Abstract
Protected areas alone cannot conserve all biodiversity; we must also conserve biodiversity within production landscapes. Little is known about spider diversity in the Cape Floristic Region (CFR) biodiversity hotspot and factors driving spider diversity in transformed landscapes. Here, we assess spatial patterns of spiders in different transformed biotopes bordering remnant fynbos natural vegetation patches, determine direction of associated edge effects, and identify environmental factors influencing spider local distribution. Spiders were sampled along replicated transects running from remnant patches into three different transformed biotopes: old-fields (abandoned farmland), vineyards, and alien tree plantations. Spider Shannon diversity within old-fields and plantations did not differ from remnant patches, which had the highest diversity, whereas vineyards had the lowest. Overall, spider diversity was consistently high around habitat boundaries, regardless of land use type. Vineyards showed sharp declines in spider diversity along the remnant-vineyard transect, compared to other transects. Spider assemblages within vineyards was significantly different compared to remnant patches and old-fields, whereas other land-uses showed greater similarity. Plant species richness within the transformed biotope core increased overall spider diversity, benefiting plant-dwelling assemblages, but negatively influencing ground-dwelling assemblages. Herbaceous plant cover was driving assemblages within vineyards, whereas Restionaceae plant cover drove assemblages within old-fields. Furthermore, amount of natural vegetation in the landscape influenced spider assemblages within transformed biotopes. Our results show that old-fields have great potential to increase structural and functional connectivity within agricultural mosaics, and their rehabilitation is recommended. Furthermore, increasing plant diversity throughout the transformed landscape can soften the landscape and benefit spider diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid anthropogenic change has resulted in loss of biodiversity (Pimm et al. 2014), with insects of particular concern (Cardoso et al. 2020). Private and proclaimed protected areas alone will not conserve all biological diversity as > 80% of land is not formally protected (Fischer et al. 2013). This realization has sparked considerable interest in conservation of biodiversity within production landscapes through sparing remnants of natural vegetation (Ekroos et al. 2016; Balmford et al. 2018). Sparing these remnants effectively conserves a variety of farmland biodiversity (Phalan et al. 2011; Vrdoljak and Samways 2014; Gaigher et al. 2015; Theron et al. 2020), as well as enhancing ecosystem services for crop production (Isaacs et al. 2008; Carvalheiro et al. 2011).
The surrounding transformed landscape, when managed correctly, can also contribute to conservation of farmland biodiversity (Perfecto and Vandermeer 2010). In addition, the functioning of source patches in terms of arthropod-mediated ecosystem services, benefits from the sustainable management of the surrounding landscape (Garibaldi et al. 2019). However, high intensity management within the production landscape influences an organisms’ ability to move within it (Martin and Fahrig 2015), which in turn, influences the organisation of species, impacting ecosystem functioning. In contrast, farmland conservation measures, such as increasing the amount of vegetation within the production landscape can help soften it, leading to improved arthropod-mediated ecosystem services (Isaacs et al. 2008). However, these management decisions need to be guided by land use management that considers arthropod response to land use (Birkhofer et al. 2018). Therefore, studying species’ responses within the transformed landscape is important for understanding how species utilise diverse elements throughout the landscape and neighbouring regions (Rosas-Ramos et al. 2018). In addition, assessing distribution patterns across habitat edges of different land uses is important for identifying and understanding the mechanisms of hard and soft boundaries needed to increase successful arthropod boundary crossings throughout the landscape mosaic (Guiller et al. 2016).
The Cape Floristic Region (CFR) biodiversity hotspot is renowned globally for its exceptionally high plant diversity and endemism (Myers et al. 2000), yet has historically undergone extensive land transformation and fragmentation (Rouget et al. 2003), creating a complex tapestry of different land uses, as well as edge effects at biotope boundaries. The relative value, in terms of biodiversity, of these different land uses within the CFR agricultural landscape is poorly understood, with only a few studies documenting the diversity patterns of ground-dwelling arthropods (Gaigher and Samways 2010, 2014), parasitoids and predatory arthropods (Gaigher et al. 2016), flower-visiting insects (Kehinde and Samways 2012, 2014; Vrdoljak and Samways 2014), and grasshoppers (Adu-Acheampong et al. 2017) across farmland mosaics of the CFR. Although conservation of remnants is critical for maintaining unique biodiversity in these landscapes, certain transformed landscape elements can also make a significant contribution to landscape-scale biodiversity (Dominik et al. 2018). Thus, understanding how arthropods respond to different landscape elements throughout the farmland mosaic will help CFR conservancies to better inform land managers on sustainable land use practices which promote arthropod conservation.
Spiders are highly mobile predators, and functionally important components of food webs in natural ecosystems (Nyffeler and Sunderland 2003). Their high mobility enables them to be one of the first groups to colonise new areas (Blandenier 2009), making them ideally suited to study the functional aspect of landscape connectivity within an agricultural mosaic. However, to date, sampling effort for spiders within CFR remnants of natural vegetation has been low (Dippenaar-Schoeman et al. 2015). Furthermore, spider diversity within different land use types of CFR agricultural mosaics is even less well known (Gaigher and Samways 2010, 2014; Gaigher et al. 2016; Arvidsson et al. 2020). Studying their distribution patterns throughout the mosaic can provide a better understanding of the degree to which different landscape elements can facilitate persistence within a fragmented landscape (Rosas-Ramos et al. 2018). Managing the production landscape with the conservation of arthropod predators in mind, will promote the protection of ecosystem services, such as the biological control of crop pests (Birkhofer et al. 2018), benefiting not only biodiversity, but crop production as well (Garibaldi et al. 2019).
Here, we focus on patterns of spider diversity within different land use types throughout the agricultural mosaic of the CFR. Specifically, we assess spider diversity and assemblage patterns across edges of remnant sclerophyllous fynbos vegetation typical of the CFR, and three different land-use types, to address the following: (1) whether patterns of spider diversity and assemblage structure differ between different human modified land-use types, (2) how the associated edge effects differ between different transformed biotopes, and the direction of these edge effects, (3) which features of the transformed landscape can help soften transformed areas and improve functional connectivity throughout the agricultural mosaic, and (4) how plant communities drive spider assemblage patterns within modified landscapes. These findings would contribute to greening initiatives within other modified landscapes, especially where arthropod conservation can be promoted.
