Publication Data, Oxford
Gonçalves MVP, Cruz MJM, Alencar CMM et al (2018) Geoquímica e qualidade da água subterrânea no município de Serra do Ramalho, Bahia (BR). Eng Sanit Ambient 23:159–172. https://doi.org/10.1590/s1413-41522018167893
González-Megías A, Gómez JM, Sánchez-Piñero F (2007) Diversity-habitat heterogeneity relationship at different spatial and temporal scales. Ecography 30:31–41. https://doi.org/10.1111/j.0906-7590.2007.04867.x
Hüppop K (2005) Adaptation to low food. In: Culver D, White WB (eds) Encyclopediaof caves. Ensevier, Amsterdam, pp 4–10
Hutchinson GE (1959) Homage to Santa Rosalia or why are there so many kinds of animals? Am Nat 93(870):145–159. https://doi.org/10.1086/282070
ICMBio (2018) Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume VII—Invertebrados, 1st edn. Brasília, DF. https://www.icmbio.gov.br/portal/component/content/article/10187
Jaffé R, Prous X, Zampaulo R, Giannini TC, Imperatriz-Fonseca VL, Maurity C et al (2016) Reconciling mining with the conservation of cave biodiversity: a quantitative baseline to help establish conservation priorities. PLoS ONE 11(12):e0168348. https://doi.org/10.1371/journal.pone.0168348
Jaffé R, Prous X, Calux A, Gastauer M, Nicacio G, Zampaulo RA et al (2018) Conserving relics from ancient underground worlds: assessing the influence of cave and landscape features on obligate iron cave dwellers from the Eastern Amazon. PeerJ 6:e4531. https://doi.org/10.7717/peerj.4531
Klopfer PH, MacArthur RH (1960) Niche size and faunal diversity. Am Nat 94(877):293–300. https://doi.org/10.1086/282130
Kozel P, Pipan T, Mammola S, Culver DC, Novak T (2019) Distributional dynamics of a specialized subterranean community oppose the classical understanding of the preferred subterranean habitats. Invertebr Biol 138(3):e12254. https://doi.org/10.1111/ivb.12254
Ladle RJ, Firmino JV, Malhado AC, Rodríguez-Durán A (2012) Unexplored diversity and conservation potential of Neotropical hot caves. Conserv Biol 26(6):978–982. https://doi.org/10.1111/j.1523-1739.2012.01936.x
Leal-Zanchet AM, Souza ST, Ferreira RL (2014) A new genus and species for the first recorded cave-dwelling Cavernicola (Platyhelminthes) from South America. ZooKeys 442:1–15. https://doi.org/10.3897/zookeys.442.8199
Lobo HAS, Boggiani PC, Perinotto JAJ (2015) Speleoclimate dynamics in Santana Cave (Petar, São Paulo State, Brazil): general characterization and implications for tourist management. Int J Speleol 44(1):61–73. https://doi.org/10.5038/1827-806X.44.1.6
Lunghi E, Manenti R (2020) Cave communities: from the surface border to the deep darkness. Diversity 12(5):167. https://doi.org/10.3390/d12050167
Lunghi E, Manenti R, Ficetola GF (2014) Do cave features affect underground habitat exploitation by non-troglobitespecies? Acta Oecol 55:29–35. https://doi.org/10.1016/j.actao.2013.11.003
Lunghi E, Manenti R, Ficetola GF (2017) Cave features, seasonality, and subterranean distribution of non-obligate cave dwellers. PeerJ 5:e3169. https://doi.org/10.7717/peerj.3169
MacArthur RH, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101:377–385. https://doi.org/10.1086/282505
Mammola S (2019) Finding answers in the dark: caves as models in ecology fifty years after Poulson and White. Ecography 42(7):1331–1351. https://doi.org/10.1111/ecog.03905
Mammola S, Isaia M (2017) Spiders in caves. Proc R Soc B. https://doi.org/10.1098/rspb.2017.0193
Mammola S, Isaia M (2018) Day-night and seasonal variations of a subterranean invertebrate community in the twilight zone. Subterr Biol 27:31–51. https://doi.org/10.3897/subtbiol.27.28909
Mammola S, Piano E, Giachino PM, Isaia M (2017) An ecological survey of the invertebrate community at the epigean/hypogean interface. Subterr Biol 24:27–52. https://doi.org/10.3897/subtbiol.24.21585
Mammola S, Arnedo MA, Fišer C, Cardoso P et al (2020) Environmental filtering and convergent evolution determine the ecological specialization of subterranean spiders. Funct Ecol 35(5):1064–1077. https://doi.org/10.1111/1365-2435.13527
Manenti R, Lunghi E, Ficetola GF (2015) The distribution of cave twilight-zone spiders depends on microclimatic features and trophic supply. Invertebr Biol 134(3):242–251. https://doi.org/10.1111/ivb.12092
Mehrabi Z, Slade EM, Solis A, Mann DJ (2014) The Importance of microhabitat for biodiversity sampling. PLoS ONE 9(12):e114015. https://doi.org/10.1371/journal.pone.0114015
Moseley M (2008) Size matters: scalar phenomena and a proposal for an ecological definition of ‘cave’. Cave Karst Science 35(3):89–94
