Abstract
Domestic gardens provide valuable ecosystem services including supporting biodiversity. These gardens typically consist of a mixture of native and non-native plants yet the relative value of these plants for invertebrates is largely unknown. To address this a replicated field experiment was established with plots planted with one of three assemblages of non-invasive perennial and shrubby garden plants (treatments), based on plant origin [British native, near-native (Northern Hemisphere) and exotic (Southern Hemisphere)]. Over 4 years soil-surface-active invertebrates were recorded by pitfall trap and canopy cover measured. The abundance of soil-surface-active invertebrates increased with canopy cover. Abundance was higher on the native treatment, but there were seasonal differences, with proportionately more invertebrates found on the exotic plots during the winter. Invertebrate herbivores, parasitoids and the Carabidae gave similar results to total abundance. Omnivores were most abundant on the near-native treatment and detritivores showed no difference between treatments. The abundance of the Araneae increased as canopy cover decreased. Analysis of diversity for groups meeting minimum data requirements indicated that abundance measures gave a good representation of diversity. The experiment demonstrated that gardens and other cultivated ornamental plantings support a wide range of soil-surface-active invertebrates regardless of the plants’ origin and the more plant matter (canopy cover) available the greater the abundance. More invertebrates will be supported by gardens and cultivated planting schemes that offer dense year-round vegetation cover combined with plantings biased towards native and near-native plants. Some areas of low vegetation cover will however, support some groups such as the Araneae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Domestic gardens (defined in Gaston et al. 2005) have a positive effect in urban environments by providing air cooling, flood mitigation, habitat resources and improvements to human health and wellbeing (Cameron et al. 2012). They can also play a vital role in maintaining invertebrate abundance and diversity, counterbalancing declines caused by increased urbanisation (Smith et al. 2006a, b, c; Owen 2010; Sánchez-Bayo and Wyckhuys 2019). Gardens are often the largest component (18–27% in the UK) of urban green infrastructure (Loram et al. 2007) and a large proportion (at least 48%) of those who manage these areas (gardeners) encourage animal life ‘wildlife’ (Davies et al. 2009). In addition many gardens have a diversity and density of plants exceeding that of natural or semi-natural habitats (Smith et al. 2006c; Loram et al. 2007, 2008). There are more than 76,000 plant taxa (ca. 14,000 distinct species) that gardeners can purchase in the UK (Cubey 2018), a country where the plant taxa considered native or naturalized is less than 5000 (Stace 2010). This availability is reflected in Britain’s garden flora which is a mixture of native and non-native species; non-native species making up at least 70% of the entire flora and 55% in an ‘average’ single garden (Loram et al. 2008). There is therefore a need for evidence based information on the value of native and non-native plantings in order to provide accurate advice to those who want to manage domestic gardens and other ornamental plantings to support invertebrates. It has often been assumed that invertebrates are best supported in gardens through native plantings (e.g. Baines 2016) yet there is little applicable evidence that this is the case. Many studies into the effects of non-native plants on invertebrate abundance, diversity and biomass focus on the effects of invasive species which can dominate native and semi-natural floras. Here the reduced plant diversity is reflected in lower invertebrate abundance and diversity (see review by Litt et al. 2014). Other work has indicated that non-native early successional plants support fewer invertebrates than native equivalents (Ballard et al. 2013) and non-native trees support fewer herbivores than native trees (Helden et al. 2012; Burghard and Tallamy 2013). These results are not however necessarily applicable to the managed domestic garden. Garden plant composition and structure is maintained and determined by human intervention, comprises a mixture of plant types with relatively few trees, and most garden plants have not become invasive (Stace and Crawley 2015). This paucity of evidence has been partially addressed in a field experiment that investigated the relative contribution of three categories of garden plant (defined by geographic origin: native, near-native and exotic). The experiment tested the null hypothesis that “there is no difference in invertebrate abundance and diversity associated with assemblages (artificial communities) of British native and non-native garden plants”. The effects of plant origin on abundance of two major ecological groups have been reported (Salisbury et al. 2015, 2017): the flower visitors (pollinators) and above ground plant-associated invertebrates. It was established that regardless of plant origin a greater floral resource resulted in an increase in pollinator visits, with pollinator abundance corresponding to peak flowering times and that the more plant matter available the greater the abundance of plant-associated invertebrates. The effects of plant origin were: (i) overall more pollinators visited native and near-native flowers; (ii) relatively fewer pollinators visited flowers of exotic plants in early summer but more visited later in the season; (iii) more plant-associated invertebrates were found on the native treatment though for several primary functional groups (including herbivores and some predator groups) near-native plants supported only marginally fewer individuals than the native plants, with exotic plants being less favoured. It is important to represent as many ecological functional groups as possible as responses may differ depending on ecological functionality (Noss 1990) therefore here we report on the abundance and diversity of a further major ecological group; the soil-surface-active invertebrates.
Materials and methods
The design and methodology of the experiment has been previously described (Salisbury et al. 2015, 2017) and only brief details are given below.
Plot and treatment layout
The experiment was replicated on two sites at Wisley, Surrey, UK (Deers Farm and Howard’s Field). At each site, 18 3 × 3 m plots were planted with an assemblage of 14 plant species treatments originating from one of three geographical regions, referred to as native, near-native or exotic (defined below). The plots were surrounded with wood (treated softwood, 150 mm × 25 mm at Deers Farm; 150 mm × 50 mm at Howard’s Field), with a 1 m guard row separating the plots. The wooden surrounds were drilled with 25 mm diameter holes at 25 mm intervals, level with the soil surface laterally throughout their length, allowing the free movement of soil-surface-active fauna into and out of the plots. The plot size represented a typical size for UK garden borders (Smith et al. 2006c). At each site the three treatments were randomly assigned to plots within each of six blocks oriented in an east-west direction (see Online Resource 1 Fig. S1). In order to maximise the representativeness of the plant species within plant origin treatments, there were three sets of 14-plant assemblages, termed plant groups ‘A’, ‘B’ and ‘C’, each formed from an overall list of 24 plant species (Online Resource Table S1). The three plant groups were assigned to the six blocks using restricted randomization, to ensure an even distribution along the north-south direction.
