Abstract
Rising global demand for palm oil comes mainly at the expense of tropical forests, and palm plantations are increasing steadily in Latin America. Conversion of forest to oil palm agriculture accelerates biodiversity loss by dramatically altering availability and abundance of resources. Assessing the impact of these monocultures on highly diverse keystone groups such as bats is of particular ecological and economic importance. We compared phyllostomid bat assemblages in mature lowland forest interior, at forest margins and within oil palm plantations in southwestern Costa Rica. A total of 45 mist-netting nights in the late dry season yielded 1235 individual captures from 31 species. Bat assemblages in oil palm plantations were clearly distinct from those at forest sites and exhibited lower species richness, similar to results reported for Southeast Asia. Assemblage structure within oil palm plantations was characterized by increased relative abundance of common frugivorous Stenodermatinae and the loss of rare species, mainly disturbance-sensitive animalivorous Phyllostominae. Although plantations may serve as flyways for highly mobile matrix-tolerant species, even small oil palm plantations seem to act as effective barriers for many others, particularly understory gleaning animalivores. By decreasing landscape permeability even for highly mobile animals such as bats, oil palm agriculture may consequently reduce population connectivity and foster faunal impoverishment, which in turn can diminish crucial ecosystem services provided by bats. The advancing expansion of these monocultures in Latin America calls for appropriate precautionary conservation measures to protect and preserve biodiversity in oil palm growing countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities, particularly agricultural production, cause major land use changes and extensive deforestation in tropical regions, including Latin America (Aide et al. 2013). What remains is a patchwork landscape consisting of fragmented near-natural habitats embedded in a human-dominated matrix of diverse land-use systems under different management regimes. While the prevailing large blocks of relatively undisturbed continuous forest represent refuges for many threatened species, they have been dramatically reduced in number and area and often constitute protected, but increasingly isolated areas (Laurance et al. 2012). Imminent land use changes leave substantial environmental scars and will pose further challenges for conservation, as rising global demand for food and commodities exerts continuous pressure on tropical ecosystems. Conversion of forest to agricultural areas can have severe implications for biodiversity, and prevailing conditions in these land-use systems (uniform vegetation structure, unsuitable microclimate and low abundance of resources) dramatically reduce habitat quality for many species. The evaluation of the conservation value of these human-modified landscapes and the matrix surrounding remnants of natural habitats is increasingly important and effects of agriculture on biodiversity have received particular attention in the patchwork landscapes of the Americas (reviewed in Donald 2004). Research on the impacts of oil palm agriculture, however, is still limited although these plantations are encroaching upon Neotropical ecosystems (Gutiérrez-Vélez et al. 2011). This important contemporary cash crop is typically cultivated in large-scale monocultures under high intensity management, and plantations are expanding worldwide in response to growing demand (Corley 2009). The palm oil boom has had profound ecological consequences, such as biodiversity decline and loss of crucial associated ecosystem services (Tscharntke et al. 2005) due to agricultural intensification and habitat conversion. Recognized as a main driver of deforestation and peatland loss in South East Asia (Danielsen et al. 2009), plantations also come at the expense of forests in Latin America (Gutiérrez-Vélez and DeFries 2013) and represent a new threat for native ecosystems and their inhabitants. Studies from South East Asia have consistently demonstrated that species richness declines and community composition of various taxa is altered in oil palm plantations in comparison to forested habitats (reviewed in Foster et al. 2011; Savilaakso et al. 2014), with the disappearing species usually being more specialized and dependent on features that are not provided in monoculture plantations (Fitzherbert et al. 2008). While impacts of oil palm plantations on the Neotropical fauna have been documented for some taxa (e.g. Nájera and Simonetti 2010; Livingston et al. 2013; Gilroy et al. 2014; Lees et al. 2015), studies on the responses of bats, a highly diverse keystone group with vital roles in forest ecosystem functions and dynamics, are lacking. Bats are often considered to be highly vagile and therefore more resistant to habitat changes than less mobile animals. Nevertheless, impacts of habitat disturbance (Medellín et al. 2000; Clarke et al. 2005) and forest fragmentation (Schulze et al. 2000; Meyer and Kalko 2008; Farneda et al. 2015) on Neotropical bats are evident and have brought consensus that preserving or restoring bat-friendly tropical landscapes is of high ecological and economic relevance. Locally comprising nearly half of all Neotropical mammal species, bats function as mobile links and provide critical ecosystem services such as pollination of both native plants and commercial crops, arthropod predation and seed dispersal (reviewed in Kunz et al. 2011). Thereby, bats not only contribute to regeneration of disturbed areas (Melo et al. 2009), but are also of great economic value for agriculture (Boyles et al. 2011).
New World Leaf-nosed bats (Phyllostomidae) in particular exhibit a striking functional diversity. Owing to their large variation in morphology, foraging ecology and habitat requirements, they represent an interesting group for studying impacts of habitat disturbance. We evaluated species richness, abundance and composition of phyllostomid bat assemblages in oil palm plantations, adjacent old growth forests and the intermediate transition zone of forest margins. The studied habitats differ in structural complexity, and consequently in their availability and abundance of resources such as food and roosting sites. For forest specialists and species with limited mobility or specific roosting requirements, plantations might represent serious barriers.
To provide a first ecological assessment of the impacts of oil palm agriculture on Neotropical bats, we address the responses of Phyllostomid bat assemblages and subfamilies by investigating a “best case scenario”, in a diverse landscape matrix with small plantations adjacent to blocks of mature forest and a large regional bat species pool. We predict (1) distinct composition and structure of assemblages at forest and oil palm plantation (OPP) sites, (2) a strong correlation between species richness and habitat complexity and consequently (3) lower species richness in OPP due to loss of disturbance‐sensitive species, such as members of the Phyllostominae subfamily.