Methods
Study area and sampling design
This study was carried out in the fynbos biome of the Western Cape Province of South Africa. This region forms much of the CFR, characterised by its Mediterranean climate, where average rainfall varies from < 100 to 2 000 mm, with most rain falling in winter (Goldblatt 1997). Two main soil types occur within the fynbos biome: coarse-grained sandy soils poor in essential plant nutrients, and nutrient rich clay soils, weathered from Carboniferous rocks (Goldblatt 1997). Large portions of the CFR have been transformed for agricultural and urban use and invaded by stands of alien invasive trees (Rouget et al. 2003). Agriculture, especially dairy, vineyards and deciduous fruit orchards, as well as some forestry plantations, cover 26% of the fynbos biome, mostly on nutrient rich soils of the renosterveld component (Rouget et al. 2003).
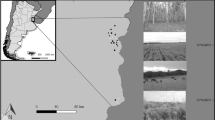
To investigate the potential influence of the production landscape on spider diversity within the CFR, a total of 18 transects were established where remnant natural vegetation was adjacent to one of three different transformed biotopes (Fig. 1). Transects ran from remnant natural vegetation into the adjacent transformed biotopes, and were sampled once per season, over two seasons (Summer 2015 and Autumn 2016). Three different transformed biotopes were sampled: old-fields (vineyards that had been abandoned for economic reasons), currently productive vineyards, and pine tree plantations. Six replicates were sampled for every combination. None of the areas contained livestock, and only very light grazing by small wild antelopes occurs within larger connected remnant patches.
Map of study area. Righthand side map shows sampling locations throughout the Cape Floristic Region. Bottom left map shows sampling plots at sampling locations. White areas represent urban, light grey represents the transformed biotopes and dark grey represents natural vegetation. Circles are sampling locations in the natural vegetation, the filled circle represents the core (interior) location and hollow circle represents the edge location. Squares are sampling area in the transformed biotopes, the filled square represents core location and hollow square represents edge location
Remnant patches were dominated by shrubs and were relatively undisturbed (no recent fires and little to no invasive plant species). Transformed biotopes all had different vegetation composition and level of disturbance (See Fig. 6 in Appendix). Old-fields contained indigenous plant communities similar to those of remnants, depending on the time since the vineyards had been removed, as well as some weedy herbaceous species. Vineyards contained a greater proportion of weedy herbaceous plant species and bare ground, depending on the cover crop management regime. All vineyards operated under integrated production regimes according to the South African Integrated Production of Wine certification programme (https://www.ipw.co.za/). In turn, plantations had low plant cover, most often weedy herbaceous plant species and some hardy native fynbos shrubs. Geographical co-ordinates of sampling locations with details of site characteristics are given in Appendix (see Table 5).
Spider sampling was undertaken at the core (interior) and edge of every transformed biotope, as well as the core and edge of every adjacent remnant natural vegetation patch. Edge plots were about 10 m from the boundary. Locations of plots along each transect were selected to be as linear as possible, while avoiding external influences from other transformed biotopes. The average transect length was 340 m. Only large remnant patches were selected to avoid core plots being influenced by edge effects (average distance of 180 m from boundary). Transects on the same farm were on average separated by 220 m.
Data collection
Pitfall trapping and vacuum sampling was used to sample spiders. Two pitfall traps (6.5 cm wide and 9 cm deep), separated by 2 m, were filled with 70% ethylene glycol to target ground-dwelling spiders. Pitfalls were left open for six days at a time, once per sampling season. After retrieving the pitfalls, the spiders where removed and stored in 75% ethanol for later identification.
A fuel powered handheld leaf blower (Stihl BG 56 C) set to vacuum with a mesh bag attached to the front of the nozzle was used to capture vegetation-dwelling spiders. Two parallel transects were walked with the vacuum sampler across the pitfall sampling locations after pitfalls were collected. To standardise vacuum sample size, the nozzle was inserted into the vegetation 50 times per vacuum transect, resulting in a total of 100 insertions per plot. Transect length varied due to amount of dead vegetation at each plot, with an average length of 75 m. Dead vegetation was not sampled. Vacuum samples were transferred into plastic bags and stored at − 40 °C before sorting.
Spiders from the pitfalls and vacuum sampling were sorted to morphospecies level (Oliver and Beattie 1996) before being identified by a spider taxonomist. All reference spider species (including juveniles) where identified to at least genus level, and where possible, to species level. However, to avoid unintentional duplication of species identified to genus level only, spiders were grouped and analysed at the genus level for all statistical analyses. Each genus was classified as either web-, plant- or ground-dwelling (Dippenaar-Schoeman et al. 2010). Furthermore, to gain insight into the response of sensitive spiders, species, where possible, were assigned an Arachnida Biodiversity Index (ABI) score. Dippenaar-Schoeman et al. (2010) developed the ABI, which uses data on a species geographic distribution and local abundance, to indicate the geographical extent of rare and range-restricted species. An ABI of 1 indicates a common widespread and habitat-generalist species, whereas an ABI of 9 indicates a highly sensitive, range-restricted species (Dippenaar-Schoeman et al. 2010).
A total of 64 plots were sampled. Two plantation transects where destroyed by a natural fire, and so only four plantation transects, but six old-field and vineyard transects, are used here. Plant surveys were conducted at every site using the point intercept line transect method. At every 1 m interval along the 50 m transect, a 5 mm thick, 3 m long rod was placed perpendicular to the ground. All plant species intercepting the rod were recorded. At every 5 m interval, vegetation height was measured, along with percentage presence of rocks. Two parallel 50 m plant transects were surveyed at each plot, spanning over the pitfall and vacuum sampling area. Plant species and their associated growth form (tree, shrub, succulent, fern, geophyte, herbaceous, graminoid and restio) were later identified for statistical analysis.
Environmental variables collected at each site included plant species richness, plant growth form cover, average vegetation height, % rockiness, amount of natural vegetation within a 500 m buffer, and topographic complexity measured as the standard deviation of the slope of the soil surface within a 50 m buffer. Amount of natural vegetation and topographic complexity was calculated in ArcMap. Along with these continuous environmental variables, additional categorical variables were created, which included land use (remnant natural vegetation, old-field, plantation, or vineyard), transect type (old-field, vineyard or plantation transects), and transect location (natural core/edge or associated transformed core/edge) (see Table 6 in Appendix).