Plant choice, planting plan and plot layout
To keep the experiment relevant to the UK gardener, plant species within each category of geographical origin were selected on the basis of suitability for garden use (i.e. horticultural merit, see Salisbury et al. 2015). To enable the experiment to continue for several years all plants selected were perennial herbs, shrubs or climbers. The plant assemblages were chosen to appear as similar as possible in terms of plant height, density and structure, with equivalent plants for each of the treatments in the same position according to a standardized plan (Online Resource 1 Fig. S2). Planting took place between spring 2009 and spring 2010.
The three plant origin treatments were defined geographically:
- (i)
Native A species that arrived in the British Isles without anthropogenic intervention, whether intentional or unintentional (Pyšek et al. 2004).
- (ii)
Near-native A species occurring naturally only in the Northern Hemisphere but not native or naturalized in the British Isles, matched in terms of general growth habit and garden usage with one of the species chosen as a native plant and taxonomically related to it at familial, and usually generic, level.
- (iii)
Exotic A species occurring naturally only in the Southern Hemisphere, matched in terms of general habit and garden usage with one of the species chosen as a native plant but not necessarily related to it at any particular taxonomic rank and not naturalized in the British Isles.
Plot and plant management followed recommended UK horticultural practice to reproduce, as far as possible, typical garden conditions including visually appealing, weed-free plots (see Salisbury et al. 2015).
Data collection
Methods for recording soil-surface-active invertebrates were adapted from established protocols (e.g. Haughton et al. 2003), modified to reflect the design of the experiment. Field recording of soil-surface-active invertebrates began in February 2010 and continued until December 2013.
Soil-surface-active invertebrates
Pitfall traps were used to capture soil-surface-active invertebrates. Although widely used to record Carabidae, spiders and Collembola, comparisons of catches between treatments must be interpreted with care. These traps reflect some combination of activity and density (abundance), they also show selectivity in the taxa captured, not necessarily giving a good estimate of total abundance (Greenslade 1964; Winder et al. 2001; Firbank et al. 2003). The catches from different treatments can however be compared, particularly if traps are operated on several occasions incorporating seasonal and annual differences, provided the ecology of the captured invertebrates is considered (Baars 1979; Winder et al. 2001; Perry et al. 2003). Pitfall trap operation in this experiment was designed to reflect these needs.
One pitfall trap was located in the centre of each plot. Each trap consisted of a colourless polypropylene cup (70 mm diameter at top, tapering to 45 mm at the bottom, 85 mm depth) sunk into the ground with the top level with the soil surface. Approximately 75 ml of a 50:50 mix of tap water and antifreeze (containing Ethanediol, Bluecol Tetrosyl Ltd., Bury, Lancs UK) was added as a preservative. A drain hole (approximately 3 mm diameter) was made just above the level of the liquid to stop traps filling with rain water. Holes in the soil for the pitfall traps were kept open between trapping occasions by using a polypropylene cup with the bottom quarter cut off to act as a sleeve into which the pitfall trap could be placed. When not in use a plastic cap was used to stop soil and other detritus filling the holes. An aluminium tent peg was used to anchor the traps, stopping corvid birds from lifting them. The traps were left in situ for 2 weeks at a time and operated every other month throughout the year to give five occasions in 2010 and six occasions in 2011, 2012 and 2013 (Online Resource 1 Table S2). At the end of each trapping occasion the entire trap contents were decanted into a plastic container. The catch was sorted and preserved in 80% ethanol, before identification under a binocular microscope in the laboratory. Identification was to a level which enabled allocation to an ecological functional group of the stage sampled. Where feasible adults were identified to species (see online Resource 1 Table S3 for a list and associated keys and other reference works used), allowing analysis of species diversity of some groups.
In addition to a total count (all invertebrates, excluding Hymenoptera: Formicidae due to their colonial nature) a primary functional group was allocated. These were: Herbivore (defined as feeding primarily on living plants); Predator (feed primarily by killing and consuming prey); Detritivore (likely to be feeding on dead material or associated fungi); Omnivore (likely to feed on a range of material of animal and plant origin). Herbivores were further divided into those with chewing mouthparts (chewing), and those with sucking mouthparts (sucking). Where species identification was possible and feeding preferences were known, those that feed on plants from more than one family were defined as Herbivore (generalist) and those that feed on only one plant family were defined as Herbivore (specialist). Total predators were subdivided into Parasitica (parasitoid Hymenoptera), and Araneae, which was further divided into web-spinners and hunters. Carabidae were subdivided into predator, herbivore and omnivore. Analysis of abundance was carried out on those groups where numbers captured were greater than 400 over the course of the experiment.
