Abstract
This study explores the possible influence of human coastal development (before and after) and protected area status (within and outside a marine protected area, MPA) on composition, density, and maximum size of fish species and guilds, including mean trophic level of the fish community, in four localities of the Yucatan Peninsula, Mexican Caribbean. Reef fish density, maximum length, species composition, and trophic guilds were recorded by SCUBA belt transects and stationary points in fore reef and lagoon reef areas at decadal intervals (1995–1998, 2006–2010, 2014–2015). Mean density of most species and guilds decreased significantly through the years, as also did mean trophic level of the fish community. Some fish species increased in length. Fish density for many species was larger outside than inside the MPA in 1995–1998; however, the difference tended to disappear in the more recent decades, which reflects either a positive effect of the MPA, or a detrimental effect of coastal development in the non-protected area. Nevertheless, the overall negative trends suggest a regional or global rather than a local cause.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatial and temporal variability in reef fish density can be influenced by biological and physical factors, such as depth (Andradi-Brown et al. 2016), coral cover (Bell and Galzin 1984), bottom rugosity (Luckhurst and Luckhurst 1978), predation (Hixon and Beets 1993), competition (Gladfelter et al. 1980), larval dynamics (Leis and McCormick 2002), recruitment variability (Booth and Brosnan 1995), or chance colonization of habitats (Sale and Dybdahl 1975). Differences in fish composition between the habitats of reef lagoon and fore reef have also been documented; Núñez-Lara and Arias-González (1998) were able to discriminate reef fish species associated with lagoon and back reef sites of low topographical complexity and species associated with high complexity reef front and reef slope sites.
Human impacts, such as fishing and coastal development, also have an influence, not only on reef fish density, but also on composition (Bianchi et al. 2000), body size (Genner et al. 2010), and relative abundance of trophic guilds (Friedlander and DeMartini 2002). Friedlander et al. (2003) found that reef fish community attributes, such as biomass, richness, abundance, and diversity, increased with higher protection, either natural (e.g. wave exposure) or human-induced (marine protected areas, MPAs).
In the Caribbean Sea, threats to coral reefs and fish assemblages emerged due to a combination of human coastal development, climate change, and overfishing (Jackson et al. 2001; Hoegh-Guldberg et al. 2007). To balance human impacts, MPAs have been implemented with restricted or forbidden fishing access; these appear to function well under certain circumstances (Roberts 1995; Halpern and Warner 2003). However, these tools can fail to protect biodiversity in the face of severe environmental degradation (Jones et al. 2004).
The decrease in coral cover and reef complexity throughout the Caribbean (Gardner et al. 2003), as well as global negative trends in mean trophic level of marine fishes (Pauly et al. 1998), call into question most existing management and conservation plans (Bellwood et al. 2004). However, MPAs have been shown to increase biomass and mean size, and hence economical value, of fish populations (Polunin and Roberts 1993). Following this rationale, and given the good results of MPAs in Belize, such as Bacalar Chico and Hol Chan (Peckol et al. 2003), the people of Xcalak town, southern Mexican Caribbean, succeeded in establishing a MPA in 2000, as the Parque Nacional Arrecifes de Xcalak (PNAX), a part of the Mesoamerican Barrier Reef System, MBRS (Hoffman 2009).
The establishment of PNAX occurred simultaneously as a coastal development 50 km north of the MPA, in Mahahual, which used to be a small fishing village. In Mahahual, tourist development, including the construction of a cruise ship pier, caused habitat loss and fragmentation (Martínez-Rendis et al. 2016). Also, intense fishing on groupers, particularly during spawning aggregations, caused the disappearance of at least one important aggregation of Nassau grouper (Epinephelus striatus) off Mahahual (Aguilar-Perera 2006).
Yeager and Arias-González (2008) examined fish communities in seagrass-beds of reef lagoons near Xcalak and Mahahual. They attributed the greater abundance found in Xcalak both to a larger extension of adjacent mangroves and to “heavy tourist use and coastal development” in Mahahual. However, Rodríguez-Zaragoza and Arias-González (2008) compared several reefs in the Mexican Caribbean and concluded that reef front and terrace habitats in Mahahual had a higher α-diversity and abundance of fishes than Xcalak and all other sites. The ubiquitous transition from hard coral to fleshy macroalgae in the Caribbean has been blamed for a “drastic” reduction in the number of fish species at Mahahual from 2000 to 2010, impacting mainly rarer species (Acosta-González et al. 2013). An additional stressor, the invasion of lionfish Pterois volitans into the area, began in 2009 (Betancur-R. et al. 2011), and a major coral-bleaching event occurred in 2011 (Hernández-Arana et al. 2014). Moreover, a decrease in coral cover has been documented for all the MBRS, in average from 23 to 13% from 2001 to 2005, and 2% in each of the next 3 years (García-Salgado et al. 2008).
