Abstract
Poaceae has numerous species that are highly invasive thorough the planet. Urochloa, native to Africa, stands out in terms of invasion and impacts in South America. However, the correct identification of the species included in this genus is complex due to their morphological similarity with other species and there is a lack of studies involving the genetic variability of some species in Brazil, which is important for understanding the invasion mechanisms and planning control measures. In this study, we evaluated the genetic variability of the invasive exotic Poaceae Urochloa arrecta in different Brazilian regions, by using the nuclear marker ITS and the intergenetic chloroplast marker trnL-trnF. The sequences obtained were compared with those available in GenBank (NCBI). The results indicated a low genetic differentiation among the sampled populations. Seven and ten distinct haplotypes were identified for ITS and trnL-trnF, respectively, and most specimens shared a single haplotype. These results indicate that a limited number of propagules were introduced in the invasive range and/or that this species reproduces mainly through asexual reproduction. This last possibility is suggested by the great regeneration and colonization rates of asexual propagules of this species. The data obtained are useful for understanding the invasion mechanisms of the group in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous aquatic macrophytes have high ecological plasticity (Thiebaut et al. 2021) and developed characteristics that allow fast dispersion, growth and reproduction (Spencer and Bowes, 1990; Hussner et al. 2021). For these reasons these plants comprise a group with many potentially invasive species (Pieterse and Murphy, 1990) and constitute a threat in ecological and economic terms to a variety of aquatic ecosystems (Kolada and Kutyla 2016; Wahl et al. 2021). Particularly in Brazil, 12 species of freshwater aquatic exotic species have been identified (Latini et al. 2016).
Among these species, representatives of Urochloa stand out for their invasive potential. These plants were disseminated in Brazilian territory as forage (Latini et al. 2016). Urochloa arrecta is a species that invades different types of aquatic ecosystems, such as ponds, canals, rivers, reservoirs and tidal habitats (Thomaz et al. 2009; Amorim et al. 2015; Diamante et al. 2020; Sato et al. 2021; Santini et al. 2022). Although Africa is the native range of this species, it is also distributed in Asia, Oceania, Europe, North and South America (GBIF.org, 2021), including anthropized areas in several Brazilian states (Fares et al. 2020a, b; Flora do Brasil 2020).
Representatives of this genus (including U. arrecta) have been responsible for negative impacts such as the reduction of the diversity of aquatic communities, replacement of native flora, increase of biotic homogenization and the blocking of canals, bridges, and water intake ducts for hydroelectric power plants (Michelan et al., 2010a; Carniatto et al. 2013; Amorim et al. 2015; Latini et al. 2016; Galvanese et al. 2022). Furthermore, U. arrecta has high plasticity and can grow as an epiphytic life form on drifting floating macrophyte mats, which enhances its dispersion and potential impacts (Michelan et al. 2018). The establishment of U. arrecta is facilitated in ecosystems where riparian vegetation is absent, since the shade caused by native vegetation can be a limiting factor for its development (Evangelista et al. 2016).
To understand the mechanism of invasion, the correct identification of invasive aquatic plant species and information on their genetic variability is fundamental (Naugzemyz et al. 2021). This information is also important to implement management strategies, as well as research into potential control agents, including biological ones (Pitelli et al. 2008). Molecular tools have been useful for the correct identification and analysis of genetic variability in macrophyte populations, including Urochloa in the Upper Paraná River basin (Machado et al. 2016; Lucio et al. 2019; Diamante et al. 2020) and in other watersheds (Collevatti et al. 2003; Cloutier et al. 2005; Azevedo et al. 2008). A previous study with U. arrecta in Itaipu reservoir (Brazil) showed a high degree of genetic homogeneity among populations in this ecosystem (Diamante et al. 2020). However, it remains to be confirmed whether this result applies or not to samples obtained in a variety of ecosystems at a larger spatial scale.
The use of the nuclear genome, specifically the internal transcribed spacer (ITS) region of nuclear ribosomal DNA, is common for molecular phylogenetic studies of plants (Torres González and Morton, 2005). Moreover, this technique has been used on different taxonomic levels of grasses (Wang et al. 2017), in addition to studies concerning molecular discrimination and identification (Diamante et al. 2020). The use of DNA chloroplast (cpDNA) allows the identification of structural and sequence polymorphisms, which may be useful for genetic studies, as well as contributing to phylogenetic reconstruction and estimation of the divergence time between species (Salariato et al. 2010; Silva et al. 2015; Machado et al. 2016; Pessoa-Filho et al. 2017) in addition to providing insights into plants population genetics and their evolution (Moore et al. 2010).