Diversity measures
Three measures of species diversity were calculated for each of the groups defined above but only where more than 400 adults were identified to species (including aggregate species). Data did not meet the criteria for analysis at the recording event, season or year level so data for each plot was combined for all 4 years of the experiment. The diversity indices calculated were those used by Brooks et al. (2003), and were chosen for the same reasons, to avoid apparent changes in species richness associated with changes in abundance, instead allowing for the strong relationship between the number of species (S) and the number of individuals sampled (N). The objective of the analysis was to quantify the degree to which plant species composition and canopy cover affect invertebrate diversity; further details are given in the data analysis section. Firstly, S was analysed using log N as a covariate, with the expectation that there is an approximately linear relationship, especially for large sample sizes. The log-series α index (Taylor et al. 1976), was chosen due to its high discriminant ability and independence of sample size. Finally an index measuring dominance (D) in the form of a simplified version of the Berger-Parker index (Berger and Parker 1970; May 1975). This was calculated as D = Nmax/N, where Nmax represented the number of individuals of the most abundant species. D was calculated for plots where the number of species recorded in each group was at least 20, as this is the minimum size for reliable results (Morris et al. 2014). For analysis, D was transformed to a logit (ln (D/[1 − D])).
Covariate: canopy cover
As in Salisbury et al. (2017) availability of vegetation resources was considered equivalent to canopy cover. Every 6 to 8 weeks an estimate of canopy cover was made. Overhead digital photographs (Sony Cyber-shot DSC- HX1 camera) of each plot were taken from the top of a 3.6 m tripod ladder (Niwaki, Somerset, UK), on the same day, ensuring the entire plot was included in the photograph. The images were cropped in processing to include only vegetation within the timber edges surrounding each plot (Adobe Photoshop CS4). A 10 × 10 grid was overlaid onto each photograph and proportion canopy cover estimated by counting the number of squares at least half-filled with vegetation. To maintain consistency the measurements were always recorded by the same two operators. The recording dates of canopy cover did not match those of emptying of the pitfall traps therefore the closest matching date was chosen, this was usually within 2 weeks and always within 4 weeks of emptying of the pitfall traps for analysis. Where no date met these criteria the covariate was recorded as a missing value, this occurred only for the second recording date in 2013 (April).
Data analyses
All analysis was carried out using GENSTAT 16th Edition (VSN International 2013). The abundance per plot for each pitfall sampling occasion, c, was transformed logarithmically to y = log10(c + 1). Three linear regression models of transformed abundance, y, on canopy cover, v, where 0 ≤ v ≤ 1, were compared for each occasion: a single line through all three plant origin treatments; three parallel lines allowing the intercept to vary with treatment; and three separate lines allowing both slope and intercept to vary with treatment. Discrimination between models was by standard partial F-tests on two degrees of freedom (Perry 1982; Hawes et al. 2003). The log-series α and logit D diversity indices were also regressed, using the three models above, on canopy cover. Preliminary analysis confirmed that regressions for the two sites were similar for all treatments, so data from the two sites were merged. Linear regression was chosen following preliminary analysis involving quadratic and other non-linear terms, which showed no significant curvature.
The mean canopy cover over all occasions was 0.626. Where three separate lines with varying slope was the best model estimated values of back-transformed abundance were computed at this mean value denoted as d0.626. This summary statistic, d0.626, facilitated a comparison between treatments for abundance on the natural scale.
Results
A total of 22,581 (21,487 adult) invertebrates (excluding Hymenoptera: Formicidae) were collected in the pitfall traps. A primary functional group (Herbivore, Predator, Detritivore or Omnivore) was allocated to 20,036 (89%) of these invertebrates (Table 1). Of the remaining 2543 invertebrates, 745 (3%) were pollinators. It was not possible to allocate a functional group to 1798 (8%) individuals as the stage collected either did not feed or feeding habit was uncertain. Detritivores were the largest group recorded with 9643 (43%) individuals, of which 54% were Collembola. Herbivores consisted of 3169 (14%) individuals of which 1635 were Herbivore (sucking) and 1534 Herbivore (chewing). It was possible to determine the host range for 1880 of the herbivores, of which the great majority (1665) were assigned to Herbivore (generalist) and 215 to Herbivore (specialist). The latter were excluded from further analysis as they were below the threshold of 400 individuals. Omnivores comprised 3715 (16%) of individuals. Total predators made up 3509 (16%) individuals; 723 of which were Parasitica and 1327 Araneae, of which 1132 were Araneae (hunters). Araneae (web-spinners) made up fewer than 400 individuals and were excluded from further analysis. There were 1883 (8%) Carabidae of which 493 were herbivores, 890 omnivores and 501 predators. Two thirds (15,075) of the adult invertebrates were identified to species or species aggregate, including 51% of herbivores (1512 of 2968), 82% of Herbivore (chewing) (1183 of 1439), 70% of predators (2203 of 3132), 100% of Araneae (1146), 97% detritivores (8942 of 9209) and 100% of Carabidae (1883).
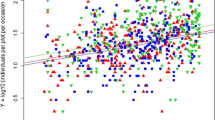
Total abundance and that of most of the primary functional groups showed a positive linear dependence of transformed abundance on the canopy cover covariate. The primary functional group Predators and the Carabidae (herbivore) subgroup did not show this positive linear dependence. The Araneae and subgroup Araneae (hunters) showed a negative linear dependence of transformed abundance on canopy cover covariate. The best fit model was three parallel lines with different intercepts for herbivores and the herbivore subgroups (sucking, chewing and generalist) and omnivores. The best fit model was three lines with different intercepts and slopes for total abundance of the Araneae (hunters), Parasitica and the Carabidae and the subgroups Carabidae (predator) and Carabidae (omnivore). There was no difference between the treatments for the Araneae. The results for these models are summarised in Table 1, Figs. 1 and 3, Online Resource 2.