Data analyses
Spider data for the two sampling methods and seasons were pooled and grouped at the genus level. Rarefaction curves plotted per transect (see Fig. 7 in Appendix) were not fully saturated, which further motivated the analysis of spiders at the genus level. Thus, spider genus richness was used as response variable in univariate analyses, and assemblage composition based on spider genera was used in multivariate analyses. All analyses were done for overall spiders, as well as web-, plant- and ground-dwelling spiders. The exponent of Shannon diversity index was calculated for all univariate response variables to allow for comparison between sampling locations. Furthermore, the exponent Shannon diversity index takes into account the rarity and commonness of species at each site. Exponent Shannon diversity index values were calculated using the vegan package (Oksanen et al. 2019). Moran’s I autocorrelation index, as well as Monte-Carlo test, in the ape package (Paradis and Schliep 2018), was used to test whether response variables and assemblages were spatially auto-correlated.
As exponent Shannon diversity showed normal and Poisson distribution, we used generalised linear mixed modelling from the glmmTMB package, which allows for more varying response distributions (Brooks et al. 2017). A Gaussian distribution was specified for overall Shannon index, ground-dwelling Shannon index, and plant-dwelling Shannon index, while a Poisson distribution was specified for web-dwelling Shannon index. In all glmm models, transect type was used as a random variable to address the hierarchically nested nature of our sampling design. A correlation matrix was first calculated to determine whether explanatory variables were correlated (see Fig. 8 in Appendix).
To address our first two aims, whether spider diversity differs between different land use types and the severity of associated edge effects, we created two models. The first model contained only land use type (4-level factor) e.g. remnant natural vegetation patches, old-fields, plantations, and vineyards, with transect type as random effect (Land use model). The second model only contained the sampling locations along the transect for every transect type (12-level factor) e.g. old-field core and edge, as well as the paired adjacent remnant natural vegetation patch core and edge, and transect type as random effect (Transect model). Post hoc tests were performed using the TukeyHSD function to identify significant differences between categorical variables.
To address our third aim, understanding the mechanisms driving spider diversity within the transformed landscape, we created a model using a subset from the overall dataset, which contained data on only the transformed biotope cores. We used plant species richness, average vegetation height, % rockiness, amount of natural vegetation, and topographic complexity, as well as transect type as a random effect, in the model (Transformed biotope model). These variables were used in a global model to perform model selection using the dredge function within the MuMIn package (Barton, 2019). After model section, model averaging was performed on a subset of the best models (AICc < 2) using the MuMIn package (Barton 2019). Averaging could not be performed when the top subset of models only contained one model. In these cases, estimates for the top model are reported.
To test the response of spider assemblages, we used the manyglm function within the mvabund package (Wang et al. 2012). This function fits multiple generalised linear models to all combinations of variables between an assemblage and environmental data set, thus allowing effects to be identified at the assemblage level. The Land use, Transect and Transformed biotope models used in the univariate modelling were also used for multivariate modelling. For the Transect model, we modelled transects separately. All the multivariate models where fitted with negative binomial distribution, assuming a quadratic mean–variance. Test statistics were calculated based on the ‘pit-trap’ resampling method, with 999 permutations. To identify significant differences between categorical variables, pairwise comparisons were performed using 999 permutation within the mvabund package (Wang et al. 2012).
To visualise the direction of spider response at the family level, we performed a fourth corner analysis using the traitglm function in the mvabund package (Wang et al. 2012). The fourth corner analysis pertains to how traits of organisms can explain variation in environmental response across assemblages. However, here, we did not use trait data, but rather spider family as a grouping variable, thereby allowing us to identify which spider families respond to specific modelled variables. The fourth corner model was fitted with negative binomial distribution and with a LASSO penalty. The LASSO penalty reduces small environmental-trait relationship coefficients to 0, thereby reducing the effects from spider families with small numbers of individuals.
Lastly, to address our fourth aim, how plant communities drive spider assemblage patterns within modified land use types, we performed non-metric multidimensional scaling (NMDS) using the metaMDS function within the vegan package (Oksanen et al. 2019). NMDS is an unconstrained ordination method that represents pairwise dissimilarity between objects in low dimensional space, where sites with similar species are plotted close together, and sites with dissimilar species are placed further apart. Furthermore, using the envfit function, environmental variables can be identified which are driving the site distribution pattern (Oksanen et al. 2019). Thus, we used NMDS to identify the influence that plant growth forms have on shaping spider assemblages among different transformed biotopes. NMDS was performed using Bray–Curtis dissimilarity. All statistical analyses were performed in R version 3.6.1 (R Core Team 2013).
Results
Spider diversity throughout the agricultural mosaic
No spatial autocorrelation was detected for univariate response variables (at p < 0.1) or assemblages (at p < 0.07). A total of 1 426 spider individuals (with juveniles included) were collected during this study, with a total of 71 spider genera from 30 families (see Table 7 in Appendix). A total of 761 individuals were plant-dwelling, 495 were ground-dwelling and 170 were web-dwelling spiders. The most diverse families were Thomisidae consisting of 11 genera, followed by Gnaphosidae and Salticidae with eight genera each. The most abundant families were Thomisidae with 256 individuals, followed by Salticidae with 222 individuals and Oxyopidae with 219 individuals. Spider families within old-fields were very similar to those of remnant natural vegetation patches in terms of diversity and abundance, with Thomisidae, Oxyopidae, Salticidae, and Philodromidae being the most abundant families within old-fields and remnants. Gnaphosidae, Oxyopidae, and Amaurobiidae were the most abundant families within vineyards, whereas, Thomisidae, Philodromidae and Amaurobiidae were the most abundant families within alien tree plantations.
When considering rare and range restricted species, numerous species of conservation concern were sampled. Diores simoni (ABI of 7) was sampled within all the different land use types. Drassodella septemmaculata (ABI of 7) occurred within an old-field and a plantation site, whereas Zelotes broomi (ABI of 7) only occurred within a plantation site. Chresiona invalida (ABI of 6) was sampled within vineyards.
Factors driving spider diversity
Overall and plant-dwelling spider diversity was significantly affected by land use type (Table 1). Post hoc tests showed that overall spider diversity was significantly lower in vineyards than in remnants and old-fields, whereas spider diversity in the other three land use types did not significantly differ (Fig. 2). Additionally, overall, plant-, and ground-dwelling spider diversity was significantly affected by transect location (Table 1). Transformed cores consistently had the lowest spider diversity compared to other sampling locations along transects (Fig. 3). Spider diversity declined sharply across the remnant-vineyard edge, with the lowest diversity of all the sampling locations in the vineyard core (Fig. 3). However, diversity at the remnant edge bordering vineyards was relatively high. Spider diversity along the old-field transects changed little, and contained the highest spider diversity compared to all other sampled plots, except for natural edge adjacent to vineyards. (Fig. 3). Spider diversity within remnant natural vegetation patches adjacent to plantations had the lowest diversity (edge and core) compared to remnants adjacent to the other land uses. Post hoc tests did not reveal any significant differences in ground-dwelling spider diversity, while plant-dwelling spider diversity showed similar patterns to that of overall spider diversity (see Fig. 9 in Appendix). Within transformed biotope cores, plant species richness had a significant positive effect on overall spider diversity, as well as on plant-dwelling spider diversity (Table 2, see Fig. 9 in Appendix).