Given the potential negative effects of introduced species on native flora and the potential economic consequences of uncontrolled growth of invasive alien species, it is important to understand the processes involved in the occupation and especially of diversification of invasive populations. In this study we evaluated the genetic diversity, at the haplotype level, of Urochloa arrecta in different ecosystems located in a large spatial scale (c. 500,000 km2) within the Brazilian territory, using the internal transcribed spacer (ITS) of nuclear DNA and the intergenic spacer located between the tRNALeu and tRNAPhe genes of chloroplast DNA (cpDNA).
Material and methods
Collecting specimens and extracting DNA
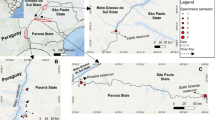
Urochloa arrecta specimens (n = 62) were collected in the states of Paraná (PR) (n = 38), Mato Grosso do Sul (MS) (n = 10), São Paulo (SP) (n = 13) and Mato Grosso (MT) (n = 1) (Fig. 1). The most distant sampling points were ca. 1,000 km apart and the coordinates of each sampling point were determined with GPS (Garmin eTrex® 30). For the collection in the state of Mato Grosso do Sul, the samplings were conducted inside an Environmental Protection Area and the authorization to collect the specimens was granted by IMASUL (No. 71/405723/2018), considering Brazilian legislation. For the other regions no authorization was required. The testimonial material of this study is deposited in the herbarium of the State University of Maringá (HUEM) and herbarium of the Federal University of Mato Grosso do Sul/ Campus—Pantanal (HCOR). The habitats where we collected samples (including the Environmental Protection Area) are altered by human interferences at different degree. Impacts include alteration of hydroperiods, deforestation, agriculture, eutrophication, and river damming (Agostinho et al. 2004; Thomaz et al. 2009; Evangelista et al. 2016).
In the field, leaf tissue samples from each U. arrecta specimen were preserved in a falcon tube with TE buffer pH 8.0 (Tris HCl (1M), EDTA (0.5mM)). Total DNA from the samples was extracted using the Promega Wizard® Genomics extraction kit according to the manufacturer's instructions. To estimate the concentration of DNA present in each sample, 1.0% agarose gel electrophoresis was performed and compared with λ-phage DNA of known concentration.
Molecular markers
In this study, we used ITS and trnL-trnF loci. Both regions were partially amplified using the primers ITS 5a–F (5′-TATCATTTAGAGGAAGGAG-3′) and ITS 4-R (5′-GCATATCAATAAGCGGAGGA-3′) (Baldwin, 1992) and trn-c-F (5′-GGAAATCGGTAGACGCTACG-3′) and trn-f-R (5′-ATTTGAACTGGTGACACGAG-3′) (Reid et al. 2006), respectively. The polymerase chain reaction (PCR) was conducted in total volume of 25 μL, containing Tris-KCl 1x reaction buffer [20mM Tris-HCl (pH 8.4), 50 mM KCl], MgCl2 (1.5 mM), primers (2.5 µM each), dNTPs (0.1 mM each), and 0.5 U Platinum™ Taq DNA Polymerase. The same markers (ITS and trnL-trnF loci) were used by Diamante et al. (2020) in another study of the same species conducted in much smaller spatial scale, which allows comparisons between both investigations.
The amplification reaction program for the ITS locus consisted of 35 cycles, with a temperature of 94 °C at 30 seconds for denaturation, 30 seconds of annealing at 55 °C, and 1 min at 72 °C for polymerization. After completion of the 35 cycles, there was a 5 min time at 72 °C for extension of incomplete fragments. For the trnL-trnF locus, the amplification reaction program started with 4 min at 92 °C, followed by 40 cycles of 15 seconds at 94 °C for denaturation, 30 seconds of annealing at 59 °C and 2 minutes at 72 °C for polymerization, with a final step of 10 min at 72 °C for extension of incomplete fragments. Subsequently, the PCR products were analyzed in 1% agarose gel and purified following the protocol of Rosenthal et al. (1993). For the sequencing reaction the Big Dye Terminator kit was used. The sequencing was performed at ACTGene Análises Moleculares Ltda, Rio Grande do Sul, using the AB-3500 automated sequencer.