Thus, environmental data from the Mahahual-Xcalak coast can be used in a Before-After/Control-Impact (BACI) design. Morales-Aranda et al. (2012) offered a preliminary BACI analysis for the area. According to Russ (2002), only 16% of the studies that attempted to detect effects of marine reserves on density/size of reef fishes included both spatial and temporal comparisons with “before” data. However, BACI designs have already been successfully used to demonstrate spillover of reef fishes from a MPA (Francini-Filho and Moura 2008), as well as the impact of lionfish on native fish abundance in the Atlantic (Ballew et al. 2016).
The present study compares the composition, density, and body size of reef fish, as well as density of ecological guilds and mean trophic level of fish communities, in the southern Mexican Caribbean. The analysis spans 20 years, before and after coastal development in Mahahual, the lionfish invasion, and the establishment of PNAX, among other changes. The objective is to detect interdecadal differences both within and outside the MPA and to test the effectiveness of the MPA.
Materials and methods
Study area
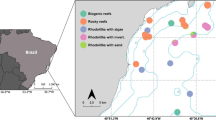
The southern coast of Quintana Roo, Mexico, comprises a fringing reef, which is part of the MBRS, on the border with Belize (Fig. 1). Southeasterly winds predominate, changing to northerly winds in November–January. Temperature ranges from the annual mean of 26 °C down to 20 °C, and mean annual precipitation is 1400 mm. Waves are usually 1.0–1.3 m high, and tides no greater than 0.5 m. The Caribbean current flows to the north, but eddies and countercurrents make current dynamics complex (Muhling et al. 2013).
This study encompassed four localities, two outside and two inside the MPA: Mahahual, an unprotected site where tourism development has risen since 2000; Xahuayxol (also spelled “Chahuaychol”), an unprotected reef, with less intense human development; Río Huach, located in the MPA; and Xcalak, also within PNAX (Fig. 1; Table 1), with a permanent lionfish culling program. Reefs in these localities share a similar structure: a 1–6 m deep sandy reef lagoon, with isolated coral heads and seagrass beds on sandy bottoms, separated by a crest from a fore reef, usually with rather well-developed spurs and grooves; the fore reef falls to a 5–40 m deep sandy terrace that extends to a much larger drop-off. For this study, we assessed fish communities only on the reef lagoon and the fore reef (to a depth of 17 m).
The most notable natural difference between our protected and unprotected localities is a better development of coastal mangrove in PNAX, due to the presence of small coastal lagoons. Another important difference is a much greater distance from the reef crest to the beach in PNAX (ca. 1000 m, compared to ca. 100 m at Mahahual). On the other hand, the spur-and-groove structure is rather similar in the fore reef across all sites, although Rodríguez-Zaragoza and Arias-González (2008) estimated a higher habitat heterogeneity in Mahahual. For more detailed descriptions of habitat complexity and coral composition, see Ruiz-Zárate et al. (1998) and Morales-Aranda et al. (2012).
Field work
Surveys were conducted every 5–8 years across three decades: 1995–1998, 2006–2010, and 2014–2015. There were at least two expeditions per year, usually coinciding with the peak of the dry and rainy seasons. The number of surveys was usually greater in the fore reef than in the reef lagoon, especially in 2014–2015 (Table 2); the lagoon is also a much shallower habitat. In the first two decades, two methods were conducted: band transects (Brock 1954, as modified by Almada-Villela et al. 2003) and stationary censuses (Bohnsack and Bannerot 1986). For the most recent period, only the stationary census method was used.
Both methods relied on SCUBA, during daylight (09:00–17:00 h). Observers were trained on underwater fish identification by using photographic guides (Humann 1989; Humann and DeLoach 2011), in estimation of the 5-m radius of the stationary census cylinder, and in estimation of fish length by practicing with moored wooden models underwater. Although twelve observers were involved in the project, > 95% of the surveys were performed by the six coauthors themselves; the first author participated in all three sampling periods. All fish were identified, counted, and their individual total length estimated to the nearest centimeter (using a 30-cm ruler fixed to the end of a 50-cm long handle). Fish smaller than ca. 2 cm were omitted, because no active search for cryptic and secretive species was attempted.