Factors influencing spider assemblage composition
Overall, web-, plant-, and ground-dwelling spider assemblages were significantly influenced by land use type (Table 3). Post hoc tests showed that the overall and plant-dwelling spider assemblages within vineyards was significantly different from that of old-fields (deviance: 112.19, p-value: 0.036; deviance: 55.95, p-value: 0.037) and remnant natural vegetation (deviance: 130.26, p-value: 0.01; deviance: 66.90, p-value: 0.013). The web-dwelling spider assemblages within plantations was significantly different from that of remnant natural vegetation (deviance: 33.14, p-value: 0.011). No difference was detected between old-fields and remnant natural vegetation or plantations. Post hoc tests did not reveal any significant differences in ground-dwelling spider assemblage structure. The fourth corner analysis revealed that abundances of Araneidae, Philodromidae, Salticidae and Thomisidae negatively correlated with vineyards, whereas Clubionidae, Gnaphosidae and Zodariidae responded positively (Fig. 4). Ammoxenidae responded negatively towards old-fields, whereas Scytodidae responded positively (Fig. 4). Lastly, Linyphiidae showed a positive response towards plantations (Fig. 4).
Overall, plant- and ground-dwelling spider assemblages were significantly influenced by transect location along the vineyard transect, whereas web-dwelling spider assemblages were significantly influenced by transect location along the plantation transect (Table 3). Post hoc tests showed that overall spider assemblages within vineyard core sites were significantly different to those of the adjacent remnant edge (deviance: 94.53, p-value: 0.046). No other significant difference was detected between any sampling locations within all other transects. For the vineyard transect, the fourth corner analysis showed that Ammoxenidae responded negatively towards the remnant edge adjacent to vineyards, and Gnaphosidae responded negatively towards remnant core adjacent to vineyards (Fig. 4). Zodariidae showed a positive response towards vineyard cores (Fig. 4).
Within the transformed core, overall and plant-dwelling spider assemblages were significantly influenced by plant species richness, amount of natural vegetation within the landscape, as well as by topographic complexity (Table 4). The web-dwelling spider assemblage was significantly influenced by rockiness (Table 4). The fourth corner analyses revealed that Gnaphosidae and Zodariidae were negatively influenced by plant species richness within the production landscape, whereas Philodromidae were positively influenced (Fig. 4). Furthermore, Zodariidae and Ammoxenidae were negatively influenced by average vegetation height, whereas Theridiidae was positively influenced (Fig. 4). Rockiness had a positive influence on Gallieniellidae and Palpimanidae, but a negative influence on Araneidae (Fig. 4). The amount of natural vegetation within the landscape had a negative influence on Oxyopidae, Philodromidae and Theridiidae, but a positive influence on Scytodidae (Fig. 4). Lastly, Araneidae was positively influenced by topographic complexity (Fig. 4).
Herbaceous plant cover and restio cover had a significant effect (r2: 0.4; p-value: 0.03 and r2: 0.43; p-value: 0.025) on spider assemblage patterns within the production landscape. Restio cover was driving spider assemblages within old-fields, whereas herbaceous plant cover was driving assemblages within vineyards (Fig. 5), which were characterised by graminoid and herbaceous plants (see Fig. 6 in Appendix). Old-fields and plantations both had similar plant growth form diversity compared to remnant patches of natural vegetation. However, old-fields had higher cover of graminoid, herbaceous, restio and shrub growth forms compared to plantations, whereas plantations had higher tree cover (see Fig. 6 in Appendix).
Discussion
Spider diversity within the agricultural mosaic
The agricultural mosaic of the CFR supports high spider diversity and diverse assemblages, with numerous rare and range-restricted spider species occurring throughout the mosaic, indicating the value of the mosaic for spider conservation. Furthermore, the number of spider individuals sampled within this study is comparable to other studies conducted within the CFR (Gaigher and Samways 2014; Gaigher et al. 2016; Theron et al. 2020). However, sampling effort for spiders within fynbos has been low (Foord et al. 2011), but with some studies focused on the agricultural mosaic (Gaigher and Samways 2010, 2014; Gaigher et al. 2016; Arvidsson et al. 2020), which suggests that more research on spider diversity within the CFR mosaic is needed.
Factors driving spider diversity within the agricultural mosaic
Remnant natural vegetation patches had the highest spider diversity, as previously reported for the CFR (Gaigher et al. 2016). Sparing remnants of natural vegetation within a production landscape is an effective tool for conserving biodiversity across farmland mosaics (Ekroos et al. 2016; Balmford et al. 2018), especially through provision of source habitats (Duelli and Obrist 2003), and as refuges during times of high disturbance within the production landscape (Gaigher and Samways 2014), while enabling species persistence across spatial and temporal scales (Saura et al. 2014). The effectiveness of these remnant habitats for biodiversity ultimately depends on the surrounding landscape. More so, if managed sustainably, is pivotal for arthropod conservation moving forward (Samways 2015), especially if different land use types with varying management intensities are maintained (Birkhofer et al. 2017).
The agricultural mosaic supports diverse spider assemblages (Birkhofer et al. 2017), thereby contributing to spider conservation at the landscape scale. Here, we found that vineyards contained different spider assemblages compared to old-fields and remnant patches. Also, vineyards had lowest spider diversity, which mirrors earlier results (Gaigher and Samways 2010, 2014). The sampled vineyards all operate under integrated production regimes. This approach allows careful use of agrochemicals, and therefore the low diversity might be caused by pesticide application. Nevertheless, actively managed vineyards can still support distinct spider assemblages, and diversity can be improved within vineyards by increasing plant species richness, specifically herbaceous plant species (Winter et al. 2018; Paiola et al. 2020). Here, we add to this, showing that herbaceous plant species influence assemblage patterns within vineyards. The effect of plant richness on spiders might be indirect via its arthropod prey species, as spiders within the CFR mosaic are strongly influenced by prey abundance (Gaigher et al. 2016). This corresponds with findings from organic fruit orchards of South Africa, showing that the type of weed species occurring within an orchard plays an important role in spider predator–prey interactions (Arvidsson et al. 2020).