Materials and methods
Study area
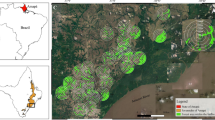
The study area is located in the Puntarenas province, the main oil palm growing region of Costa Rica. It comprises the rural area of the village La Gamba, the Golfito Wildlife Refuge and Piedras Blancas National Park, a large remnant block of perhumid tropical lowland forest mostly located on narrow ridges and steep slopes (60–345 m.a.s.l., mean annual temperature 28.5 °C, precipitation 6000 mm—Weissenhofer et al. 2008). The forest-dominant landscape around La Gamba is interspersed with agro-mosaics of settlements and fallows, water bodies, pastures, and various agricultural land-use systems, prevalently oil palm plantations (Höbinger et al. 2012). Sampling points extended over an area of roughly 23 km2 with a mean inter-site distance of 2.1 km (±1.2 SD; range 0.3–5.6 km) located near the La Gamba Biological Field Station (N 8°42″61′, W 83°12′97″), and consisted of five replicates for each of the following three habitat types: forest interior (FI), forest margin (FM) and oil palm plantation (OPP) (Fig. 1).
FI sites were situated at least 250 m from the nearest forest edge. FM sites were located several meters inwards from the forest edge and characterized by a high proportion of young secondary vegetation with high densities of Cecropia and Piper sp., herbaceous plants, shrubs and small trees. Adjacent areas included abandoned shade cacao plantations, open land, riverine areas or a creek separating the forest from oil palm plantations. All OPP sites were adjacent to forest but connected in varying extent (Fig. 1), cultivation area ranged from 12 to 40 ha and stand age was 7–14 years. Palms were regularly spaced and canopy height typically averaged between 4 and 7 m. Within plantations, several drainage ditches were present and the ground was sparsely covered with grasses but lacked understory. Piles of pruned palm fronds represented the only considerable structure on the ground and owners reported the regular use of Glyphosate herbicides.
Data collection
Bats were sampled for a total of 45 nights during the late dry season (February–May 2012), three nights per site using four ground-level mist nets (12 × 2.5 m, 5 shelves, Mesh 16 mm, Denier 75/2-ply; Ecotone, Poland). We alternated between habitat types to reduce phenological bias and captured from 17:30 to 00:30 for two consecutive nights at each of the 15 sampling points before finishing off with a full night from 17:30–5:30 at each site. Nets were slightly moved between capture nights and closed during rain, and no mist-netting took place on full moon nights. Nets were checked every 15 min. Captured bats were identified to species (Timm and LaVal 1998; Reid 2009) and basic parameters (sex, age class, reproductive condition) and measurements (body mass to the nearest 0.5 g using a Pesola spring scale, forearm to the nearest 0.5 mm using calipers) recorded. Upon release, bats were fed diluted sugar water and marked by collecting a 3 mm wing membrane sample. The study was conducted following all local regulations (Ministerio de Ambiente, Energía y Telecomunicaciones de Costa Rica MINAET, Permit-# 010-2012-SINAC).
The taxonomy used in this study follows Reid (2009) but includes revisions (Hoofer et al. 2008). While the Lonchophyllinae and Glossophaginae are treated as distinct subfamilies (Datzmann et al. 2010), for comparability with previous studies we retain Glyphonycterinae and Micronycterinae (Baker et al. 2003) within Phyllostominae. Artibeus intermedius and A. lituratus are not always reliably distinguishable in the field and were treated as one species group for analysis. To relate species richness, composition and abundance patterns to differences in habitat structure, we measured some basic vegetation parameters. At each site, the number of trees with a diameter at breast height (DBH) greater than 10 cm (thereafter: DBH >10) and the number of woody plant stems with a DBH less than 10 cm (DBH <10) were counted within a buffer of 5 m around each net, resulting in four 22 m × 10 m plots per sampling site. To roughly approximate canopy coverage (CC), non-hemispherical pictures were taken in a standardized way by placing a digital camera flat on the ground, lengthwise at the center of each mist net location. Pictures were converted in Adobe Photoshop CS4 following Stewart et al. (2007) to calculate canopy coverage (percentage of vegetation obscuring the sky) using the software Image J 1.46r (Abramoff et al. 2004). To account for effects of forest proximity, we furthermore calculated the percentage of land covered by forest (old growth and secondary) within a buffer radius of 300 m around each OPP sampling site in ARCMap 10.1 (ESRI Inc.).
Analysis
In this study, we focus on phyllostomid bat assemblages, as they are effectively sampled with ground mist nets while comprehensive acoustic survey methods are required to properly document aerial insectivores, thus non-phyllostomid captures were excluded from analysis. We assessed species richness and sample coverage, a measure of inventory completeness (expressed in the proportion of the total number of individuals in a community that belong to the species represented in the sample) with a combined method of extrapolation and rarefaction (Colwell et al. 2012) using the iNEXT software (Hsieh et al. 2013). We used 100 bootstrap runs and extrapolated to 598 individuals (representing twice the smallest sample size recorded for an individual habitat type). Both individual-based species accumulation curves and sample coverage curves were plotted, as coverage-based rarefaction provides a less biased measure of community species richness by keeping the ratio of species richness instead of compressing it, as in conventional methods (Chao and Jost 2012).
All statistical tests were performed in Statistica 7.1 (StatSoft Inc. 2005) unless stated otherwise. We ran a GLM (with Poisson distribution and log-link function) on observed phyllostomid species richness per site with habitat as categorical predictor and number of captured bat individuals as covariate. Differences in assemblage composition between habitat types were quantified using Bray-Curtis similarities calculated from square-root transformed abundance data (to increase the influence of rare species) with the software Primer (Version 5.2.9, Clarke and Gorley 2006). The resulting similarity matrix was visualized in a non-metric multidimensional scaling (NMDS), and using the meta MDS function in the R package vegan 2.2-1 (Oksanen et al. 2015), a species overlay was computed, where species scores represent the weighted average of all site-scores where an according species occurred. We calculated rarity, where a species was considered as rare if ≤5 individuals were caught, corresponding to a contribution of ≤0.4 % of all captures (N = 1235).
Analysis of similarity (ANOSIM) was used to test for effects of habitat type on species composition. Due to short distances between some of the sites, data were screened for spatial correlation by measuring inter-site-distances in ARCMap. A triangular site-distance-matrix was created and related to a Bray-Curtis similarity matrix of assemblage data, using the RELATE function in Primer.