During band transects, the divers swam a distance of 30 m and recorded fish up to 2 m left and right from the line, for a total area of 120 m2. The line was being deployed simultaneously, its end anchored to a lead weight set at the beginning of the transect. To perform stationary censuses, each diver rotated over the bottom, recording all fish (identity, number, individual length) in a 5-m-radius imaginary cylinder, including the substrate and the water column, for 5 min.
The first point was fixed, i.e. all surveys began from the same starting point (Fig. 1; Table 1). Replicates for the same dive, either by transects or stationary censuses, started at a point determined by a previously prepared list of random directions (using a compass) and distances (number of kicks, from 10 to 20). Number of replicates was 10 stationary censuses or four transects per observer per dive.
Transects were applied in the two earlier decades and stationary census was used in all three decades. Xcalak was not visited in 1997–1998, Río Huach was not visited in the year 2010, and Mahahual was not visited in 2014–2015.
Data analysis
Abundance data grouped all replicates for a given level of protection (sites within vs. outside the MPA; Table 1), habitat (fore reef vs. reef lagoon), and decade (“1990” means 1995–1998; “2000” means 2006–2010; “2010” means 2014–2015). “Fish maximum length” is the mean of the maximum lengths by each replicate for every site, habitat, and decade combination. The number of replicates varied (Table 2), but abundance data were standardized to one transect or stationary census. These data are to be interpreted as densities (specimens per 120 m2 for transects, specimens per 78.5 m2 for stationary censuses). Trophic level by species (according to Froese and Pauly 2016) was weighted by the frequency of the species (i.e., percentage of the replicates where present by site, habitat, and decade), and the average trophic level obtained for each grouping. No attempt was made to analyze by season or month, or to calculate community descriptors, e.g. diversity indices or species richness, which have been explored by other authors (e.g. Acosta-González et al. 2013).
Data were compared among decades (three levels), protection status (two), and habitat (two) by a three-way ANOVA (p < 0.05, 0.01, or 0.001), followed by Tukey’s multiple-range tests to detect pairwise significant differences. Note was taken of interactions between decade and protection status. This was done separately for each method (transects and stationary censuses). Prior to ANOVA, abundance was arcsinh-transformed, a procedure recommended because of the frequent observations of zero in the matrix (Fowler et al. 1998). Maximum length and trophic level were checked for homoscedasticity and normality and were determined to meet assumptions for ANOVA. Statistical analyses were performed in R vers. 3.2.2 (Venables and Smith 2003).
Results
In total, 156 fish species were surveyed, representing 77 genera and 40 families from 303 belt transects and 590 stationary censuses (Table 2). Ten fish species identified in the 1990’s (observations outside of stationary censuses or transect surveys not included) were not found in later surveys. Thirty-two species were encountered in the 1990’s and 2000’s, but not after 2010. Fifteen species were only seen in the 2000’s. Eight, including P. volitans, were only found after 2000; out of these, four were exclusively found after 2010 (Table 3). Some trends are evident, especially within the large-piscivore guild: e.g. there were seven species of large groupers (species of Epinephelus, Hyporthodus, and Mycteroperca) in the earliest decade, vs. only three still recorded after 2000. Butterflyfish Chaetodon aculeatus was no longer detected after 2010 and species of Hypoplectrus decreased from four in the 2000’s to only one, H. guttavarius, following 2010 (Table 3).
Density of taxa and guilds
Fifty-three species (out of 141) detected in stationary censuses showed significant declines in abundance, either from 1990 to 2000 (43 species), or from 2000 to 2010 (14 species). Only Halichoeres bivittatus and Stegastes adustus consistently decreased across all three decades (see in Online Appendix 1 average and variance of density for all species by decade, protection status, and habitat, and in Online Appendix 2 F, p, and post hoc homogeneous groups by species for the three factors plus the decade:protection status interaction, including non-significant results). Five (out of 13) trophic guilds (small benthivores, medium-sized and small herbivores, medium-sized piscivores, and medium-sized omnivores) also decreased (Fig. 2). From 2000 to 2010 only seven species increased. Eleven species displayed ambiguous patterns; for example, an increase from 1990 to 2000, followed by a decrease from 2000 to 2010 (Supplementary Table 4). No species consistently increased in time from decade to decade; five species increased only from 1990 to 2000, and two species increased from 2000 to 2010 (Supplementary Table 4). No trophic guild increased in time, and none showed ambiguous patterns.