Old-fields supported high spider diversity, and contained similar spider assemblages to remnant patches of natural vegetation. Similar patterns of diversity have previously been shown for parasitoids within the CFR production landscape (Gaigher et al. 2016). However, spider families responded differently towards old-fields, which could be due to differences in the ecological traits of different families (Schmidt et al. 2005; Gallé et al. 2019). Ammoxenidae, also known as termite hunters, showed a negative response, whereas Scytodidae, also known as spitting spiders, showed a positive response. Both these families contain mostly ground dwelling spiders and difference in response might be attributed to their different ecological traits. Additionally, the negative response of Ammoxenidae might be seasonal, corresponding to the seasonal occurrence of their termite prey (Haddad et al. 2016). Thus, sampling spiders throughout the year might provide further, nuanced results.
Plant composition within old-fields was similar to that of remnant patches when compared to other transformed biotopes, and restios were found to influence old-field spider assemblages. Old-fields retained herbaceous and graminoid species in high abundance, similar to the plant composition of vineyards, which suggests that some old-fields might suffer from legacy effects from vineyard abandonment (Helm et al. 2019). However, results from Europe indicate that fallow fields, independent of age, can still act as a source habitat with high activity density and spillover for some spider families (Birkhofer et al. 2018). Nevertheless, our results show that old-fields may be able to increase the functional connectivity within the agricultural mosaic by providing greater permeability for fynbos spiders.
Overall, spider diversity can be enhanced within the production landscape through increasing plant species richness. Here, even plantation sites were able to support fairly high spider diversity. This contrasts with previous findings that show plantations to affect spider diversity worse than vineyards (Magoba and Samways 2012). One explanation for this could be due to the plant species sampled within our plantations, as plantations had some of the same components of remnants, but in lower abundance. Web-dwelling spiders responded positively towards plantations, specifically the Linyphiidae, and these web building spiders might benefit from heterogeneity in vegetation structure within the plantations (Oxbrough et al. 2005). In general, exotic timber plantations have significant negative impact on arthropod diversity. However, spider assemblages can recover quickly once plantations are removed (Magoba et al. 2015).
How the production landscape is managed will ultimately influence the outcome of conservation goals (e.g. increasing function connectivity) within an agricultural mosaic (Driscoll et al. 2013). Not all transformed biotopes are similar, and some provide greater resistance to movement than others. This differential permeability of different land-uses is likely to influence patterns at habitat edges. Here, we consistently found highest spider diversity at the habitat boundary, except for remnant edges adjacent to plantations where we found low spider diversity. Nevertheless, the trend of aggregated richness at habitat boundaries is also supported by other studies (Evans et al. 2016; Guiller et al. 2016), and can be explained through the proliferation of generalist species that can exploit resources in multiple land uses (Pardini et al. 2009). Dennis and Fry (1992) showed that field margins can increase arthropod diversity on farmland, especially predatory arthropods, due to an increase in prey species at habitat edges. In addition, the permeability of the biotope is dependent on plant cover density within it, which can change throughout the year (Vasseur et al. 2013), impacting local arthropod distribution patterns across the production landscape.
Not all spiders here responded similarly to plant richness within the production landscape. Ground-dwelling spiders within the Gnaphosidae and Zodariidae were negatively affected by plant richness, whereas plant-dwelling spiders (Philodromidae) were positively influenced. Increasing the proportion of natural vegetation cover at the landscape scale within the agricultural mosaic had a strong positive influence on the spider assemblages, supporting results from Europe (Schmidt et al. 2008). However, there were contrasting responses, with Oxyopidae, Philodromidae and Theridiidae responding negatively, whereas Scytodidae responded positively. Oxyopidae, Philodromidae and Theridiidae are common spider families within the agricultural landscape (Dippenaar-Schoeman et al. 2013) and might benefit from increased cropland within the landscape. Furthermore, the structure of the vegetation can also play an important role for spiders, as ground dwelling spiders (Zodariidae and Ammoxenidae) responded negatively to average vegetation height. Entling et al. (2007) found that shading from vegetation was an important predictor for spiders, which might be an important driver here for ground dwelling spiders. Differential responses of different families could also be due to different dispersal abilities. Ballooning of certain spider taxa can enable relatively long-distance passive dispersal (Hogg and Daane 2010) and may obscure species responses to habitat quality (Schmidt et al. 2005). Although data are not currently available on this trait for the local taxa, this would nonetheless be an interesting topic for future assessments.
In our rocky sclerophyllous sampling area, rockiness had a negative influence on web-building spider assemblage, specifically spiders within the Araneidae, potentially due to reduced opportunity for web attachments. Additionally, rockiness positively influenced ground-dwelling families (Gallieniellidae and Palpimanidae), which supports previous findings (Theron et al. 2020). Rockiness creates microclimatic conditions that influences a variety of taxa (Crous et al. 2013), thereby adding to the heterogeneity of landscapes.
Spider conservation and management implications
An effective agro-ecological conservation framework requires incorporation of both the protection of remnant patches of natural vegetation and sustainable management of the production landscape (Perfecto and Vandermeer 2010). However, production landscapes comprise a variety of landscape elements, and to prioritise conservation effort and improve landscape management in agricultural environments, keystone structures need to be identified (Tews et al. 2004; Rosas-Ramos et al. 2018). Here we identified old-fields as an important supplementary habitat element to remnant patches, conserving high spider diversity and sustaining spider assemblages similar to those of remnant natural vegetation. Old-fields therefore increase the area of occupancy for many spider species through an increase in the structural and functional connectivity across the landscape mosaic (Tischendrof and Fahrig 2000). However, some old-fields, especially those with high amounts of graminoids and weedy herbaceous plant species, suffer from legacy effects, and active old-field restoration is recommended (Helm et al. 2019). Midoko-Iponga et al. (2005) demonstrated that by reducing weedy herbaceous species within old-fields of the CFR, native plant species establishment can be improved, thereby allowing old-fields to resemble native remnant vegetation more closely. To add to this, future work could focus on how the amount of natural vegetation in the surrounding landscape influences plant composition within old-fields, and how different plant assemblages within old-fields impact arthropod predators. Nonetheless, old-fields hold great conservation value, and their restoration should be incorporated into agri-environmental schemes, especially as fallow fields have been shown to be important for conservation biological control in Europe (Birkhofer et al. 2018).