Due to extremely high bat abundances in some OPP sites temporarily requiring closing of some nets in order to ensure safe processing of the animals, and increased precipitation interfering towards the end of the study period, our final sampling effort varied (FI 502, FM 480, OPP 384 mnh). To obtain a standardized measure of bat activity per habitat ensuring comparability among sites, relative capture rates were calculated (number of captured individuals/mist net hour) for each site. One mist-net hour (mnh) was defined as one 12 m mist net opened for 1 h. Capture rates were compared using a Kruskal–Wallis ANOVA.
Differences in relative abundances of phyllostomid subfamilies across habitats were assessed with log(x + 1) transformed data (tested for homoscedasticity with Levene’s test) in multiple ANOVAs. Subsequent calculation of a false discovery rate (FDR) following Pike (2011), using their “classical one-stage method”, provided corrected p-values (=q-values). Effects of percentage of nearby forest on relative abundances of subfamilies at OPP sites were evaluated using Spearman rank correlations.
In a principal component analysis (PCA), all three habitat variables (canopy cover, density of large diameter trees and density of small woody plants) had high factor loadings (DBH >10: 0.84, DBH <10: 0.93, CC: 0.759) with the first principal component. Moreover, recorded habitat parameters were highly correlated with each other (R ≥ 0.6 for all three combinations respectively; P = 0.019). Therefore, all three site descriptors were combined into a new meta-variable (hereafter PC1). PC1 accounted for 71.7 % of total variance in the original data and differed significantly among habitats (Fig. 2, ANOVA, F 2,12 = 11.95, P = 0.001). PC1 was subsequently used as a measure of habitat structure to test for effects of vegetation structure on species richness using a linear regression analysis. To evaluate effects of differences in vegetation structure on bat species composition, a partial Mantel test (with inter-site distances of mist-netting locations as covariate) relating the Bray-Curtis similarities to differences in PC1 values was computed with the software Past 3.06 (Hammer et al. 2001). The associated distance matrix for the latter variable consisted of calculated Euclidean distances.
Habitat structure of forest interior (FI), forest margin (FM) and oil palm plantation (OPP) sites, as quantified by the first factor (PC1) of a PCA on three site descriptors. Provided are mean PC1 values ± SE (box) and 95 % CI (whiskers). Low values of PC1 for FI and FM illustrate high structural complexity, respectively high values of OPP equate to reduced habitat structure
Results
General findings
In a total sampling effort of 1366 mist net hours, 1364 bats belonging to 36 species (respectively 37, see notes about Artibeus intermedius in Methods) from 4 families were caught. Subsequent analyses focus exclusively on the Phyllostomidae, comprising the majority in terms of all species and captured individuals (Table 1, 98.7 % of all captures, 31 species). Recaptures (108) and unidentified escapes were excluded from analyses. Out of the remaining 1235 individuals, 299 (27 species) were captured in FI, 393 (23 species) in FM and 543 (20 species) in OPP. Of the 20 species recorded in OPP, 17 were also found within the forest habitats. Furthermore, 17 species (54.8 %) were shared between the 3 habitat types. More than 35 % of all species (11) were restricted to the forest habitats and never recorded in OPP, and 7 out of 31 species (22.6 %) were exclusively captured in FI, where we also documented two species previously not recorded in the area (Glyphonycteris sylvestris and Lampronycteris brachyotis).
Three out of the four most abundant species across all habitat types belonged to the subfamily Stenodermatinae (Table 1). Species with highest capture frequency in OPP were also those generally most abundant in the area. Artibeus jamaicensis was the most abundant species overall, representing 25 % of all captures. Furthermore, it had the highest relative abundance in OPP (38.9 % of all individuals captured in this habitat), while Dermanura watsoni represented the species with highest capture frequencies in both forest habitats (FI 27.1 %, FM 26.0 %). Uroderma bilobatum was frequently captured in OPP (15.5 %) but in comparably low numbers at FI (1.7 %) and FM (3.3 %). Ranking fifth in the most common species of all captures and predominantly caught at FM was Carollia castanea (FI 8.0 %, FM 16.9 %, OPP 2.3 %).
A total of 13 from 31 species were classified as rare (Table 1), corresponding to 2.6 % of all captured phyllostomid individuals. FI had the highest abundance of rare bats compared to FM and OPP (FI N = 19, FM N = 6, OPP N = 7; Chi square test: χ 2 = 22.19, df = 2, P < 0.001). Eleven out of 27 species caught in FI were rare, whereas at FM and OPP sites only 5 out of 23, respectively 4 out of 20, recorded species were rare. However, differences in richness of rare species were not significant (χ 2 = 3.21, df = 2, P = 0.201).
Species richness, faunal coverage and species-abundance distributions
Species richness decreased from FI towards OPP, illustrated by the curve tail length in a rank abundance plot, which also suggests higher dominance of common species due to a steeper slope (see Online Resource Fig. S2). In rarefaction-extrapolation curves (Fig. 3a) the confidence intervals of both FI and FM as well as FM and OPP overlap, whereas the clear gap between FI and OPP demonstrates significant differences in species richness both at the smallest number of individuals captured (n = 299, FI) and when extrapolated to 600 individuals.
Individual-based integrated extrapolation-rarefaction curves (a) and species richness as a function of sample coverage (b). for forest interior (black line, triangles), forest margins (green line, squares) and oil palm plantations (red line, circles). Dashed lines indicate extrapolated data and shaded areas represent 95 % confidence intervals
When inspected at the sample coverage level, curves for the individual habitats are distinct and confidence intervals do not overlap (Fig. 3b). Species coverage ranged from 86.5 to 99.4 % and was lowest at sites with highest species richness. Corroborated by the rarefaction-extrapolation, the curve for OPP had almost reached an asymptote, indicating that most species were recorded and the assemblage was adequately sampled, while curves for forest habitats are still increasing and detection of further species can be expected (Fig. 3a). Observed species richness on site level, in contrast, did not differ significantly among habitats (Wald’s \(\chi_{2.12}^{2}\) = 2.361, P > 0.3). Species richness was weakly, but significantly related to the habitat meta-variable PC1, indicating a reduction of bat richness with decreasing habitat complexity (r 2 = 0.29, P = 0.038, see Online Resource Fig. S1). Capture rates differed significantly between habitat types (Kruskal–Wallis ANOVA: H 2,12 = 6.540, P = 0.038) and were highest in OPP (1.4 ± 0.7 individuals mnh−1), intermediate at FM (0.8 ± 0.1) and lowest in FI (0.6 ± 0.2). Differences in capture rates between sites were related to changes in habitat structure (partial Mantel test with spatial distance between sites as covariate: r part = 0.37, P = 0.0485).