Interdecadal declines in abundance of fish guilds in the southern Mexican Caribbean: a small benthivores; b medium-sized herbivores; c small herbivores; d medium-sized piscivores; e medium-sized omnivores. Bars are 95% confidence intervals. Density is given as arcsinh(individuals/78.5 m2). Outline images from Froese and Pauly (2016)
Based on transects, 57 species (out of 124) declined in abundance (only the 1990’s and 2000’s were compared). For 28 species the trend is significant in both datasets (Supplementary Table 4). All guilds that decreased according to the stationary census database also declined as per the transect database (Supplementary Table 4), except for small herbivores and small benthivores, which did not display any significant pattern.
By protection status, 36 species were more abundant outside the MPA, whereas only three were more abundant inside (Supplementary Table 4). No trophic guild showed differences by protection status. With regard to the interaction term decade:protection status, 16 species displayed significantly higher abundance outside the MPA in 1990 vs. other combinations, whereas only six species were more abundant inside the MPA in 1990; in 2010, four species were more abundant outside the MPA, and three inside (Supplementary Table 4). The decline was therefore greater in the non-protected area.
Mean largest size
Seventeen fish species increased in body size from 2000 to 2010; four, from 1990 to 2000; five more showed a consistent increase in size from decade to decade. Lactophrys triqueter decreased in size from 1990 to 2000; Abudefduf saxatilis and Sparisoma rubripinne also decreased during that period, but then increased from 2000 to 2010, whereas Stegastes leucostictus showed the opposite pattern. Concerning the protected status, only Haemulon carbonarium was larger inside the MPA, whereas Melichthys niger was larger outside (Supplementary Table 5).
Trophic level
Trophic level weighted by species frequency decreased from 0.433 in the 1990’s to 0.189 in the 2010’s, with a significant difference ( F = 5.90, p < 0.05) between those decades (Fig. 3). There was no difference by protection status. By habitat, the fore reef had a higher trophic level (weighted by species frequency) than the reef lagoon (F = 7.91, p < 0.05), 0.382 vs. 0.252. Separating the decadal effect by habitat, the decreasing pattern holds for the fore reef (0.547 in 1990–0.193 in 2010; F = 8.61, p < 0.05), but becomes non-significant for the reef lagoon.
Discussion
Most fish species and guilds displayed declining patterns in abundance. Even for those which were non-significant, the trend is in general the same, the lack of statistical significance probably related to sample size (number of individuals sighted). Although different guilds showed similar trends, the decrease in trophic level indicates that predators have suffered the most, especially in the fore reef. This scenario is worse than that found by Paddack et al. (2009), who observed that only three trophic groups declined: herbivores, invertivores, and generalist carnivores, with no change for piscivores, omnivores, and planktivores. In our study, large-bodied guilds did not show significant trends (probably because of their overall scarcity), but several species were not found in the later decades. Fishermen in the Mexican Caribbean assert that large piscivores (Epinephelus itajara, Hyporthodus nigritus, and sharks) have become less frequent, less abundant, and also smaller in body size. Similarly, species that aggregate to spawn have been especially vulnerable (García-Téllez 2002; Aguilar-Perera 2006; Graham et al. 2009). Rates of reduction detected in our study are comparable to those measured in quite different taxa and other areas in the Caribbean; they are consistent with the general decline for fishes, both exploited and non-exploited, described in the meta-analysis by Paddack et al. (2009).
Given the general deterioration of reefs in the Mexican Caribbean over the past 20 years, we expected a lower abundance not only of large carnivores, but also of specialist and sensitive taxa or guilds. Such is the case for angelfishes (Pomacanthidae), which eat ectoparasites from large groupers (themselves very scant) as juveniles and sponges as adults (Hourigan et al. 1989). Additionally, butterflyfishes are known to be useful indicators of ecosystem health elsewhere (Pratchett et al. 2006), and Ch. capistratus and Ch. striatus displayed decreasing trends, although Ch. ocellatus did not, and Ch. aculeatus was not found after 2010.