Protecting semi-natural habitat features within the production landscape helps conserve farmland biodiversity in the CFR (Vrdoljak and Samways 2014; Gaigher et al. 2016). Here we add to this evidence that improving the proportion of natural and semi-natural vegetation throughout the landscape sustains spider assemblages across the production mosaic. It is well known that establishing vegetation within the inter-rows of vineyards can increases farmland biodiversity (Winter et al. 2018; Katayama et al. 2019). Therefore, increasing the complexity and quality of the transformed landscape, via establishment of cover crops, protecting remnant natural vegetation and restoring old-fields, will increase natural enemy diversity, soften the transformed landscape, and allow for more effective habitat boundary crossings. This will help ensure that agro-ecological mosaics function as close as possible to natural systems, thereby providing guarantees for biodiversity and better resilience in these times of great environmental change.
Data availability
Data will be made available upon request.
References
Adu-Acheampong S, Samways MJ, Landmann T, Kyerematen R, Minkah R, Mukundamago M, Moshobane CM (2017) Endemic grasshopper species distribution in an agro-natural landscape of the Cape Floristic Region, South Africa. Ecol Eng 105:133–140. https://doi.org/10.1016/j.ecoleng.2017.04.037
Arvidsson F, Addison P, Addison M, Haddad CR, Birkhofer K (2020) Weed species, not mulching, affect web-building spiders and their prey in organic fruit orchards in South Africa. Ecosphere 11(3):e03059. https://doi.org/10.1002/ecs2.3059
Balmford B, Green RE, Onial M, Phalan B, Balmford A (2018) How imperfect can land sparing be before land sharing is more favourable for wild species? J Appl Ecol 56:73–84. https://doi.org/10.1111/1365-2664.13282
Barton K (2019) MuMIn: multi-model inference. R package version 1.43.6. https://CRAN.R-project.org/package=MuMIn
Birkhofer K, Gossner MM, Diekötter T, Drees C, Ferlian O, Maraun M, Scheu S, Weisser WW, Wolters V, Wurst S, Zaitsev AS, Smith HG (2017) Land-use type and intensity differentially filter traits in above- and below-ground arthropod communities. J Anim Ecol 86:511–520. https://doi.org/10.1111/1365-2656.12641
Birkhofer K, Fevrier V, Heinrich AE, Rink K, Smith HG (2018) The contribution of CAP greening measures to conservation biological control at two spatial scales. Agric Ecosyst Environ 255:84–94. https://doi.org/10.1016/j.agee.2017.12.026
Blandenier G (2009) Ballooning of spiders (Araneae) in Switzerland: general results from an eleven-year survey. Br Arachnol Soc 14:308–316. https://doi.org/10.13156/arac.2009.14.7.308
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) glmmTMB Balances speed and flexibility among packages for zero-inflated generalized linear mixed modelling. R J 9:378–400
Cardoso P, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushima CS, Gaigher R, Habel JC, Hallmann CA, Hill MJ, Hochkirch A, Kwak ML, Mammola S, Noriega JA, Orfinger AB, Pedraza F, Pryke JS, Roque FO, Simaika SJ, JP, Stork NE., Suhling F, Vorster C, Samways MJ, (2020) Scientists’ warning to humanity on insect extinctions. Biol Conserv 242:108426. https://doi.org/10.1016/j.biocon.2020.108426
Carvalheiro LG, Veldman R, Shenkute AG, Tesfay GB, Pirk CWW, Donaldson JS, Nicolson SW (2011) Natural and within-farmland biodiversity enhances crop productivity. Ecol Lett 14:251–259. https://doi.org/10.1111/j.1461-0248.2010.01579.x
Crous CJ, Samways MJ, Pryke JS (2013) Exploring the mesofilter as a novel operational scale in conservation planning. J Appl Ecol 50:205–214. https://doi.org/10.1111/1365-2664.12012
Dennis P, Fry GL (1992) Field margins: can they enhance natural enemy population densities and general arthropod diversity on farmland? Agric Ecosyst Environ 40:95–115. https://doi.org/10.1016/0167-8809(92)90086-Q
Dippenaar-Schoeman AS, Haddad CR, Foord S, Lyle R, Lotz L, Helberg L, Mathebula S, van den Berg A, Marais P, van den Berg AM, van Niekerk E, Jocqué R (2010) First atlas of the spiders of South Africa (Arachnida: Araneae). South African National Survey of Arachnida Technical Report 2010 version 1
Dippenaar-Schoeman AS, van den Berg AM, Haddad CR, Lyle R (2013) Current knowledge of spiders in South Africa agroecosystems (Arachnida: Araneae). Trans R Soc S Afr 68:57–74. https://doi.org/10.1080/0035919X.2012.755136
Dippenaar-Schoeman AS, Haddad CR, Foord SH, Lyle R, Lotz LN, Marais P (2015) South African National Survey of Arachnida (SANSA): review of current knowledge, constraints and future needs for documenting spider diversity (Arachnida: Araneae). Trans R Soc S Afr 70:245–275. https://doi.org/10.1080/0035919X.2015.1088486
Dominik C, Seppelt R, Horgan FG, Settele J, Václavík T (2018) Landscape composition, configuration, and trophic interactions shape arthropod communities in rice agroecosystems. J Appl Ecol 55:2461–2472. https://doi.org/10.1111/1365-2664.13226
Driscoll DA, Banks SC, Barton PS, Lindenmayer DB, Smith AL (2013) Conceptual domain of the matrix in fragmented landscapes. Trends Ecol Evol 28:605–613. https://doi.org/10.1016/j.tree.2013.06.010
Duelli P, Obrist MK (2003) Regional biodiversity in an agricultural landscape: the contribution of seminatural habitat islands. Basic Appl Ecol 4:129–138. https://doi.org/10.1078/1439-1791-00140
Ekroos J, Ödman AM, Andersson GKS, Birkhofer K, Herbertsson L, Klatt BK, Olsson O, Olsson PA, Persson AS, Prentice HC, Rundlöf M, Smith HG (2016) Sparing land for biodiversity at multiple spatial scales. Front Ecol Evol 3:145. https://doi.org/10.3389/fevo.2015.00145
Entling W, Schmidt MH, Bacher S, Brandl R, Nentwig W (2007) Niche properties of Central European spiders: shading, moisture and the evolution of the habitat niche. Glob Ecol Biogeogr 16:440–448. https://doi.org/10.1111/j.1466-8238.2006.00305.x