Dependence of total abundance of soil-surface-active invertebrates (excluding Hymenoptera: Formicidae) on plant canopy. Observed data and fitted regressions for everything (n = 22,581), over all years 2010–2013 combined. Native (N) green, downward triangles; near-native (Z) blue, squares; exotic (E) red, upward triangles. Estimated intercepts and slope are shown in Table 1. The fitted regressions differ (F5,704 = 18.41, P = 0.043)
The observed data and fitted relationships for the total 22,581 soil-surface-active invertebrates collected are shown in Fig. 1; estimates of regression coefficients and significance tests are given for each year in Table 2. The model showed significant differences between the treatments although at typical values of canopy cover the difference is marginal with roughly a third of the catch coming from each treatment; 33% on the native compared to 34% on the near-native and 33% on the exotic treatments. The treatment with the lowest intercept (near-natives) had the highest slope and the treatment with the highest intercept (exotics) had the lowest slope. Thus at higher levels of canopy cover the native treatment has a noticeably higher abundance compared to the exotic or near-native, and at low levels of canopy cover exotics had higher abundance (Fig. 1). This pattern is repeated in each of the 4 years of the experiment (Table 2), although there are some differences in treatment with highest and lowest intercept. This result is mirrored by the seasonal changes in canopy cover, where exotic beds tended to have smaller differences between maximum and minimum levels of canopy cover throughout the year compared to the native or near-native treatments. Changes in the mean proportion of canopy cover and mean invertebrate abundance per plot between sampling occasions, within each of the 4 years, are shown in Fig. 2. All treatments followed a similar pattern of changes in canopy cover. In 2011 and 2012 canopy cover increased most from April to June, in 2010 this was May to July, while in 2013 canopy cover data in April are absent and the trend is unknown. Peak canopy cover was reached in August in all years. Soil-surface-active invertebrate abundance increased most from February to June in all years. For the majority of spring and summer recording events the native treatment had the highest canopy cover, the exotic treatment the lowest. During the winter recording events (December and February) the exotic treatment had canopy cover greater than or equal to the near-native treatment in all years and was often similar to that of the native beds. During this period invertebrate abundance on the exotic beds was, with the exception of February 2013, higher or equal to that of the other treatments.
The Detritivore functional group showed a positive linear response to canopy cover but no difference between the treatments (Fig. 3).
Dependence of soil-surface-active detritivore on plant canopy. Observed data and fitted regressions for ground dwelling detritivore (n = 9645), over all years 2010–2013 combined. Native (N) green, downward triangles; near-native (Z) blue, squares; exotic (E) red, upward triangles. Estimated intercepts and slope are shown in Table 1. The fitted regression is significant (F1,708 = 42.44, P < 0.001) and is the same for all three treatments
Relative herbivore abundance was highest on the native plots which was approximately 30% higher than the near-native plots and the exotic plots (Online Resource Fig. S2.1). Abundance of the herbivore subgroups analysed was also highest on the native beds, for Herbivore (sucking) being about 24% higher than on the exotic plots and 34% higher than on the near-native plots (Online Resource Fig. S2.2). Herbivore (chewing) were 11% more abundant on the native plots than on the near-native plots and 22% more abundant than on the exotic treatment (Online Resource Fig. S2.3). Herbivore (generalist) abundance was 19% higher on the native plots than the near-native and 22% higher than the exotic treatment (Online Resource Fig. S2.4). Abundance of omnivores was highest on the near-native plots, being nearly 30% higher than the native and exotic plots (Online Resource Fig. S2.5).
Total predator abundance showed no relationship with canopy cover (Online Resource Fig. S2.6) although the predatory subgroups did show a relationship. The Araneae and Araneae (hunters) showed decreasing abundance with increasing canopy cover (Online Resource Figs. S2.7, S2.8). There was no difference between the treatments for total Araneae. The best fitting model was three lines with differing negative slopes for Araneae (hunters), at typical values of canopy cover being 8% and 11% more abundant on the exotic treatment than on the native and near-native treatments respectively. The Parasitica gave three lines with differing positive slopes with the highest abundance on the native treatment which was 10% higher than on the near-native plots and 12% higher than on the exotic plots (Online Resource Fig. S2.9).
For Carabidae three lines with different slopes gave the best fitting model, with a positive relationship to canopy cover (Online Resource Fig. S2.10). The Carabidae (predator) and Carabidae (omnivore) subgroups gave similar results (Online Resource Figs. S2.11, S2.13). At typical values of canopy cover abundance of these groups was highest on the near-native and lowest on the exotic plots; total Carabids and Carabidae (predator) were 10% higher on the near-native compared to the native and about a third higher for the near-native treatment than the exotic. At typical values of canopy cover there were 5% fewer Carabidae (omnivore) captured on the native beds and 20% fewer on the exotic treatment compared to the near-native treatment.
Diversity
Diversity measures were calculated for the functional groups Detritivore, Herbivore, Herbivore (chewing), total Predators, Araneae and the taxonomic group Carabidae. Other groups did not meet the criterion of more than 400 adults identified to species. Numbers of individuals were lower than for total abundance as those specimens not identified to species were not included in the analysis.
As expected when S was analysed using log N as a covariate, there was a significant positive linear relationship, with the exception of the Detritivores (Table 3, Fig. 4, Online Resource Figs. 2.13–2.18). Dominance showed a linear relationship with canopy cover for the Predator primary functional group and the Carabidae, but gave no relationship for the other groups analysed (Table 4, Online Resource Figs. 2.19–2.24). Predators overall showed a marginal drop in dominance from 34% at the lowest value of canopy cover (0.43) to 31% at the highest value (0.75) (Online Resource Fig. 2.19). The Carabidae showed a larger drop in dominance from 44% to 34% over the same values of canopy cover (Online Resource Figs. 2.24). The log-series α index gave a postive linear relationship with canopy cover for Predator, Araneae, Detritivore and the Carabidae, there was however no relationship for Hebivores and subgroup Herbivore (chewing) (Figs. 5, 6, Online Resource Figs. 2.25–28).