Small herbivores, mostly foragers of substrate-fixed algae, also decreased in abundance. The same pattern was seen for larger herbivores, e.g. parrotfishes, contrary to preliminary analyses (see below). An increase in herbivore abundance was expected, due to the increase of their main food item since the mass mortality of herbivore sea urchin, Diadema antillarum (Carpenter 1990), although the relevance of herbivore abundance and algal cover has been contested (Suchley et al. 2016). Several authors have warned about the change, global but especially stark in the Caribbean, from coral to algal phases in reefs (Martínez-Rendis et al. 2016), and Álvarez-Filip et al. (2011) predicted a loss of architectural complexity in coral reefs would lead to the demise of small-bodied species, because of their need for refuges in the reef structure.
It is also true that such trophic cascades and indirect ecological effects may be secondary to overfishing. In the case of large piscivores, especially species vulnerable because of their reproductive aggregations, this has been obvious for many years (Sadovy and Eklund 1999). It is likely that even species protected for decades do not fully recover from historical impacts. However, facing the demise of such traditional resources, fishers have turned to other species, such as parrotfishes and angelfishes (pers. obs.).
In some of our sites, Morales-Aranda et al. (2012) detected significant differences in the fore reef, but not in the reef lagoon. Overall abundance was greater in the earlier decade. The MPA showed higher abundance of M. niger, H. flavolineatum, H. carbonarium, Pomacanthus arcuatus, and Lutjanus mahogoni. These results do not hold true for the present study, with most increases becoming non-significant or even turning into decreases when analyzed across the entire study area. The preference of M. niger for the MPA is clear in by both datasets, but this species also experienced a decrease from 1990 to 2000.
The tendency of several species to increase in maximum observed length over the years seems counterintuitive. Because several large piscivores have declined to the point they are no longer detected in our surveys, this might be the effect of predator release. However, Barley et al. (2017) found exactly the opposite in Australia, comparing predator-depleted reefs versus sites where sharks are common.
Our results are more consistent when compared by decade than by protection status; however, a definite majority of species display greater abundance outside rather than inside the MPA. The result may be discouraging, taken at face value. However, according to the interaction term between protection status and decade, the difference was greater in the nineties than in the decade of 2010, meaning that the decline was more intense in the non-protected area. Arias-González (1998) explained the diversity in Mahahual, which was greater than elsewhere in the southern Mexican Caribbean coast, in terms of natural factors (habitat complexity and heterogeneity); our findings suggest that these “natural advantages” of the non-protected area have been eroded through the years. On the other hand, Morales-Aranda et al. (2012) mentioned, as another possible advantage of the region outside the MPA, the influence of Chinchorro Bank, located 30 km offshore from Mahahual, which affords protection against hurricanes and may be a source for recolonization, a situation which should not change because of human influence.
This pattern supports a positive effect of the MPA (not reverting, but at least slowing down declines), a detrimental effect of coastal development in the non-protected sites, or both. However, the MPA itself, PNAX, in addition to its probably natural lower diversity and abundance, is not pristine. Despite its protected status, it experiences frequent poaching, habitat destruction from shipwrecks, and even the use of dynamite in 2004 to open access to the Zaragoza Canal, a direct route to Chetumal (Fig. 4).
Nevertheless, the overall negative trends suggest a regional or global rather than (or stronger than) a local cause. In addition to the lionfish invasion, the global change in ocean chemistry and dynamics will bring about massive extirpation of hermatypic corals in the western Caribbean (Melo Merino 2013). Local success stories have had no significant impact on the reduction of biodiversity loss (Butchart et al. 2010). To address such international problems, MPAs, preferably provided strict no-take status (Soler et al. 2015), should explicitly be integrated into international networks to enhance biological connectivity between them (Pittman et al. 2014).