Evans TR, Mahoney MJ, Cashatt ED, Noordijk J, de Snoo G, Musters CJM (2016) The impact of landscape complexity on invertebrate diversity in edges and fields in an agricultural area. Insects 7:1–16. https://doi.org/10.3390/insects7010007
Fischer J, Brittain C, Klein A (2013) Biodiversity-friendly farming. Encyclopaedia of biodiversity. Academic Press, Boca Raton, pp 418–429
Foord SH, Dippenaar-Schoeman AS, Haddad CR (2011) South African spider diversity: African perspectives on the conservation of a mega-diverse group. Changing diversity in a changing environment. Intech, Croatia, pp 163–182
Gaigher R, Samways MJ (2010) Surface-active arthropods in organic vineyards, integrated vineyards and natural habitat in the Cape Floristic Region. J Insect Conserv 14:595–605. https://doi.org/10.1007/s10841-010-9286-2
Gaigher R, Samways MJ (2014) Landscape mosaic attributes for maintaining ground living spider diversity in a botanical hotspot. Insect Conserv Divers 7:470–479. https://doi.org/10.1111/icad.12070
Gaigher R, Pryke JS, Samways MJ (2015) High parasitoid diversity in remnant natural vegetation, but limited spillover into the agricultural matrix in South African vineyard agroecosystems. Biol Conserv 186:69–74. https://doi.org/10.1016/j.biocon.2015.03.003
Gaigher R, Pryke JS, Samways MJ (2016) Old fields increase habitat heterogeneity for arthropod natural enemies in an agricultural mosaic. Agric Ecosyst Environ 230:242–250. https://doi.org/10.1016/j.agee.2016.06.014
Gallé R, Happe A, Baillod AB, Tscharntke T, Batáry P (2019) Landscape configuration, organic management, and within-field position drive functional diversity of spiders and carabids. J Appl Ecol 56:63–72. https://doi.org/10.1111/1365-2664.13257
Garibaldi LA, Pérez-Méndez N, Garratt MPD, Gemmill-Herren B, Miguez FE, Dicks LV (2019) Policies for ecological intensification of crop production. Trends Ecol Evol 34:282–286. https://doi.org/10.1016/j.tree.2019.01.003
Goldblatt P (1997) Floristic diversity in the Cape flora of South Africa. Biodivers Conserv 6:359–377. https://doi.org/10.1023/A:1018360607299
Guiller C, Affre L, Albert CH, Tatoni T, Dumas E (2016) How do field margins contribute to the functional connectivity of insect-pollinated plants? Landsc Ecol 31:1747–1761. https://doi.org/10.1007/s10980-016-0359-9
Haddad CR, Brabec M, Pekár S, Fourie R (2016) Seasonal population dunamics of a specialized termite-eating spider (Araneae: Ammozenidae) and its prey (Isoptera: Hodotermidtidae). Pedobiologia 59:105–110. https://doi.org/10.1016/j.pedobi.2016.03.003
Helm J, Dutoit T, Saatkamp A, Bucher SF, Leiterer M, Römermann C (2019) Recovery of Mediterranean steppe vegetation after cultivation: legacy effects on plant composition, soil properties and functional traits. Appl Veg Sci 27:71–84. https://doi.org/10.1111/avsc.12415
Hogg BN, Daane KM (2010) The role of dispersal from natural habitat in determining spider abundance and diversity in California vineyards. Agric Ecosyst Environ 135:260–267. https://doi.org/10.1016/j.agee.2009.10.004
Isaacs R, Tuell J, Fiedler A, Gardiner M, Landis D (2008) Maximizing arthropod-mediated ecosystem services in agricultural landscapes: the role of native plants. Front Ecol Environ 7:196–203. https://doi.org/10.1890/080035
Katayama N, Bouam I, Koshida C, Baba YG (2019) Biodiversity and yield under land-use types in orchard/vineyard landscapes: a meta-analysis. Biol Conserv 229:125–133. https://doi.org/10.1016/j.biocon.2018.11.020
Kehinde T, Samways MJ (2012) Endemic pollinator response to organic vs. conventional farming and landscape context in the Cape Floristic Region biodiversity hotspot. Agric Ecosyst Environ 146:162–167. https://doi.org/10.1016/j.agee.2011.10.020
Kehinde T, Samways MJ (2014) Effects of vineyard management on biotic homogenization of insect–flower interaction networks in the Cape Floristic Region biodiversity hotspot. J Insect Conserv 18:469–477. https://doi.org/10.1007/s10841-014-9659-z
Magoba RN, Samways MJ (2012) Comparative footprint of alien, agricultural and restored vegetation on surface-active arthropods. Biol Invasions 14:165–177. https://doi.org/10.1007/s10530-011-9994-x
Magoba RN, Samways MJ, Simaika JP (2015) Soil compaction and surface-active arthropods in historic, agricultural, alien, and recovering vegetation. J Insect Conserv 19:501–508. https://doi.org/10.1007/s10841-015-9771-8
Martin AE, Fahrig L (2015) Matrix quality and disturbance frequency drive evolution of species behavior at habitat boundaries. Ecol Evol 5:5792–5800. https://doi.org/10.1002/ece3.1841
Midoko-Iponga D, Krug CB, Milton SJ (2005) Competition and herbivory influence growth and survival of shrubs on old fields: Implications for restoration of renosterveld shrubland. J Veg Sci 16:685–692. https://doi.org/10.1111/j.1654-1103.2005.tb02411.x
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Nyffeler M, Sunderland KD (2003) Composition, abundance and pest control potential of spider communities in agroecosystems: a comparison of European and US studies. Agric Ecosyst Environ 95:579–612. https://doi.org/10.1016/S0167-8809(02)00181-0
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) vegan: Community Ecology Package. R package version 2.5-6. https://CRAN.R-project.org/package=vegan
Oliver I, Beattie AJ (1996) Invertebrate morphospecies as surrogates for species: a case study. Conserv Biol 10:99–109. https://doi.org/10.1046/j.1523-1739.1996.10010099.x
Oxbrough AG, Gittings T, O’Halloran J, Giller PS, Smith GF (2005) Structural indicators of spider communities across the forest plantation cycle. For Ecol Manag 212:171–183. https://doi.org/10.1016/j.foreco.2005.03.040
Paiola A, Assandri G, Brambilla M, Zottini M, Pedrini P, Nascimbene J (2020) Exploring the potential of vineyards for biodiversity conservation and delivery of biodiversity-mediated ecosystem services: a global-scale systematic review. Sci Total Environ 706:135839. https://doi.org/10.1016/j.scitotenv.2019.135839