Moseley M (2009) Are all caves ecotones? Cave Karst Science 36(2):53–58
Moura VMA, Bichuette ME (2008) Perspectivas de proteção ambiental na Serra do Ramalho. Belo Horizonte 20:76–85
Novak T, Matjaž P, Saška L et al (2012) Duality of terrestrial subterranean fauna. Int J Speleol 41:181–188. https://doi.org/10.5038/1827-806X.41.2.5
Oliver I, Beattie AJ (1996) ) Invertebrate morphoespecies as surrogates for species: a case study. Conserv Biol 10:99–109. https://doi.org/10.1046/j.1523-1739.1996.10010099.x
Osborne RAL (2019) Saving and conserving the caves: reflections on 37 years of listings, disputes, submissions, and court cases. Aust J Earth Sci 66(6):767–778. https://doi.org/10.1080/08120099.2018.1489895
Paixão EA, Ferreira RL, Paixão CA (2017) Spatial distribution and seasonal behavior of Endecous aguassay and Eidmanacris lencionii (Orthoptera: Grylloidea, Phalangopsidae) in an artificial iron ore cave. Speleobiology Notes 9:23–33. https://doi.org/10.5563/spbn.v9i0.85
Pellegrini TG, Ferreira RL (2016) Are inner cave communities more stable than entrance communities in Lapa Nova show cave? Subterr Biol 20:15–37. https://doi.org/10.3897/subtbiol.20.9334
Pellegrini TG, Sales LP, Aguiar P, Ferreira RL (2016) Linking spatial scale dependence of land-use descriptors and invertebrate cave community composition. Subterr Biol 18:17–38. https://doi.org/10.3897/subtbiol.18.833
Pipan T, Deharveng L, Culver DC (2020) Hotspots of subterranean biodiversity. Diversity 12(5):209. https://doi.org/10.3390/d12050209
Polhemus DA, Ferreira RL (2018) Two unusual new genera of cavernicolous Hydrometridae (Insecta: Heteroptera) from eastern Brazil. Tijdschr Entomol 161(1):25–38. https://doi.org/10.1163/22119434-00002072
Prevorčnik S, Ferreira RL (2012) Brasileirinidae, a new isopod family (Crustacea: Isopoda) from the cave in Bahia (Brazil) with a discussion on its taxonomic position. Zootaxa 3452(1):47–65. https://doi.org/10.11646/zootaxa.3452.1.2
Prous X, Ferreira RL, Martins RP (2004) Ecotone delimitation: Epigean–hypogean transition in cave ecosystems. Austral Ecol 29(4):374–382. https://doi.org/10.1111/j.1442-9993.2004.01373.x
Prous X, Ferreira RL, Jacobi CM (2015) The entrance as a complex ecotone in a Neotropical cave. Int J Speleol 44:177–189. https://doi.org/10.5038/1827-806X.44.2.7
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Rabelo LM, Souza-Silva M, Ferreira RL (2018) Priority caves for biodiversity conservation in a key karst area of Brazil: comparing the applicability of cave conservation indices. Biodivers Conserv 27(9):2097–2129. https://doi.org/10.1007/s10531-018-1554-6
Rylands AB, Brandon K (2005) Brazilian protected areas. Conserv Biol 19(3):612–618. https://doi.org/10.1111/j.1523-1739.2005.00711.x
Romero A (2009) Cave biology: life in darkness. Cambridge University Press, New York
Rosenzweig ML (1981) A theory of habitat selection. Ecology 62(2):327–335. https://doi.org/10.2307/1936707
Sendra A, Reboleira ASPS (2012) The world’s deepest subterranean community—Krubera-Voronja Cave (Western Caucasus). Int J Speleol 41:221–230. https://doi.org/10.5038/1827-806X.41.2.9
Sharratt NJ, Picker M, Samways M (2000) The invertebrate fauna of the sandstone of the caves of the Cape Peninsula (South Africa): patterns of endemism and conservation priorities. Biodivers Conserv 9:107–143. https://doi.org/10.1023/A:1008968518058
Shmueli G (2010) To explain or to predict? Stat Sci 25(3):289–310. https://doi.org/10.1214/10-STS330
Simões MH, Souza-Silva M, Ferreira RL (2015) Cave physical attributes influencing the structure of terrestrial invertebrate communities in Neotropics. Subterr Biol 16:103–121. https://doi.org/10.3897/subtbiol.16.5470
Sket B (1999) The nature of biodiversity in hypogean waters and how it is endangered. Biodivers Conserv 8:1319–1338. https://doi.org/10.1023/A:1008916601121
Sket B (2008) Can we agree on an ecological classification of subterranean animals? J Nat Hist 42:1549–1563. https://doi.org/10.1080/00222930801995762
Souza-Silva M, Ferreira RL (2016) The first two hotspots of subterranean biodiversity in South America. Subterr Biol 19:1–21. https://doi.org/10.3897/subtbiol.19.8207
Souza-Silva M, Martins RP, Ferreira RL (2011a) Cave lithology determining the structure of the invertebrate communities in the Brazilian Atlantic Rain Forest. Biodivers Conserv 20:1713–1729. https://doi.org/10.1007/s10531-011-0057-5
Souza-Silva M, Parentoni RM, Ferreira RL (2011b) Trophic dynamics in a neotropical limestone cave. Subterr Biol 9:127–138. https://doi.org/10.3897/subtbiol.9.2515