References
Acosta-González G, Rodríguez-Zaragoza FA, Hernández-Landa RC, Arias-González JE (2013) Additive diversity partitioning of fish in a Caribbean coral reef undergoing shift transition. PLoS ONE 8:e65665. doi:10.1371/journal.pone.0065665
Aguilar-Perera A (2006) Disappearance of a Nassau grouper spawning aggregation off the southern Mexican Caribbean coast. Mar Ecol Prog Ser 327:289–296. doi:10.3354/meps327289
Almada-Villela PC, Sale PF, Gold-Bouchot G, Kjerfve B (2003) Manual de métodos para el programa de monitoreo sinóptico del SAM. Documento técnico del SAM No. 4. CCAD, SAM, Belize
Álvarez-Filip L, Gill JA, Dulvy NK (2011) Complex reef architecture supports more small-bodied fishes and longer food chains on Caribbean reefs. Ecosphere 2(10):1–17. doi:10.1890/ES11-00185.1
Andradi-Brown DA, Gress E, Wright G et al (2016) Reef fish community biomass and trophic structure changes across shallow to upper-mesophotic reefs in the Mesoamerican barrier reef, Caribbean. PLoS ONE 11:1–19. doi:10.1371/journal.pone.0156641
Arias-González JE (1998) Trophic models of protected and unprotected coral reef ecosystems in the South of the Mexican Caribbean. J Fish Biol 53:236–255. doi:10.1111/j.1095-8649.1998.tb01030.x
Ballew NG, Bacheler NM, Kellison GT, Schueller AM (2016) Invasive lionfish reduce native fish abundance on a regional scale. Sci Rep 6:32169. doi:10.1038/srep32169
Barley SC, Meekan MG, Meeuwig JJ (2017) Species diversity, abundance, biomass, size and trophic structure of fish assemblages on coral reefs in relation to shark abundance. Mar Ecol Prog Ser 565:163–179. doi:10.3354/meps11981
Bell JD, Galzin R (1984) Influence of live coral cover on coral-reef fish communities. Mar Ecol Prog Ser 15:265–274. doi:10.3354/meps015265
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833
Betancur-R R, Hines A, Acero A et al (2011) Reconstructing the lionfish invasion: insights into Greater Caribbean biogeography. J Biogeogr 38:1281–1293. doi:10.1111/j.1365-2699.2011.02496.x
Bianchi G, Gislason H, Graham K et al (2000) Impact of fishing on size composition and diversity of demersal fish communities. ICES J Mar Sci 57:558–571. doi:10.1006/jmsc.2000.0727
Bohnsack JA, Bannerot SP (1986) A stationary visual census technique for quantitatively assessing community structure of coral reef fishes. NOAA Tech Rep NMFS 41:1–15
Booth DJ, Brosnan DM (1995) The role of recruitment dynamics in rocky shore and coral reef fish communities. Adv Ecol Res 26:309–385
Brock VE (1954) A preliminary report on a method of estimating reef fish populations. J Wildl Manag 18:297–308. doi:10.2307/3797016
Butchart SHM, Walpole M, Collen B et al (2010) Global biodiversity: indicators of recent declines. Science 328(5982):1164–1168. doi:10.1126/science.1187512
Carpenter RC (1990) Mass mortality of Diadema antillarum I. Long-term effects on sea urchin population-dynamics and coral reef algal communities. Mar Biol 104:67–77. doi:10.1038/470444a
Fowler J, Cohen L, Jarvis P (1998) Practical statistics for field biology, 2nd edn. Wiley, Chichester
Francini-Filho RB, de Moura RL (2008) Evidence for spillover of reef fishes from a no-take marine reserve: an evaluation using the before-after control-impact (BACI) approach. Fish Res 93:346–356. doi:10.1016/j.fishres.2008.06.011
Friedlander AM, DeMartini EE (2002) Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Mar Ecol Prog Ser 230:253–264. doi:10.3354/meps230253
Friedlander AM, Brown EK, Jokiel PL et al (2003) Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs 22:291–305. doi:10.1007/s00338-003-0317-2
Froese R, Pauly DA (2016) Fish Base. http://www.fishbase.de/. Accessed 1 Jun 2016
García-Salgado MÁ, Nava-Martínez GG, Vasquez M, et al (2008) Declining trend on the Mesoamerican Reef System marine protected areas. In: Proceedings 11th Int Coral Reef Symp Ft Lauderdale, Florida, 7–11 July 2008. pp 883–888
García-Téllez N (2002) Situación actual de la cherna (Epinephelus itajara Lichtenstein, 1822) en la costa de Quintana Roo, Méx., con énfasis en la bahía de Chetumal. Dissertation. El Colegio de la Frontera Sur, Chetumal