Paradis E, Schliep K (2018) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528. https://doi.org/10.1093/bioinformatics/bty633
Pardini R, Faria D, Accacio GM, Laps RR, Mariano-Neto E, Paciencia MLB, Dixo M, Baumgarten J (2009) The challenge of maintaining Atlantic forest biodiversity: a multi-taxa conservation assessment of specialist and generalist species in an agro-forestry mosaic in southern Bahia. Biol Conserv 142:1178–1190. https://doi.org/10.1016/j.biocon.2009.02.010
Perfecto I, Vandermeer J (2010) The agroecological matrix as alternative to the land sparing/agriculture intensification model. Proc Natl Acad Sci USA 107:5786–5791. https://doi.org/10.1073/pnas.0905455107
Phalan B, Balmford A, Green RE, Scharlemann JW (2011) Minimising the harm to biodiversity of producing more food globally. Food Policy 36:S62–S71. https://doi.org/10.1016/j.foodpol.2010.11.008
Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO (2014) The biodiversity of species and their rates of extinction, distribution, and protection. Science 344:1246752
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Rosas-Ramos N, Baños-Picón L, Tobajas E, de Paz V, Tormos J, Asís JD (2018) Value of ecological infrastructure diversity in the maintenance of spider assemblages: a case study of Mediterranean vineyard agroecosystems. Agric Ecosyst Environ 265:244–253. https://doi.org/10.1016/j.agee.2018.06.026
Rouget M, Richardson DM, Cowling RM, Lloyd JW, Lombard AT (2003) Current patterns of habitat transformation and future threats to biodiversity in terrestrial ecosystems of the Cape Floristic Region, South Africa. Biol Conserv 112:63–85. https://doi.org/10.1016/S0006-3207(02)00395-6
Samways MJ (2015) Future-proofing insect diversity. Curr Opin Insect Sci 12:71–78. https://doi.org/10.1016/j.cois.2015.09.008
Saura S, Bodin O, Fortin M (2014) Stepping stones are crucial for species long distance dispersal and range expansion through habitat networks. J Appl Ecol 51:171–182. https://doi.org/10.1111/1365-2664.12179
Schmidt MH, Roschewitz I, Thies C, Tscharntke T (2005) Differential effects of landscape and management on diversity and density of ground-dwelling farmland spiders. J Appl Ecol 42:281–287. https://doi.org/10.1111/j.1365-2664.2005.01014.x
Schmidt MH, Thies C, Nentwig W, Tscharntke T (2008) Contrasting responses of arable spiders to the landscape matrix at different spatial scales. J Biogeogr 35:157–166. https://doi.org/10.1111/j.1365-2699.2007.01774.x
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92. https://doi.org/10.1046/j.0305-0270.2003.00994.x
Theron KJ, Gaigher R, Pryke JS, Samways MJ (2020) High quality remnant patches in a complex agricultural landscape sustain high spider diversity. Biol Conserv 243:108480. https://doi.org/10.1016/j.biocon.2020.108480
Tischendrof L, Fahrig L (2000) On the usage and measurement of landscape connectivity. Oikos 90:7–19. https://doi.org/10.1034/j.1600-0706.2000.900102.x
Vasseur C, Joannon A, Aviron S, Burel F, Maynard J, Baudry J (2013) The cropping system mosaic: how does the hidden heterogeneity of agricultural landscapes drive arthropod populations? Agric Ecosyst Environ 166:3–14. https://doi.org/10.1016/j.agee.2012.08.013
Vrdoljak SM, Samways MJ (2014) Agricultural mosaics maintain significant flower and visiting insect biodiversity in a global hotspot. Biodivers Conserv 23:133–148. https://doi.org/10.1007/s10531-013-0588-z
Wang Y, Naumann U, Wright S, Warton DI (2012) mvabund: an R package for model-based analysis of multivariate data. Methods Ecol Evol 3:471–474. https://doi.org/10.1111/j.2041-210X.2012.00190.x
Winter S, Bauer T, Strauss P, Kratschmer S, Paredes D, Popescu D, Landa B, Guzmán G, Gómez JA, Guernion M, Zaller JG, Batáry P (2018) Effects of vegetation management intensity on biodiversity and ecosystem services in vineyards: a meta-analysis. J Appl Ecol 55:2484–2495. https://doi.org/10.1111/1365-2664.13124
Acknowledgements
This project was funded by Mondi Group. Fauna specimens were collected with permit no. AAA007-00144-0056 provided by Cape Nature. Spider species were identified by Prof Ansie Dippenaar-Schoeman. Centre for Geographical Analysis provided access to Stellenbosch University 5 m resolution digital elevation model. Lastly, access to sampling locations granted by multiple landowners and wine farms.
Funding
This work was funded by Mondi Group.
Author information
Authors and Affiliations
Contributions
KJT: conceptualization, methodology, formal analysis, investigation, writing—original draft, writing—review & editing, visualization. RG: conceptualization, methodology, validation, formal analysis, resources, writing—review & editing. JSP: conceptualization, methodology, validation, formal analysis, writing—review & editing. MJS: conceptualization, methodology, validation, writing—review & editing, funding acquisition.
Corresponding author
Ethics declarations
Conflicts of interest
Not applicable.
Additional information
Communicated by Nigel E. Stork.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Figs. 6, 7, 8, 9 and Tables 5, 6, 7.
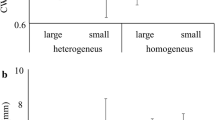
Significant results obtained through generalized linear mixed effect models. Characters above box plots indicate significant differences. a Plant-dwelling exponent of the Shannon index and land use type, b Plant-dwelling exponent of the Shannon index and transect location, c Overall exponent of the Shannon index and plant richness, and d Plant-dwelling exponent of the Shannon index and plant richness
Rights and permissions
About this article
Cite this article
Theron, K.J., Gaigher, R., Pryke, J.S. et al. Abandoned fields and high plant diversity support high spider diversity within an agricultural mosaic in a biodiversity hotspot. Biodivers Conserv 29, 3757–3782 (2020). https://doi.org/10.1007/s10531-020-02048-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-020-02048-9