Assemblage composition
An NMDS ordination of Bray-Curtis similarities indicates similar species compositions of FI and FM, clustering together and well segregated from OPP sites (Fig. 4). The stress value of 0.18 confirms a reasonable visualization of similarity relationships between sites. Species composition was significantly affected by habitat type (one-way ANOSIM, Global R = 0.429, P = 0.001) and subsequent pair-wise tests indicated distinct assemblages only between the two forest habitats and OPP sites (ANOSIM, R Statistic = 0.546 for FI-OPP and 0.668 FM-OPP, both P = 0.008). Tests for effects of intra-site distances on assemblage composition did not indicate spatial correlation (Rho = 0.083, P = 0.306).
Species composition of bat assemblages in forest interior (triangles), at forest margins (squares) and in oil palm plantations (circles), ordinated by non-metric multidimensional scaling (NMDS) based on Bray-Curtis similarities (using √ × transformed abundances), including an overlay of species positions in reduced ordination space (codes represent a combination of the first three letters of genus and species name, respectively; compare Table 1)
Subfamilies
In four out of six subfamilies, relative abundances aggregated over all representative species differed significantly across habitat type (Fig. 5, ANOVA results with FDR-corrected P-values).
Stenodermatinae were the most abundant group in all habitats, comprising more than half of the captures in forest habitats (FI 55 %, FM 50 %) and 84 % in OPP. This increase in relative abundance by nearly one-third in OPP in comparison to forest habitats comes with significantly lower relative abundances of Carolliinae, Phyllostominae and Lonchophyllinae in OPP. Neither Glossophaginae nor Desmondontinae (represented only by Desmodus rotundus) seemed to be affected by habitat type. Relative abundances of individual subfamilies in OPP were not affected by forest cover within 300 m buffer (all Spearman rank correlations P > 0.5).
Discussion
Our case study from Costa Rica represents a scenario of habitat conversion where the adjacency of oil palm plantations to large blocks of old growth forest permits for wildlife to temporarily use this land use system, while still being able to retreat to the forest. However, even in this landscape where forest specialists could reach agricultural areas with relative ease, we observed dramatic differences between oil palm and forests in species richness, abundance, and assemblage structure. Highest species richness was observed in forest interior, the habitat type of greatest structural complexity and natural resource diversity, and intermediate at forest margins, the ecotone between the other habitats. Corroborating studies in South East Asian oil palm plantations (Danielsen and Heegaard 1995; Fukuda et al. 2009), species richness was also lowest in plantations in our study area, despite entirely different extents of oil palm cultivation.
Species accumulation curves for forest habitats are still increasing when extrapolated to larger sample sizes, indicating that these assemblages have not been exhaustively surveyed yet, as suggested by lower sample coverage in forest habitats. This may be due to certain constraints of our study, such as sampling during a relatively short time span and only during one late dry season but not the wet season. Nevertheless, richness differed significantly between OPP and FI, but not between FI and FM nor FM and OPP, indicating potential “spillover effects” between adjacent habitat types. Proximity to natural habitat positively affects species richness in agricultural land use systems (Laube et al. 2008), and observed OPP bat species numbers are likely artificially boosted by transients and spillover species from adjoining forest habitats (Lucey et al. 2014; Yue et al. 2015). Should the extent of oil palm cultivation increase in the region, we would expect even more dramatic differences between agricultural and unmanaged land uses.
Species composition in OPP was clearly distinct from those at FI and FM sites. The similarity of the latter could indicate that assemblages in our study area do not exhibit strong edge sensitivity, which can substantially affect species composition in fragmented habitats (Meyer et al. 2008). It may also relate to methodological limitations, i.e. more sampling would be required to increase detection probability (especially for rare species) in these complex habitats, as indicated by a lower sample-coverage. In contrast, high sample coverage in OPP and a leveled-off species accumulation curve indicate near complete sampling of the impoverished bat assemblages in plantations. Increased relative abundances of certain Stenodermatinae and declines of Carolliinae, Lonchophyllinae and Phyllostominae seem to shape the deviating assemblages in OPP. These compositional shifts in the structure of Neotropical bat assemblages are commonly observed in response to habitat disturbance. Even low-intensity anthropogenic impact such as selective logging can alter bat species composition or activity patterns (Presley et al. 2009), whereas certain frugivorous and nectarivorous species may even increase in slightly to moderately disturbed habitats (Schulze et al. 2000; Meyer and Kalko 2008). This frequently observed pattern could result from greater ecological resilience or may simply reflect locally increased resource availability (flowers, fruits) in areas of moderate disturbance. Edge habitats like forest margins often provide high densities of early successional plants (e.g. Cecropia, Piper, Solanum and Vismia spp.) that many understory frugivores, such as the Carolliinae, feed on.