Gardner TA, Côté IM, Gill JA et al (2003) Long-term region-wide declines in Caribbean corals. Science 301(5635):958–961
Genner MJ, Sims DW, Southward AJ et al (2010) Body size-dependent responses of a marine fish assemblage to climate change and fishing over a century-long scale. Glob Chang Biol 16:517–527. doi:10.1111/j.1365-2486.2009.02027.x
Gladfelter WB, Ogden JC, Gladfelter EH (1980) Similarity and diversity among coral reef fish communities: a comparison between tropical western Atlantic (Virgin Islands) and tropical central Pacific (Marshall Islands) patch reefs. Ecology 61:1156–1168
Graham RT, Rhodes KL, Castellanos D (2009) Characterization of the goliath grouper Epinephelus itajara fishery of southern Belize for conservation planning. Endanger Species Res 7:195–204. doi:10.3354/esr00187
Halpern BS, Warner RR (2003) Matching marine reserve design to reserve objectives. Proc R Soc B-Biol Sci 270:1871–1878. doi:10.1098/rspb.2003.2405
Hernández-Arana HA, López-Adame H, Vega-Zepeda A (2014) An overview of the coral bleaching event in the central and southern Mexican Caribbean in 2011. Reef Encount 29:32–33
Hixon MA, Beets JP (1993) Predation, prey refuges, and the structure of coral-reef fish assemblages. Ecol Monogr 63:77–101
Hoegh-Guldberg O, Mumby PJ, Hooten AJ et al (2007) Coral reefs under rapid climate change and ocean acidification. Science 318(5857):1737–1742
Hoffman DM (2009) Institutional legitimacy and co-management of a marine protected area: implementation lessons from the case of Xcalak Reefs National Park, Mexico. Hum Organ 68:39–54
Hourigan TF, Stanton FG, Motta PJ et al (1989) The feeding ecology of three species of Caribbean angelfishes (family Pomacanthidae). Environ Biol Fishes 24:105–116. doi:10.1007/BF00001281
Humann P (1989) Reef fish identification, 1st edn. New World, Florida
Humann P, DeLoach N (2011) Reef fish identification, 3rd edn. New World, Florida
Jackson JBC, Kirby MX, Berger WH et al (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–637
Jones GP, McCormick MI, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves. Proc Natl Acad Sci 101:8251–8253. doi:10.1073/pnas.0401277101
Leis JM, McCormick MI (2002) The biology, behavior, and ecology of the pelagic, larval stage of coral reef fishes. In: Sale PF (ed) coral reef fishes. Academic Press, San Diego, pp 171–199
Luckhurst BE, Luckhurst K (1978) Analysis of the influence of substrate variables on coral reef fish communities. Mar Biol 49(4):317–323
Martínez-Rendis A, Acosta González G, Hernández-Stefanoni JL, Arias-González JE (2016) Quantifying the reefscape transformation of a coastal Caribbean coral reef during a phase shift and the associated coastal landscape change. Mar Ecol 37:697–710. doi:10.1111/maec.12334
Melo Merino SM (2013) Cambios potenciales en la distribución de corales arrecifales (Scleractinia) del Pacífico Oriental y Atlántico Occidental, como consecuencia del cambio climático. Dissertation. Universidad Nacional Autónoma de México, Mexico City
Morales-Aranda AA, Schmitter-Soto JJ, Herrera-Pavón RL (2012) Evaluación de un área marina protegida en el Caribe: un análisis antes-después/control-impacto con peces arrecifales. In: del Moral-Flores LF, Martinez Pérez JA, Franco López J et al (eds) Investigación ictiológica en México. Temas selectos en honor al Dr. José Luis Castro-Aguirre. Universidad Nacional Autónoma de México/Sociedad Ictiológica Mexicana, Mexico City, pp 231–245
Muhling BA, Smith RH, Vásquez-Yeomans L et al (2013) Larval fish assemblages and mesoscale oceanographic structure along the Mesoamerican Barrier Reef System. Fish Oceanogr 22:409–428. doi:10.1111/fog.12031
Núñez-Lara E, Arias-González JE (1998) The relationship between reef fish community structure and environmental variables in the southern Mexican Caribbean. J Fish Biol 53:209–221. doi:10.1111/j.1095-8649.1998.tb01028.x
Paddack MJ, Reynolds JD, Aguilar-Zúñiga C et al (2009) Recent region-wide declines in Caribbean reef fish abundance. Curr Biol 19:590–595. doi:10.1016/j.cub.2009.02.041