Dependence of species (S) of soil-surface-active Herbivore (chewing) on abundance (N). Observed data and fitted regressions for ground dwelling Herbivore (Chewer) (N = 1183, S = 84), over all years 2010–2013 combined. Native (N) green, downward triangles; near-native (Z) blue, squares; exotic (E) red, upward triangles. Estimated intercept and slope are shown in Table 3. The fitted regressions (intercepts) differ (F3,32 = 14.5, P = 0.011)
Dependence of diversity measure log-series α index (Alpha) of soil-surface-active detritivores on Canopy cover. Observed data and fitted regressions for ground dwelling predators (N = 8942, species number = 97), over all years 2010–2013 combined. Native (N) green, downward triangles; near-native (Z) blue, squares; exotic (E) red, upward triangles. Estimated intercept and slope are shown in Table 5. The fitted regressions (intercepts) differ (F3,32 = 4.09, P = 0.022)
There were limited treatment effects for the groups across the measures of diversity; Herbivore (chewing) showed the greatest values for the S log N comparison on the near-native plots (Fig. 4), and lowest on the exotic plots. For the log-series α index; Detritivores gave the highest value on the near-native plots and lowest on the native plots (Fig. 5) whilst the Carabidae was highest on the exotic plots and lowest on the natives (Fig. 6). The measure of dominance gave no treatment differences.
Dependence of diversity measure log-series α index (Alpha) of soil-surface-active Carabidae on Canopy cover. Observed data and fitted regressions for ground dwelling predators (N = 1883, species number = 48), over all years 2010–2013 combined. Native (N) green, downward triangles; near-native (Z) blue, squares; exotic (E) red, upward triangles. Estimated intercept and slope are shown in Table 5. The fitted regressions (intercepts) differ (F3,32 = 370, P = 0.005)
Discussion
Regardless of garden plant origin abundance of soil-surface-active invertebrates shows a positive response to increasing vegetation resource (canopy cover), a result consistent with that found for plant-associated invertebrates (Salisbury et al. 2017). This effect has also been previously observed in gardens (Smith et al. 2006b) and the wider environment (Dennis et al. 1998; Koricheva et al. 2000). Across plant origin treatments this can result in differences in the relative numbers of invertebrates captured in different seasons due to changes in relative amount of vegetation resource. In this experiment comparatively more soil-surface-active invertebrates were captured on the exotic treatment compared to the other treatments in the winter months, when this treatment also had relatively higher levels of canopy cover. The higher levels of canopy cover at this time are likely to be due to differences in the number of evergreen or semi-evergreen plants in each plot—native plots contained either 3 or 4, near-native plots 5 or 6 and exotic plots 6 or 9. This factor may also provide some explanation for the small dissimilarities recorded between the treatments at typical values of canopy cover. Differences in abundance of soil-surface-active invertebrates were more pronounced at high and low levels of canopy cover and overall plantings of native herbaceous perennials and shrubs support greater numbers of soil-surface-active invertebrates than groups of near-native or exotic plants. Higher levels of canopy cover occurred in the spring and summer when invertebrate numbers were also higher. This result is similar to that reported for plant-associated invertebrates (Salisbury et al. 2017) and to those found for invertebrate herbivores in North America (Zuefle et al. 2008; Ballard et al. 2013; Burghart and Tallamy 2013). It is possible that this pattern of increasing abundance with increasing canopy cover may have been underestimated in this experiment. Pitfall trap catch can be influenced by vegetation density, for example in the Carabidae vegetation impedes movement, the more vegetation the smaller the proportion of invertebrates captured (Greenslade 1964).
These results demonstrate that to gain insight into soil-surface-active invertebrates supported by garden plant communities, the responses of different functional groups should be examined. Whilst several functional groups (the Carabidae (and its predatory and omnivore subgroups), omnivores and herbivores) followed a similar pattern to total abundance, other functional groups did not. The primary Predator and Carabidae (herbivore) functional groups showed no relationship with canopy cover whilst the Araneae showed a negative relationship. There were also differences in the responses to the different plant origin treatments.
The abundance of detritivores did not appear to depend on the geographic origin of plant resources but only on the amount of vegetative cover. This is similar to results of other studies (Proches et al. 2008; Hartley et al. 2010) and it may be expected that detritivores exploit resources (for example decomposing parts of plants) irrespective of plant identity or origin resulting in little difference between treatments. It was found that the abundance of plant-associated detritivores was higher on native treatments (Salisbury et al. 2017). This could be due to differences in the detritivorous taxa on the soil-surface compared to the plant-associated; the Collembola comprised 54% of the soil-surface-active detritivores but 97% of the plant-associated detritivores.
The soil-surface-active herbivores and its subgroups derived greatest benefit from native plants, a result likely driven by food or other resources such as plant structural differences not detected by the methodology, and is similar to the results for plant-associated invertebrates (Salisbury et al. 2017). Total abundance of herbivores and the three herbivore subgroups was highest on the native treatment, followed in most cases by the near-native treatment; the exception was herbivore (suckers) where abundance on the exotic treatment was higher than on the near-native treatment. Greater abundance of herbivores on native plants is consistent with other works comparing invertebrate abundance on native and non-native plants (Proches et al. 2008; Hartley et al. 2010; Helden et al. 2012; Salisbury et al. 2017). It is possible that the herbivore (suckers) group contained species that were particularly abundant on one or more of the exotic plants leading to the higher numbers observed compared to the near-native treatment. The responses of herbivores to plant origin can be highly variable depending on plant and invertebrate species and in some cases exotic plants can support similar abundances of invertebrates to native plants, especially if there are successful generalists present (Tallamy 2004; Proches et al. 2008; Zuefle et al. 2008), and there is evidence that herbivore abundance is affected by natural enemy (predator) abundance (Koricheva et al. 2000).