Species absent in oil palm plantations are usually those with specialized diets or roosting requirements and small home ranges, while assemblages in OPP are often dominated by a few abundant generalistic species (Fitzherbert et al. 2008). Similarly, species caught in high abundances in our studied OPP plantations were mainly generalistic and rather open-habitat tolerant. Generally, those affected most by forest conversion are often rare and range-restricted species (Scales and Marsden 2008), and in the Neotropics, several Phyllostominae are known to be adversely affected by habitat disturbance. These mainly animalivorous gleaners often have rather small home ranges (Kalko et al. 1999). Adapted to foraging in the forest understory, they seem to avoid clearings or other structurally simplified or degraded habitats, instead showing strong affiliation to forest interior (e.g. Medellín et al. 2000; Clarke et al. 2005; Meyer and Kalko 2008). Likewise, Phyllostominae were mainly encountered in intact forest habitats and only represented by few captures in our study. Species present were similar to those previously reported from managed coffee plantations (Williams-Guillén and Perfecto 2010), indicating a certain flexibility in habitat use. Phyllostomus discolor is fairly common in the study area and was also captured in all three sampled habitats during our study. Traits such as relatively large body size and high mobility, combined with dietary flexibility might make it less susceptible to habitat disturbance than other members of this subfamily. Trachops cirrhosus may use larger foraging areas than other Phyllostominae of similar size (Kalko et al. 1999) and seems to be one of the few animalivorous bats able to find food in OPP—generalistic amphibian prey such as Physalaemus pustulosus can be fairly abundant in mud puddles forming in plantations after rainfall. Micronycteris microtis represents the most commonly encountered species of this genus in Central America (Reid 2009) and has previously been recorded in agro-ecosystems like coffee plantations (Castro-Luna and Galindo-González 2012). However, other Phyllostominae seem to be more disturbance-sensitive, such as Micronycteris hirsuta, a species frequently captured in the area but restricted to intact forest interior (Freudmann, unpublished data). Despite their apparent vagility, certain species may be reluctant to cross an unfavourable matrix (Henry et al. 2007). Particularly species adapted to highly cluttered environments and with small home ranges and comparably low mobility seem most threatened by habitat disturbance and fragmentation (Meyer et al. 2008; Farneda et al. 2015). At our study sites, mean canopy cover decreased from forest interior towards oil palm plantations and no woody plants were present in the sparse understory of OPP. Forest margins showed highest understory density and large-diameter trees were most abundant in forest interior. The observed decrease in bat species richness towards lower structural habitat complexity illustrates negative impacts of habitat simplification and consequences such as reduced resource availability for many species foraging in the understory.
Vertical stratification of Neotropical bat assemblages might be a key factor to differential responses of species to habitat alteration. For gleaners with small home ranges and a strong dependence on understory structures for hunting, the contrast between OPP and natural habitat is apparently greater than for highly mobile frugivores, which may use lower strata for commuting but feed on canopy fruits (Rex et al. 2011).
The matrix surrounding a species’ habitat appears more permeable the more closely it resembles its natural habitat structure (Eycott et al. 2012), and OPP seem to function as a hostile matrix or even dispersal barrier for many species in the study area. Overall, forest conversion in favor of agriculture is detrimental for biodiversity and OPP are unlikely to provide any resources for most species we recorded there. Nevertheless, agricultural land-use systems like OPP could have some value for some bat species by reducing isolation distances between forest patches separated by open areas such as pastures. Neotropical bats generally rely on linear structures such as forest trails, live fences, riparian vegetation or gallery forests as flyways in open areas (e.g. Estrada and Coates-Estrada 2001) and many species typically do not traverse open habitats. The canopy structure of oil palms may provide increased protection from predation when commuting, and higher capture rates in OPP despite the absence of food resources indicate that small-scale plantations in proximity to forest can apparently function as flyways for certain species. Similarly, Eucalyptus and rubber plantations can hold surprising bat diversity but also seem to serve as stepping-stones between more valuable habitat patches (Barlow et al. 2007; Heer et al. 2015) rather than functioning as suitable habitat by themselves.
The spatial distribution of resources like food and roosting sites influences a species’ landscape perception (Pinto and Keitt 2008) and consequently affects movement patterns. Transient individuals caught in profuse numbers in OPP were not only those that are generally more abundant in the area, but mainly canopy feeding frugivores (Rex et al. 2011) characterized by a rather high mobility (Meyer et al. 2008). Even small species such as Uroderma bilobatum or Dermanura watsoni can be considerably mobile in relation to their size (Meyer et al. 2009; Ripperger et al. 2015), and clearly, those engaging in strenuous commuting flights to find scattered ephemeral food (e.g., fruiting fig trees) are more likely to be encountered in corridor-like habitats such as OPP. Their matrix-tolerance appears to be higher than that of understory frugivores feeding on steady-state resources clustered in successional areas, but the majority of Neotropical bats still require intact forest to reliably supply their foraging and roosting demands and are unlikely to thrive in areas dominated by high intensity agricultural habitats such as oil palm plantations. Even common species such as Artibeus lituratus, Carollia perspicillata and Dermanura watsoni, all of which have been shown to forage in degraded habitats, depend on forest habitats for roosting (Trevelin et al. 2013; Ripperger et al. 2015). Furthermore, at least 18 species of Phyllostomids use leaf tents for roosting but most of the plants used to construct tents are generally not available in high-intensity agricultural land such as OPP. Neither night nor day roosts were observed in OPP (A. Freudmann, unpublished observations), indicating unsuitability of either oil palm fronds or microclimate.
Implications for conservation
Intensification and expansion of agriculture in response to growing world food demands require appropriate policies to protect and preserve biodiversity. While the protection of large areas of contiguous forest is indispensable, retaining and connecting habitat remnants becomes increasingly important in areas of high anthropogenic impact. Agricultural landscapes dominated by monocultures such as oil palm lack habitat features that are crucial for maintaining high biodiversity (Danielsen et al. 2009) and clearly do not exhibit the carrying capacity to support the remarkable bat diversity of the Neotropics, where more than 100 species can occur sympatrically (Rex et al. 2008). Habitat loss, reduced connectivity and fragmentation can have severe implications for bats (Henry et al. 2007; Meyer et al. 2009; Ripperger et al. 2013), and in return may affect forest dynamics and ecosystem functioning. Subfamilies affected most by OPP include species that are of great relevance for certain ecosystem services: gleaning insectivores contributing to crop pest reduction by preying on herbivorous insects (Maas et al. 2015) and specialized nectar bats that are important pollinators but sensitive to habitat disruption (Quesada et al. 2004).