Genetic analysis
The obtained sequences were edited and aligned by the algorithm ClustalW (Thompson et al. 1994), using the programs BioEdit (Hall 1999) and MEGA 7 (Kumar et al. 2016), respectively. Sequence similarity values were obtained by comparison with GenBank data, obtained using Blastn.
In addition to the sequences obtained in this study, we also obtained sequences from Urochloa arrecta available in GenBank from the state of Paraná, Mexico and Zimbabwe (Supplementary Material—Table 1). Distance p values were calculated between sequences by the program MEGA 7. 0 and the nucleotide and haplotype diversity indices were obtained by DnaSP v5 (Librado and Rozas 2009). It was possible, by analyzing the alignments of both genes, to estimate the frequency of each haplotype in the different sampled populations. A haplotype network was generated using the program PopArt (Leigh and Bryant 2015). Access to the genetic heritage of the specimens was authorized through the electronic system Sisgen—National System for the Management of Genetic Heritage and Associated Traditional Knowledge (number A0D62EA) and the sequences obtained were deposited in GenBank: MW853712—MW853763; ON710859—ON710862 (ITS) and MW863262—MW863307; ON778459—ON778462 (trnL-trnF).
Results
Fifty-six ITS sequences with 517 base pairs, after alignment and editing, and fifty trnL-trnF sequences (844 bp) were obtained for Urochloa arrecta specimens from the states of Paraná, São Paulo, Mato Grosso do Sul and Mato Grosso. Although all samples were tested with both markers, the number of sequences differs between them because for some samples the amplification was not successful. For the ITS region, 14 variable sites and 7 haplotypes were identified, while for trnL-trnF, 14 variable sites and 10 haplotypes were identified. For both markers, H1 was the most frequent haplotype, shared by individuals from the states studied (Table 1). The haplotype diversity indices (h) and nucleotide (π) were, respectively, 0.1946 and 0.00105 for ITS and 0.5044 and 0.00110 for trnL-trnF.
The genetic similarity of the ITS sequences of Urochloa obtained in this study for each haplotype ranged from 98.65 to 100% with GenBank sequences from U. arrecta. The p-distance values obtained also confirm this genetic similarity, ranging from 0 to 1.8% between the collected specimens and those obtained from the database. The analysis of the trnL-trnF region also confirms the genetic proximity between the specimens, since the sequences obtained in this study for each haplotype found were 98.64–100% with sequences from the GenBank of U. arrecta, the p-distance values ranged from 0 to 0.4% between the collected specimens and those obtained from the database, confirming this similarity.
The haplotype network obtained with the ITS marker (Fig. 2A) shows that all specimens collected in the state of São Paulo (n = 13) have the same haplotype (H1), shared with samples obtained from GenBank (Itaipu Reservoir (PR) and Guaraguaçu River (PR)) and with individuals from other Brazilian localities (Paraná, Mato Grosso and Mato Grosso do Sul). The state that showed the greatest genetic variation was Paraná, with the presence of 4 of the 7 haplotypes obtained in Urochloa. The sequence comes from Zimbabwe, a country in Africa, where Urochloa is native, constituted a distinct haplotype (p distance of 0.4% to 1.8% relative to the others) (Supplementary material—table 2). Among the sequences obtained in this study, the most distinct haplotype was H6, with a p-distance of 1.4–1.9% relative to the other haplotypes.
On the other hand, the haplotype network obtained with the trnL-trnF marker (Fig. 2B) shows that not only state of Paraná presented the greatest haplotype variation, with the presence of 7 of the 10 haplotypes obtained in Urochloa, but specimens from the states of Mato Grosso do Sul and São Paulo also showed genetic variation. Mato Grosso do Sul presented 5 of the 10 haplotypes obtained and state of São Paulo presented 3 haplotypes. The sequences available in GenBank (from state of Paraná and Mexico) shared the most common haplotype (H1) for this marker with 100% similarity with the other specimens (Supplementary material—Table 3). Among the sequences obtained in this study, the most distinct haplotype was H5 (from state of Paraná), showing a p-distance of 0.4% to 0.7% compared to the other haplotypes.
The states of Paraná and Mato Grosso do Sul stand out for the presence of several unique haplotypes, both for the ITS region and for trnL-trnF.
Discussion
The genetic analysis indicates low genetic differentiation among the populations studied since most of the specimens evaluated share the most frequent haplotype for both markers. The haplotype H1 occurred in most of the individuals for the ITS and trnL-trnF regions, respectively, covering, besides the specimen from Mexico, the four Brazilian states analyzed. A phylogeographic structure was not observed, since the same haplotype could be found in specimens from distinct countries, while distinct haplotypes were obtained from geographically close specimens.