Pauly DA, Christensen V, Dalsgaard J et al (1998) Fishing down marine food webs. Science 279:860–863. doi:10.1126/science.279.5352.860
Peckol PM, Curran HA, Floyd EY et al (2003) Assessment of selected reef sites in northern and southern central Belize, including recovery from bleaching and hurricane disturbances (stony corals, algae and fish). Atoll Res Bull 496:146–171. doi:10.5479/si.00775630.496-8.146
Pittman SJ, Monaco ME, Friedlander AM et al (2014) Fish with chips: tracking reef fish movements to evaluate size and connectivity of Caribbean marine protected areas. PLoS ONE. doi:10.1371/journal.pone.0096028
Polunin NVC, Roberts CM (1993) Greater biomass and value of target coral-reef fishes in two small Caribbean marine reserves. Mar Ecol Prog Ser 100:167–176. doi:10.3354/meps100167
Pratchett MS, Wilson SK, Baird AH (2006) Declines in the abundance of Chaetodon butterflyfishes following extensive coral depletion. J Fish Biol 69:1269–1280. doi:10.1111/j.1095-8649.2006.01161.x
Roberts CM (1995) Rapid build-up of fish biomass in a Caribbean marine reserve. Conserv Biol 9:815–826. doi:10.1046/j.1523-1739.1995.09040815.x
Rodríguez-Zaragoza FA, Arias-González JE (2008) Additive diversity partitioning of reef fishes across multiple spatial scales. Caribb J Sci 44:90–101
Ruiz-Zárate MÁ, Hernández-Landa RC, González-Salas C et al (1998) Condition of coral reef ecosystems in central-southern Quintana Roo, Mexico (Part 1: stony corals and algae). Atoll Res Bull 496:318–337. doi:10.5479/si.00775630.496-18.318
Russ GR (2002) Yet another review of marine reserves as reef fishery management tools. In: Sale PF (ed) Coral Reef Fishes. Academic Press, London, pp 421–443
Sadovy Y, Eklund A-M (1999) Synopsis of biological data on the Nassau grouper, Epinephelus striatus (Bloch, 1792), and the jewfish, E. itajara (Lichenstein, 1822). FAO Fish Synopsis 68
Sale PF, Dybdahl R (1975) Determinants of community structure for coral reef fishes in an experimental habitat. Ecology 56:1343–1355
Soler GA, Edgar GJ, Thomson RJ et al (2015) Reef fishes at all trophic levels respond positively to effective marine protected areas. PLoS ONE 10:e0140270. doi:10.1371/journal.pone.0140270
Suchley A, McField MD, Álvarez-Filip L (2016) Rapidly increasing macroalgal cover not related to herbivorous fishes on Mesoamerican reefs. PeerJ 4:e2084. doi:10.7717/peerj.2084
Venables WN, Smith DM (2003) An Introduction to R, Version 1.0. The R Development Core Team
Yeager LA, Arias-González JE (2008) Preliminary survey of fish community composition in seagrass habitat in two back-reef lagoons of the southern Mexican Caribbean. Gulf Caribb Res 20:41–47
Acknowledgements
We thank the community of Xcalak and the Mexican Comisión Nacional de Áreas Naturales Protected areas for granting permission to work at PNAX, for supporting us logistically, and for discussing these ideas with us. The projects were financed by the Mexican agencies Consejo Nacional de Ciencia y Tecnología (CONACYT) and Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO), in addition to the Mesoamerican Barrier Reef Fund, and part of the results were included in a M.Sc. thesis by AAMA and a Ph.D. dissertation by DMC. We thank Héctor Salvat, Mauricio Espadas, Héctor Gamboa, Aristeo Hernández, Rudy Lara, Sandra Mendoza, Brenda Murillo, and Susana Perera for field assistance. Janneth Padilla processed the map. Humberto Bahena allowed us to use of his aerial photograph of the Zaragoza Canal destruction. Thanks are due also to to Avery E. Scherer, who improved our English, and to the anonymous reviewers of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Angus Jackson.
This article belongs to the Topical Collection: Coastal and marine biodiversity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmitter-Soto, J.J., Aguilar-Perera, A., Cruz-Martínez, A. et al. Interdecadal trends in composition, density, size, and mean trophic level of fish species and guilds before and after coastal development in the Mexican Caribbean. Biodivers Conserv 27, 459–474 (2018). https://doi.org/10.1007/s10531-017-1446-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-017-1446-1