Despite the vagility of bats, even small oil palm plantations adjacent to forest can act as effective barriers by restricting the movements of understory-dependent species. Discrepancies between large, isolated plantations and forest interior can be even more severe, as elucidated by a decline in species richness, abundance, and similarity of orchid bee communities in oil palm plantations with increasing distance from forest (Livingston et al. 2013). Similarly, oil palm plantations in Brazilian Amazonia were found to contain species-poor avian assemblages comparable to those of pastures, mainly consisting of generalistic species of low conservation concern and lacking range-restricted and forest-associated species (Lees et al. 2015). Consequently, OPP contribute to an impoverishment of the local fauna and may prevent the utilization of isolated forest remnants embedded in an agricultural matrix, conducing forest fragmentation and habitat degradation as well as diminishment of ecosystem services provided by bats. Particularly in the Neotropics, where more than 80 % of the rain forest plants are dispersed by frugivorous vertebrates (Howe and Smallwood 1982), bats function as crucial mobile links. Even small bats can disperse relatively large seeds (Melo et al. 2009)—a fundamental and increasingly important task, particularly in areas of anthropogenic impact where other seed dispersers (such as frugivore primates or birds) have become rare or are reluctant to cross open and disturbed habitats.
While plantations in our study area mainly derive from conversion of other crop fields or degraded pastures, and large scale forest conversion is unlikely due to complex topography (Höbinger et al. 2012), in other Neotropical regions they frequently emerge from previously forested areas (Gutiérrez-Vélez et al. 2011), and suitable land for oil palm cultivation is largely still under forest cover and in areas with high degrees of endemism (Stickler et al. 2007). Restricting establishment of plantations to pastures or other agricultural areas of lower ecological and economic value is frequently suggested as an alternative to forest conversion and indeed, OPP in the grasslands of the Colombian Llanos were found to harbor higher faunal species richness of several taxa than cattle pastures (Gilroy et al. 2014). Further proposed mitigation measures for oil palm dominated landscapes include maintaining ground vegetation cover, partial reforestation of plantations with native trees, retention of forest remnants within plantations (Lucey et al. 2014) or growing oil palm in a mixed plantation matrix (Bhagwat & Willis 2008; Nájera and Simonetti 2010). Certain agro-ecosystems are more biodiversity-compatible than conventional agriculture (Jose 2012) and can harbor relatively high bat species richness (Estrada et al. 1993; Harvey and Villalobos 2007; Williams-Guillén and Perfecto 2010). Diversified systems in particular can enhance the permeability of the agricultural matrix and thereby increase habitat connectivity (Castro-Luna and Galindo-González 2012), but enriching plantations with understory crops likely impedes efficiency of harvesting. Thus, second to preserving remaining natural habitat, the creation of buffer zones seems to be one of the few realistic biodiversity-friendly practices in regard to oil palm proliferation. Establishing vegetation strips around plantations would not hinder harvesting as much as consistent undergrowth in mixed plantations, and bank reinforcement of drainage ditches with vegetation and actively promoting growth along these drainages could re-enhance landscape connectivity. Riparian corridors, even when located in open habitat such as pastures, can harbor considerable species richness and facilitate the persistence of bat species sensitive to the agricultural matrix (de la Peña-Cuéllar et al. 2015). Even single scattered trees can have substantial impacts on species richness in cleared or agricultural landscapes (Galindo-González and Sosa 2003) and corridor-like structures such as gallery forests and live fences are of great importance for wildlife transients in agricultural areas (Harvey et al. 2005). Along with the protection of larger tracts of natural habitat and preservation of remnants that can have substantial conservation value for Neotropical bats (Ripperger et al. 2015), these buffer zones can facilitate movement for animals reluctant to enter plain OPP or other agricultural areas. Retaining natural habitat and creating stepping stones to increase landscape permeability is crucial for the conservation of contrast-sensitive forest specialists, and will also provide source populations of ecosystem service providers creating direct economic value and incentives for the oil palm industry. Further comprehensive assessments of the impact of oil palm plantations on the Neotropical fauna are required in order to deliver conclusive arguments for improving sustainable and biodiversity-compatible cultivation and establishing according conservation measures.
References
Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11(7):36–42
Aide TM, Clark ML, Grau HR, López-Carr D, Levy M et al (2013) Deforestation and reforestation of Latin America and the Caribbean (2001–2010). Biotropica 45:262–271
Baker RJ, Hoofer SR, Porter CA, Van Den Bussche RA (2003) Diversification among new world leaf-nosed bats: an evolutionary hypothesis and classification inferred from digenomic congruence of DNA sequence. Occas Papers Mus, Texas Tech Univ 230:1–32
Barlow J, Gardner TA, Araujo IS, Avila-Pires TCS, Bonaldo AB et al (2007) Quantifying the biodiversity value of tropical primary, secondary and plantation forests. Proc Natl Acad Sci USA 104:18555–18560
Bhagwat SA, Willis KJ (2008) Agroforestry as a solution to the oil-palm debate. Conserv Biol 22(6):1368–1369
Boyles JG, Cryan PM, McCracken GF, Kunz TH (2011) Economic importance of bats in agriculture. Science 332(6025):41–42
Castro-Luna AA, Galindo-González J (2012) Enriching agroecosystems with fruit-producing tree species favors the abundance and richness of frugivorous and nectarivorous bats in Veracruz, Mexico. Mamm Biol 77(1):32–40