In terms of diversity, aquatic plants generally have a lower number of species than terrestrial plants, and a low intraspecific variability (Nakamura and Kadono 2000; Amsellem et al. 2000; Lambertini et al. 2010; Lucio et al. 2019). Low intraspecific genetic variability had also been shown for Urochloa arrecta populations colonizing one reservoir in South Brazil (Diamante et al. 2020). In this study, a larger number of individuals was used as well as a wider sampling area than Diamante et al. (2020), including the states with the highest concentration of Urochloa (Latini et al. 2016), and the low genetic differentiation among populations from different locations was still observed. Somaclonal variation might be an explanation for the presence of different haplotypes, since there are records of this variation, which becomes heritable, in plants of various groups (Kaeppler et al. 2000; Bairu et al. 2011).
Some studies observed that the processes involved in species invasion can result in genetic differences between the introduced population and that from its native location (Bossdorf et al. 2005; Dietiz and Edwards 2006). Samples of U. arrecta in this study had the same haplotype as the sample from Mexico. However, when compared to the sample from Zimbabwe, the native location, there was a haplotype difference, in which Zimbabwe sample showed a unique haplotype, different from those present in the Brazilian territory. However, there are no other sequences available for this species sampled in its native location. Therefore, it is not yet possible to infer whether the population introduced into Brazil has a low or high genetic variation when compared to specimens from the native region.
As noted in previous studies, it is common for introduced populations to show lower genetic diversity in the introduced area when they do not experience environmental pressures (Lucio et al. 2019; Diamante et al. 2020). That could explain the low genetic variability observed in this study, as well as the mode of dispersion by asexual structures, since Urochloa is able to propagate by stem fragments and stolons (Michelan et al. 2010b; Amorim et al. 2015) and by rhizomes, or any other fragment that can be transported by water flow (Pott et al. 2011; Michelan et al. 2018). Furthermore, experiments related to the regeneration of U. arrecta showed that it recovers very efficiently through asexual fragments (Michelan et al. 2010b). In contrast, genetic diversity can be greater than expected when the number of introduced individuals is high or a high number of introduction events occur (Estoup et al. 2016).
Our results point out that propagule dispersal is a likely means of reproduction of this species and this could explain the high frequency of a unique haplotype for both markers. In cases where the pattern of low variability occurs, besides being indicative of the presence of a single founder genotype, it may also indicate that the maintenance of the population occurs through vegetative reproduction and dispersal of propagules to different locations (Lucio et al. 2019). In addition to clonal reproduction, we speculate that the introduction of a small number of individuals may also help to explain the low genetic variability found within a given population. This process acts as a genetic bottleneck, limiting the genetic variation in invasive populations compared to populations from natural sites (Estoup et al. 2016; Diamante et al. 2020). In the case of specimens of Urochloa in this study, most of them were sampled in the upper Paraná River basin, where they could disperse by river flow, being found as far as the Itaipu Reservoir, where they share the same haplotype, although some sampling points within this reservoir were located more than 80 km apart (Diamante et al. 2020).
Although we have a limitation regarding the number of individuals in some of the areas sampled, as in the state of Mato Grosso, the presence of a widely dispersed haplotype in a large area is consistent with the expectation of clonal reproduction (Santamaría, 2002). The reproductive system of a plant determines opportunities for adaptive evolution, since it has an important influence on different population genetic parameters, including genetic recombination, effective population size, gene flow and partitioning of genetic diversity within and between populations (Barrett et al. 2008).
The success of exotic species, such as Urochloa arrecta, can be limited by the combination of different factors acting as filters (biotic, abiotic and dispersal), in which biotic resistance could be the main explanation for failure (Pearson et al. 2014); however, the species may be favored in disturbed habitats (Leal et al., 2022), as the ones where we collected our samples. The predominance of habitats that have been altered by human interference could help explaining the existence of populations that are similar in terms of genetic, probably well adapted to disturbances, and indeed we were not able to identify specific haplotypes in particular regions. In addition, geographic factors may contribute to the existence of clones. For example, habitat connectivity provided by the main river channel, as occurs in the Paraná River and the Itaipu reservoir, could be the explanation for the low genetic variability, at least for the specimens collected in this basin.