The omnivores are a heterogeneous group that could be expected to exploit a wide range of plant, fungal and animal matter, regardless of origin. It is therefore not clear why this group was found in greater abundance on the near-native treatment, whilst the differences between the native and exotic treatments were small.
The groups of predators analysed appeared to utilise different resources or activity differed with canopy cover and across the treatments. The lack of a response to canopy cover by total predators is explained by the opposing relationships of the subgroups; the Araneae showed a negative relationship with canopy cover whilst the Parasitica gave a positive relationship. Decreasing numbers of Araneae with increasing canopy cover was more pronounced in the Araneae (hunters) subgroup. Plant-associated Araneae and its hunting subgroup were found to increase with plant density (Salisbury et al. 2017). This may be indicative of the behaviour of this group, those soil-surface hunting species having different requirements to those hunting on plants, though little is known about the habitat requirements of most spiders (Bell et al. 2001). It is also possible as with the Carabidae (Greenslade 1964) that lower vegetation density allows more movement over the soil-surface and potentially results in a greater catch in pitfall traps.
Plant origin did not appear to be a driver of abundance in total Araneae perhaps indicating that the availability of prey items or time spent searching for them is similar in each treatment. The Araneae (hunters) subgroup had a higher abundance on the exotic plot. As pitfall traps give an indication of activity it is possible that this is a reflection of time spent searching for prey by this group.
The higher abundance of Parasitica on the native plots compared to the near-native and exotic plots is similar to that observed on plant-associated Parasitica collected from plants (Salisbury et al. 2017). This indicates greater availability prey on this treatment and is supported by the result observed for herbivores.
Taking into account that pitfall traps record a combination of the activity and abundance of carabids (Greenslade 1964; Winder et al. 2001; Firbank et al. 2003), there was more activity of total Carabidae and its subgroups Carabidae (predator) and Carabidae (omnivore) on the near-native and native plots, perhaps reflecting a greater abundance of prey or other resources. The Carabidae (herbivore) was the only group analysed other than the total predators to show no relationship with canopy cover, but this group only just exceeded the threshold of 400 individuals for analysis, so perhaps not much should be made of this result.
Diversity responses
The results from diversity measurements reflect those from abundance, therefore the abundance measures used are a good indication of the effects of canopy cover and plant origin treatments on diversity. This is most clearly demonstrated in the linear relationship observed with the analysis of S log N, for all groups analysed except the detritivores. As with abundance the alpha (α) diversity measurement suggests that as the amount of vegetation increases so does species diversity, with the exception of the herbivorous functional groups. Similarly the dominance of one species for soil-surface-active predators and Carabidae decreases as canopy cover increases, indicating an increase in diversity. These results are to be expected as they have been found in other garden-based studies (Smith et al. 2006a, b) and may reflect available niches. Herbivores showed no increase in diversity with canopy for either alpha (α) or dominance measures, perhaps due to herbivore diversity being closely linked to plant diversity rather than amount of plant material (see Smith et al. 2006c) and in this experiment plant diversity was a constant (14 plant species per plot). There was limited evidence of differences in diversity between the plant origin treatments; the exceptions were Detritivores and Carabidae which gave the lowest alpha (α) diversity on the native plots and a higher intercept of the S Log N comparison for Herbivore (chewing) on the native and near-native treatments compared to the exotic. The result with the Herbivore (chewing) is to be expected and likely to be due to the availability of suitable (native and near-native) plant food species and is similar to that of Hartley et al. (2010) and Proches et al. (2008). It has not been possible to make comparisons of diversity measures from other sampling methods used in this experiment, such as plant-associated invertebrates collected by Vortis suction sampler, due to numbers of any group not reaching the minimum criteria for analysis. The results from the pitfall traps, however, indicate that the abundance measures used in previous work (Salisbury et al. 2017) provide a good comparison between the treatments. The exception is the Collembola which were also recorded from soil samples and will be dealt with separately.
Conclusions
Our conclusions relate to the relative foraging preferences or activity and diversity of invertebrates between three plant origin treatments. While absolute abundances and diversity might differ for different locations and plot sizes, we consider the relative differences reported will be robust to changes in spatial scale for British gardens. The results reinforce the view of Davis et al. (2011) that the focus should be much more on the functions of non-native and native species and much less on their origins, this is particularly relevant to gardens and similar ornamental plantings.
This experiment has shown that the more vegetation (canopy cover) ornamental plantings have, the greater the abundance of soil-surface-active invertebrates they will support, regardless of plant origin (nativeness). This was also found to be true of plant-associated invertebrates in the same experiment (Salisbury et al. 2017). The seasonal differences in vegetation cover between plant treatments play a role, for example the exotic plants used provided relatively more cover in winter compared to the other treatments and invertebrate abundance responded likewise. Despite the overwhelmingly positive effect of increasing plant cover on almost all invertebrate groups investigated it is clear that some groups may benefit from low canopy cover, for example the Araneae (hunters).
The native and near-native treatments supported more soil-surface-active invertebrates than the exotic treatment. This was also true for most of the functional groups analysed (with the exception of the detritivores) and indicates that native and near-native plants provide the most resources for invertebrates in gardens. Analysis of diversity for those groups meeting minimum data requirements showed that abundance measures gave a good picture of diversity.