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93(12):2533–2547
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Clarke FM, Pio DV, Racey PA (2005) A comparison of logging systems and bat diversity in the Neotropics. Conserv Biol 19(4):1194–1204
Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX et al (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol 5(1):3–21
Corley RHV (2009) How much palm oil do we need? Environ Sci Policy 2712(2):134–139
Danielsen F, Heegaard M (1995) Impact of logging and plantation development on species diversity: a case study from Sumatra. In: Sandbukt Ø (ed) Management of tropical forests: towards an integrated perspective. University of Oslo, Oslo, pp 73–92
Danielsen F, Beukema H, Burgess ND, Parish F, Brühl CA et al (2009) Biofuel plantations on forested lands: double jeopardy for biodiversity and climate. Conserv Biol 23(2):348–358
Datzmann T, von Helversen O, Mayer F (2010) Evolution of nectarivory in phyllostomid bats (Phyllostomidae Gray, 1825, Chiroptera: Mammalia). BMC Evol Biol 10:165
de la Peña-Cuéllar E, Benítez-Malvido J, Avila-Cabadilla LD, Martínez-Ramos M, Estrada A (2015) Structure and diversity of phyllostomid bat assemblages on riparian corridors in a human-dominated tropical landscape. Ecol Evol 5(4):903–913
Donald PF (2004) Biodiversity impacts of some agricultural commodity production systems. Conserv Biol 18(1):17–38
Estrada A, Coates-Estrada R (2001) Bat species richness in live fences and in corridors of residual rain forest vegetation at Los Tuxtlas, Mexico. Ecography 24(1):94–102
Estrada A, Coates-Estrada R, Meritt D (1993) Bat species richness and abundance in tropical rain forest fragments and in agricultural habitats at Los Tuxtlas, Mexico. Ecography 16(4):309–318
Eycott AE, Stewart GB, Buyung-Ali LM, Bowler DE, Watts K, Pullin AS (2012) A meta-analysis on the impact of different matrix structures on species movement rates. Landsc Ecol 27(9):1263–1278
Farneda FZ, Rocha R, López-Baucells A, Groenenberg M, Silva I et al (2015) Trait-related responses to habitat fragmentation in Amazonian bats. J Appl Ecol. doi:10.1111/1365-2664.12490
Fitzherbert EB, Struebig MJ, Morel A, Danielsen F, Brühl CA, Donald PF, Phalan B (2008) How will oil palm expansion affect biodiversity? Trends Ecol Evol 23:538–545
Foster WA, Snaddon JL, Turner EC, Fayle TM, Cockerill TD et al (2011) Establishing the evidence base for maintaining biodiversity and ecosystem function in the oil palm landscapes of South East Asia. Phil Trans R Soc B: Biol Sci 366(1582):3277–3291
Fukuda D, Tisen OB, Momose K, Sakai S (2009) Bat diversity in the vegetation mosaic around a lowland dipterocarp forest of Borneo. Raffles Bull Zool 57(1):213–221
Galindo-González J, Sosa VJ (2003) Frugivorous bats in isolated trees and riparian vegetation associated with human-made pastures in a fragmented tropical landscape. Southwest Nat 48(4):579–589
Gilroy JJ, Prescott GW, Cardenas JS, Castañeda PGDP, Sánchez A, Rojas-Murcia LE et al (2014) Minimizing the biodiversity impact of Neotropical oil palm development. Glob Change Biol 21(4):1531–1540
Gutiérrez-Vélez VH, DeFries R (2013) Annual multi-resolution detection of land cover conversion to oil palm in the Peruvian Amazon. Remote Sens Environ 129:154–167
Gutiérrez-Vélez VH, DeFries R, Pinedo-Vásquez M, Uriarte M, Padoch C et al (2011) High-yield oil palm expansion spares land at the expense of forests in the Peruvian Amazon. Environ Res Lett 6(4):044029
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):9
Harvey CA, Villalobos JAG (2007) Agroforestry systems conserve species-rich but modified assemblages of tropical birds and bats. Biodivers Conserv 16(8):2257–2292
Harvey CA, Villanueva C, Villacís J, Chacón M, Muñoz D et al (2005) Contribution of live fences to the ecological integrity of agricultural landscapes. Agric Ecosyst Environ 111(1):200–230
Heer K, Helbig-Bonitz M, Fernandes RG, Mello MA, Kalko EKV (2015) Effects of land use on bat diversity in a complex plantation-forest landscape in northeastern Brazil. J Mammal. doi:10.1093/jmammal/gyv068
Henry M, Pons J, Cosson JF (2007) Foraging behaviour of a frugivorous bat helps bridge landscape connectivity and ecological processes in a fragmented rainforest. J Anim Ecol 76(4):801–813
Höbinger T, Schindler S, Seaman BS, Wrbka T, Weissenhofer A (2012) Impact of oil palm plantations on the structure of the agroforestry mosaic of La Gamba, southern Costa Rica: potential implications for biodiversity. Agrofor Syst 85(3):367–381
Hoofer SR, Solari S, Larsen PA, Bradley RD, Baker RJ (2008) Phylogenetics of the fruit-eating bats (Phyllostomidae: Artibeina) inferred from mitochondrial DNA sequences. Occas Papers Mus, Texas Tech Univ 277:1–15
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Evol Syst 13:201–228
Hsieh TC, Ma KH, Chao A (2013) iNEXT online: interpolation and extrapolation (Version 10). [Software] Available from https://chao.shinyapps.io/iNEXT/
Jose S (2012) Agroforestry for conserving and enhancing biodiversity. Agrofor Syst 85(1):1–8
Kalko EKV, Friemel D, Handley CO, Schnitzler HU (1999) Roosting and foraging behavior of two Neotropical gleaning bats, Tonatia silvicola and Trachops cirrhosus (Phyllostomidae). Biotropica 31(2):344–353
Kunz TH, Braun de Torrez E, Bauer D, Lobova T, Fleming TH (2011) Ecosystem services provided by bats. Ann NY Acad Sci 1223(1):1–38
Laube I, Breitbach N, Böhning-Gaese K (2008) Avian diversity in a Kenyan agroecosystem: effects of habitat structure and proximity to forest. J Ornithol 149(2):181–191
Laurance WF, Useche DC, Rendeiro J, Kalka M, Bradshaw CJ et al (2012) Averting biodiversity collapse in tropical forest protected areas. Nature 489(7415):290–294
Lees AC, Moura NG, de Almeida AS, Vieira ICG (2015) Poor prospects for Avian biodiversity in Amazonian oil palm. PLoS One 10(5):e0122432
Livingston G, Jha S, Vega A, Gilbert L (2013) Conservation value and permeability of neotropical oil palm landscapes for orchid bees. PLoS One 8(10):e78523