Souza-Silva M, Martins RP, Ferreira RL (2015) Cave conservation priority index to adopt a rapid protection strategy: a case study in Brazilian Atlantic Rain Forest. Environ Manage 55:279–295. https://doi.org/10.1007/s00267-014-0414-8
Souza-Silva M, Iniesta LFM, Ferreira RL (2020) Invertebrates diversity in mountain Neotropical quartzite caves: which factors can influence the composition, richness, and distribution of the cave communities? Subterr Biol 33:23–43. https://doi.org/10.1080/00222930801995762
Sprent P, Smeeton NC (2000) Applied nonparametric statistical methods. Chapman and Hall/CRC, Boca Raton
Stein A, Beck J, Meyer C, Waldmann E, Weigelt P, Kreft H (2015) Differential effects of environmental heterogeneity on global mammal species richness. Global Ecol Biogeogr 24(9):1072–1083. https://doi.org/10.1111/geb.12337
Sugai LM, Ochoa-Quintero JM, Costa-Pereira R, Roque R (2015) Beyond aboveground. Biodivers Conserv 24:2109–2112. https://doi.org/10.1007/s10531-015-0918-4
Tilman D (1982) Resource competition and community structure. Monographs in population biology. Princeton Univ. Press, Princeton
Tobin BW, Hutchins BT, Schwartz BF (2013) Spatial and temporal changes in invertebrate assemblage structure from the entrance to deep-cave zone of a temperate marble cave. Int J Speleol 42:203–214. https://doi.org/10.5038/1827-806X.42.3.4
Van Beynen PE, Van Beynen KM (2011) Human disturbance of karst environments. In: Beynen PEV (ed) Karst management. Springer, Dordrecht, pp 379–397
Vargas-Mena JC, Cordero-Schmidt E, Rodriguez-Herrera B et al (2020) Inside or out? Cave size and landscape effects on cave-roosting bat assemblages in Brazilian Caatinga caves. J Mammal 101(2):464–475. https://doi.org/10.1093/jmammal/gyz206
Wang X, Auler AS, Edwards L, Cheng H, Cristalli PS et al (2004) Wet periods in northeastern Brazil over the past 210 kyr linked to distant climate anomalies. Letters to Nature 432(9):740–743. https://doi.org/10.1038/nature03067
Whittaker RH (1952) A study of summer foliage insect communities in the Great Smoky Mountains. Ecol Monogr 22(1):1–44. https://doi.org/10.2307/1948527
Wynne JJ, Howarth FG, Sommer S, Dickson BG (2019) Fifty years of cave arthropod sampling: techniques and best practices. Int J Speleol 48(1):33–48. https://doi.org/10.5038/1827-806X.48.1.2231
Yang Z, Liu X, Zhou M, Ai D, Wang G et al (2015) The effect of environmental heterogeneity on species richness depends on community position along the environmental gradient. Sci Rep 5(1):1–7. https://doi.org/10.1038/srep15723
Acknowledgements
Roberta Fernanda Ventura Cerqueira was sponsored by FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) with 12 months of grants. The data used in this paper was part of her master thesis on Ecology at Universidade Federal de São João del-Rei. We thank Joaquim Ferreira and family, residents, who provide accommodation in Agrovila 23. Joaquim also was part of the research team. We are thankful to the Bambuí Speleological group for providing the cave maps. The CEBS team that helped in field work (Dayvison, Denizar, Ellen, Gabrielle, and Gilson). We also thank Leopoldo F. O. Bernardi (Acari), Rafaela Bastos (Isopoda), Maysa F. V. R. Souza (Palpigradi) and Rodrigo Souza (Orthoptera) for their help in fauna identification. We also thank the VALE/SA company for all support provided to CEBS/UFLA. RLF is grateful to CNPq for support for Research No. 308334/2018-3. Thais Giovannini Pellegrini is grateful to Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG and VALE/SA for financial support.
Author information
Authors and Affiliations
Contributions
RLF formulated the idea and contributed to the literature review. RLF, MSS, RFVC built the sample design collected the data and identified invertebrates. TGP and MSS performed the statistical analysis. MSS wrote the manuscript with collaboration from all co-authors. RLF and TGP reviewed the manuscript and contributed to the discussion of the results.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
All authors consent to participate in the work.
Consent to publish
All authors consent to publish the work.
Additional information
Communicated by B.D. Hoffmann.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Souza-Silva, M., Cerqueira, R.F.V., Pellegrini, T.G. et al. Habitat selection of cave-restricted fauna in a new hotspot of subterranean biodiversity in Neotropics. Biodivers Conserv 30, 4223–4250 (2021). https://doi.org/10.1007/s10531-021-02302-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-021-02302-8