Results from the soil-surface-active invertebrates have enabled us to refine advice to gardeners and managers of cultivated green spaces who wish to provide maximum resources for invertebrate abundance, given previously regarding pollinators and plant-associated invertebrates (Salisbury et al. 2015, 2017). That is, to select plantings biased towards native and near-native plants, managed to provide dense vegetation cover. Some exotics can also be considered as these will be inhabited by some invertebrates and, if flowering, support pollinators and can potentially provide relatively more resource in winter (compared to native and near-native plants). In addition, some areas of low vegetation density may benefit some invertebrate groups such as the Araneae.
Data accessibility
Soil-surface-active invertebrate data is available in Online Resource 3.
References
Baars MA (1979) Catches in pitfall traps in relation to mean densities of carabid beetles. Oecologia 41:25–46
Baines C (2016) RHS companion to wildlife gardening. Frances Lincoln, London
Ballard M, Hough-Goldstein J, Tallamy D (2013) Arthropod communities on native and nonnative early successional plants. Environ Entomol 42:851–859
Bell JR, Wheater PC, Cullen WR (2001) The implications of grassland and heathland management for the conservation of spider communities: a review. J Zool 255:377–387
Berger WH, Parker FL (1970) Diversity of planktonic foraminifera in deep-sea sediments. Science 168:1345–1347
Brooks DR, Bohan DA, Champion GT, Haughton AJ, Hawes C, Heard MS, Clark SJ, Dewar AM, Firbank LG, Perry JN, Rothery P, Scott RJ, Woiwod IP, Birchall C, Skellern MP, Walker JH, Baker P, Bell D, Browne EL, Dewar AJD, Fairfax CM, Garner BH, Haylock LA, Horne SL, Hulmes SE, Mason NS, Norton LR, Nuttall P, Randle Z, Rossall MJ, Sands RJN, Singer EJ, Walker MJ (2003) Invertebrate responses to the management of genetically modified herbicide-tolerant and conventional spring crops. I. Soil-surface-active invertebrates. Philos Trans R Soc Lond B 358:1847–1862
Burghardt KT, Tallamy DW (2013) Plant origin asymmetrically impacts feeding guilds and life stages driving community structure of herbivorous arthropods. Divers Distrib 19:1553–1565
Cameron RWF, Blanuasa T, Taylor JE, Salisbury A, Halstead AJ, Henricot BB, Thompson K (2012) The domestic garden – Its contribution to urban green infrastructure. Urban For Urban Gree 11:129–137
Cubey J (Editor in Chief) (2018) RHS Plant Finder 2018. The Royal Horticultural Society, London
Davies ZG, Fuller RA, Loram A, Irvine KN, Sims V, Gaston KJ (2009) A national scale inventory of resource provision for biodiversity within domestic gardens. Biol Conserv 142:761–771
Davis MA, Chew MK, Hobbs RJ, Lugo AE, Ewel JJ, Vermeij GJ, Brown JH, Rosenzweig ML, Gardener MR, Carroll SP, Thompson K, Pickett STA, Stromberg JC, Del Tredici P, Suding KN, Ehrenfeld JG, Grime JP, Mascaro J, Briggs JC (2011) Don’t judge species on their origins. Nature 474:153–154
Dennis P, Young MR, Gordon IJ (1998) Distribution and abundance of small insects and arachnids in relation to structural heterogeneity of grazed, indigenous grasslands. Ecol Entomol 23:253–264
Firbank LG, Heard MS, Woiwod IP, Hawes C, Haughton AJ, Champion GT, Scott RJ, Hill MO, Dewar AM, Squire GR, May MJ, Brooks DR, Bohan DA, Daniels RE, Osborne JL, Roy DB, Black HIJ, Rothery P, Perry JN (2003) An introduction to the farm-scale evaluations of genetically modified herbicide-tolerant crops. J Appl Ecol 40:2–16
Gaston KJ, Warren PH, Thompson K, Smith RM (2005) Urban domestic gardens (IV): the extent of the resource and its associated features. Biodivers Conserv 14:3327–3349
Greenslade PJM (1964) Pitfall trapping as a method for studying populations of Carabidae (Coleoptera). J Anim Ecol 33:301–310
Hartley MK, Rogers WE, Siemann E (2010) Comparisons of arthropod assemblages on an invasive and native trees: abundance, diversity and damage. Arthropod-Plant Inte 4:237–245
Haughton AJ, Champion GT, Hawes C, Heard MS, Brooks DR, Bohan DA, Clark SJ, Dewar AM, Firbank LG, Osborne JL, Perry JN, Rothery P, Roy DB, Scott RJ, Woiwod IP, Birchall C, Skellern MP, Walker JH, Baker P, Browne EL, Garner BH, Haylock LA, Horne SL, Mason NS, Sands RJN, Walker MJ, Bell D, Fairfax CM, Hulmes SE, Norton LR, Nuttall P, Randle Z, Rossall MJ, Singer EJ (2003) Invertebrate responses to the management of genetically modified herbicide-tolerant and conventional spring crops. II. Within-field epigeal and aerial arthropods. Philos Trans R Soc B 358:1847–1862
Hawes C, Haughton AJ, Osborne JL, Roy DB, Clark SJ, Perry JN, Rothery P, Boha DA, Brooks DR, Champion GT, Dewar AM, Heard MS, Woiwod IP, Daniels RE, Young MW, Parish AM, Scott RJ, Firbank LG, Squire GR (2003) Responses of plants and invertebrate trophic groups to contrasting herbicide regimes in the Farm Scale Evaluations of genetically modified herbicide-tolerant crops. Philos Trans R Soc B 358:1899–1913