Lucey JM, Tawatao N, Senior MJ, Chey VK, Benedick S et al (2014) Tropical forest fragments contribute to species richness in adjacent oil palm plantations. Biol Conserv 169:268–276
Maas B, Karp DS, Bumrungsri S, Darras K, Gonthier D et al (2015) Bird and bat predation services in tropical forests and agroforestry landscapes. Biol Rev. doi:10.1111/brv12211
Medellín RA, Equihua M, Amin MA (2000) Bat diversity and abundance as indicators of disturbance in Neotropical rainforests. Conserv Biol 14:1666–1675
Melo FP, Rodriguez-Herrera B, Chazdon RL, Medellín RA, Ceballos GG (2009) Small tent-roosting bats promote dispersal of large-seeded plants in a Neotropical forest. Biotropica 41(6):737–743
Meyer CF, Kalko EKV (2008) Assemblage-level responses of phyllostomid bats to tropical forest fragmentation: land-bridge islands as a model system. J Biogeogr 35(9):1711–1726
Meyer CF, Fründ J, Lizano WP, Kalko EKV (2008) Ecological correlates of vulnerability to fragmentation in Neotropical bats. J Appl Ecol 45(1):381–391
Meyer CF, Kalko EKV, Kerth G (2009) Small-scale fragmentation effects on local genetic diversity in two Phyllostomid bats with different dispersal abilities in Panama. Biotropica 41(1):95–102
Nájera A, Simonetti JA (2010) Can oil palm plantations become bird friendly? Agrofor Syst 80(2):203–209
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR et al (2015) R Package ‘vegan’. Community ecology package, Version 2.2-1, http://www.cran.r-project.org/package=vegan
Pike N (2011) Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol 2(3):278–282
Pinto N, Keitt TH (2008) Scale-dependent responses to forest cover displayed by frugivore bats. Oikos 117(11):1725–1731
Presley SJ, Willig MR, Castro-Arellano I, Weaver SC (2009) Effects of habitat conversion on temporal activity patterns of phyllostomid bats in lowland Amazonian rain forest. J Mammal 90(1):210–221
Quesada M, Stoner KE, Lobo JA, Herrerías-Diego Y, Palacios-Guevara C et al (2004) Effects of forest fragmentation on pollinator activity and consequences for plant reproductive success and mating patterns in bat-pollinated Bombacaceous trees. Biotropica 36(2):131–138
Reid FA (2009) A field guide to the mammals of central America & Southeast Mexico, 2nd edn. Oxford University Press, New York
Rex K, Kelm DH, Wiesner K, Kunz TH, Voigt CC (2008) Species richness and structure of three Neotropical bat assemblages. Biol J Linnean Soc 94(3):617–629
Rex K, Michener R, Kunz TH, Voigt CC (2011) Vertical stratification of Neotropical leaf-nosed bats (Chiroptera: Phyllostomidae) revealed by stable carbon isotopes. J Trop Ecol 27(3):211–222
Ripperger SP, Tschapka M, Kalko EKV, Rodriguez-Herrera B, Mayer F (2013) Life in a mosaic landscape: anthropogenic habitat fragmentation affects genetic population structure in a frugivorous bat species. Conserv Genet 14(5):925–934
Ripperger SP, Kalko EKV, Rodríguez-Herrera B, Mayer F, Tschapka M (2015) Frugivorous bats maintain functional habitat connectivity in agricultural landscapes but rely strongly on natural forest fragments. PLoS One 10(4):e0120535
Savilaakso S, Garcia C, Garcia-Ulloa J, Ghazoul J, Groom M et al (2014) Systematic review of effects on biodiversity from oil palm production. Environ Evid 3(1):1–21
Scales BR, Marsden SJ (2008) Biodiversity in small-scale tropical agroforests: a review of species richness and abundance shifts and the factors influencing them. Environ Conserv 35(02):160–172
Schulze MD, Seavy NE, Whitacre DF (2000) A comparison of the phyllostomid bat assemblages in undisturbed Neotropical forest and in forest fragments of a slash-and-burn farming mosaic in Petén, Guatemala. Biotropica 32(1):174–184
Stewart AM, Edmisten KL, Wells R, Collins GD (2007) Measuring canopy coverage with digital imaging. Commun Soil Sci Plant Anal 38(7–8):895–902
Stickler C, Coe M, Nepstad D, Fiske G & Lefebvre P (2007) Readiness for REDD? A preliminary assessment of global forested land suitability for agriculture. Woods Hole Massachusetts: Woods Hole Research Center. http://www.whrc.org/resources/publications/pdf/WHRC_REDD_crop_suitability.pdf Accessed July 02 2015
Timm RM, LaVal RK (1998) A field key to the bats of Costa Rica. Center of Latin American studies. Univ Kansas Occ Pub Ser 22:1–30
Trevelin LC, Silveira M, Port-Carvalho M, Homem DH, Cruz-Neto AP (2013) Use of space by frugivorous bats (Chiroptera: Phyllostomidae) in a restored Atlantic forest fragment in Brazil. Forest Ecol Manag 291:136–143
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol Lett 8(8):857–874
Weissenhofer A, Huber W, Mayer V, Pamperl S, Weber A & Aubrecht G (2008) Natural and Cultural History of the Golfo Dulce Region, Costa Rica. Stapfia 88, Biologiezentrum des Oberösterreichischen Landesmuseums Linz
Williams-Guillén K, Perfecto I (2010) Effects of agricultural intensification on the assemblage of Leaf-Nosed bats (Phyllostomidae) in a coffee landscape in Chiapas, Mexico. Biotropica 42(5):605–613
Yue S, Brodie JF, Zipkin EF, Bernard H (2015) Oil palm plantations fail to support mammal diversity. Ecol Appl doi: http://dx.doi.org/10.1890/14-1928.1
Acknowledgments
Our thanks go to the plantation owners who kindly provided permission to work on their land, the La Gamba field station staff for administrative and logistic support and Ralph Hertlein for assistance in the field. Thanks to Konrad Fiedler and Marc-Oliver Adams for feedback and statistical advice, as well as three anonymous reviewers for providing helpful comments on an earlier version of the manuscript.
Funding
AF received partial funding by the University of Vienna (KWA and research scholarship) and the Austrian Federal Ministry of Science, Research and Economy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by Kirsty Park.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Freudmann, A., Mollik, P., Tschapka, M. et al. Impacts of oil palm agriculture on phyllostomid bat assemblages. Biodivers Conserv 24, 3583–3599 (2015). https://doi.org/10.1007/s10531-015-1021-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-015-1021-6