Helden AJ, Stamp GC, Leather SR (2012) Urban biodiversity: comparison of insect assemblages on native and non-native trees. Urban Ecosyst 15:611–624
Koricheva J, Mulder CPH, Schmid B, Joshi J, Huss-Danell K (2000) Numerical responses of different trophic groups of invertebrates to manipulations of plant diversity in grasslands. Oecologia 125:271–282
Litt RL, Cord EE, Fulbright TE, Schuster GL (2014) Effects of invasive plants on arthropods. Conserv Biol 28:1532–1549
Loram A, Tratalos J, Warren PH, Gaston KJ (2007) Urban domestic gardens (X): the extent structure of the resource in five major cities. Landsc Ecol 22:601–615
Loram A, Thompson K, Warren PH, Gaston KJ (2008) Urban domestic gardens (XII): the richness and composition of the flora in five UK cities. J Veg Sci 19:321–330
May RM (1975) Patterns of species abundance and diversity. In: Cody ML, Diamond JM (eds) Ecology and evolution of communities. Harvard University Press, Cambridge, pp 81–120
Morris EK, Caruso T, Buscot F, Fischer M, Hancock C, Maier TS, Meiners T, Muller C, Obermaier E, Prati D, Socher SA, Sonnemann I, Waschke N, Wubet T, Wurst S, Rillig MC (2014) Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol Evol 4:3514–3524
Noss RF (1990) Indicators for monitoring biodiversity: a hierarchical approach. Conserv Biol 4:355–364
Owen J (2010) Wildlife of a garden. A thirty year study. Royal Horticultural Society, London
Perry JN (1982) Fitting split-lines to ecological data. Ecol Entomol 7:421–435
Perry JN, Rothery P, Clark SJ, Heard MS, Hawes C (2003) Design, analysis and statistical power of the Farm-Scale Evaluations of genetically modified herbicide-tolerant crops. J Anim Ecol 40:17–31
Proches E, Wilson JRU, Richardson DM, Chown SL (2008) Herbivores, but not other insects, are scarce on alien plants. Austral Ecol 33:691–700
Pyšek P, Richardson DM, Rejmánek M, Webster GL, Williamson M, Kirschner J (2004) Alien plants in checklists and floras: towards better communication between taxonomists and ecologists. Taxon 53:131–143
Salisbury A, Armitage J, Bostock H, Perry J, Tatchell M, Thompson K (2015) Enhancing gardens as habitats for flower-visiting aerial insects (pollinators): should we plant native or exotic species? J Appl Ecol 52:1156–1164
Salisbury A, Al-Beidh S, Armitage J, Bird S, Bostock H, Platoni A, Tatchell M, Thompson K, Perry J (2017) Enhancing gardens as habitats for plant-associated invertebrates: should we plant native or exotic species? Biodivers Conserv 26:2657–2673
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27
Smith RM, Gaston KJ, Warren PH, Thompson K (2006a) Urban domestic gardens (VI): environmentalcorrelates of invertebrate species richness. Biodivers Conserv 15:2415–2438
Smith RM, Gaston KJ, Warren PH, Thompson K (2006b) Urban domestic gardens (VIII): environmental correlates of invertebrate abundance. Biodivers Conserv 15:2515–2545
Smith RM, Thompson K, Hodgson JG, Warren PH, Gaston KJ (2006c) Urban domestic gardens (IX): composition and richness of the vascular plant flora, and implications for native biodiversity. Biol Conserv 129:312–322
Stace C (2010) New flora of the British Isles, 3rd edn. Cambridge University Press, Cambridge
Stace CA, Crawley MJ (2015) Alien plants (Collins New Naturalist Library, Book 129). Harper Collins, London
Tallamy DW (2004) Do alien plants reduce insect biomass? Conserv Biol 18:1689–1692
Taylor LR, Kempton RA, Woiwod IP (1976) Diversity statistics and the log-series model. J Anim Ecol 45:255–272
VSN International (2013) GenStat for windows, 16th edn. VSN International, Hemel Hempstead
Winder L, Holland JM, Perry JN, Woolley C, Alexander CJ (2001) The use of barrier-connected pitfall trapping for sampling predatory beetles and spiders. Entomol Exp Appl 98:249–258
Zuefle ME, Brown WP, Tallamy DW (2008) Effects of non-native plants on the native insect community of Delaware. Biol Invasions 10:1159–1169
Acknowledgements
We would like to thank the project volunteers James Backshall, Crystal Duncan, Carolyn Hewitt, Helen Latham, Linda Moyes, Judi O’Prey, John Ricketts, Kathy Stones and Jenna Watt who maintained the plots and the members of RHS Wisley curatorial staff who assisted. Thanks are also due to those who provided help and advice including Jonty Denton, David Brooks, Suzanne Clark, Colin Crosbie, Victoria Coupland, Jim Gardiner, Gerard Clover, Alistair Griffiths, Andrew Halstead, Alison Haughton and Steve Head (the Wildlife Gardening Forum), David Notton, Juliet Osborne, Claire Ozanne, Laura Robins, Peter Shaw, Richard Sanford, Ian Waghorn and Roger Williams.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jens Wolfgang Dauber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Urban biodiversity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salisbury, A., Al-Beidh, S., Armitage, J. et al. Enhancing gardens as habitats for soil-surface-active invertebrates: should we plant native or exotic species?. Biodivers Conserv 29, 129–151 (2020). https://doi.org/10.1007/s10531-019-01874-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-019-01874-w