The presence of few or unique haplotypes in a population, as evidenced in our study, does not preclude high invasiveness. In fact, some studies report that not only genetic diversity, but also the phenotypic plasticity has been an important factor to explain the invasion success of these plants (Richardson and Pysek, 2006). Among these phenotypic characteristics, we can cite the U. arrecta aggressive potential regarding competition for nutrients, fast growth and allocation of individual resources when compared to other species (Bianco et al., 2015). Compared to plants native to aquatic environments, U. arrecta also stands out as it presents high resilience to drought (Michelan et al. 2010b; Leal et al. 2022).
Another evidence that can be highlighted, from the comparison of haplotype networks, is that the trnL-trnF region showed a higher haplotype diversity when compared to the ITS. This can be explained by the fact that the chloroplastidial DNA region are susceptible to accumulating mutations, since regions with a higher degree of variation have been found in the chloroplast genome (Collevatti et al. 2003; Cloutier et al. 2005). This region has already proven useful in studying the genetic variability of different aquatic plants (Salariato et al. 2010; Silva et al. 2015; Machado et al. 2016; Lucio et al. 2019; Diamante et al. 2020).
In summary, we observed that populations of U. arrecta are highly homogeneous when comparing trnL-trnF and ITS markers. These results suggest an important role of asexual reproduction for this plant, which probably spreads through fragmentation toward distant ecosystems. In addition to the data obtained, molecular studies, including other DNA regions, such as microsatellite markers, will be necessary to comprehend the low genetic variability which occurs within the Brazilian territory.
Data availability
The datasets generated during and analysed during the current study are available in the GenBank repository under accession numbers: MW853712—MW853763; ON710859—ON710862 (ITS) and MW863262—MW863307; ON778459—ON778462 (trnL-trnF).
References
Agostinho AA, Thomaz SM, Gomes LC (2004) Threats for biodiversity in the floodplain of the Upper Paraná River: effects of hydrological regulation by dams. Ecohydrol Hydrobiol 4:255–268
Amorim SR, Umetsu CA, Camargo AFM (2015) Effects of a non-native species of Poaceae on aquatic macrophyte community composition: a comparison with a native species. J Aquat Plant Manage 53:191–196
Amsellem L, Noyer JL, Bourgeois T, Hossaert-Mckey M (2000) Comparison of genetic diversity of the invasive weed Rubus alceifolius Poir. (Rosaceae) inits native range and in areas of introduction, using amplified fragment length polymorphism (AFLP) marker. Mol Ecol 9:443–455. https://doi.org/10.1046/j.1365-294x.2000.00876.x
Azevedo VCR, Kanashiro M, Grattapaglia D, Ciampi AY (2008) Variabilidade no cpDNA em Manilkara huberi, espécie sob manejo sustentável na Amazônia brasileira. Pesq Agrop Brasileira 43(7):859–867. https://doi.org/10.1590/S0100-204X2008000700010
Bairu MW, Aremu AO, Van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63:147–173. https://doi.org/10.1007/s10725-010-9554-x
Baldwin BG (1992) Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: an example from the compositae. Mol Phylogenet Evol 1:3–16. https://doi.org/10.1016/1055-7903(92)90030-K
Barrett SCH, Colautti RI, Eckert CG (2008) Plant reproductive systems and evolution during biological invasion. Mol Ecol 17:373–383. https://doi.org/10.1111/j.1365-294X.2007.03503x
Bianco S, Carvalho LB, Bianco MS, Yamauchi AKF (2015) Crescimento e nutrição mineral de Urochloa arrecta. Planta Daninha 33:33–40. https://doi.org/10.1590/S0100-83582015000100004
Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11. https://doi.org/10.1007/s00442-005-0070-z
Carniatto N, Thomaz SM, Cunha ER, Fugi R, Ota RR (2013) Effects of an invasive alien Poaceae on aquatic macrophytes and fish communities in a neotropical reservoir. Biotropica 45:747–754. https://doi.org/10.1111/btp.12062
Cloutier D, Póvoa JSR, Procópio LC, Leão NVM, Wadt LD, Ciampi AY, Schoen DJ (2005) Chloroplast DNA variation of Carapa guianensis in the Amazon basin. Silvae Genetica. https://doi.org/10.1515/sg-2005-0039
Collevatti RG, Grattapaglia D, Hay JD (2003) Evidences for multiple maternal lineages of Caryocar brasiliense populations in the Brazilian Cerrado based on the analysis of chloroplast DNA sequences and microsatellite haplotype variation. Mol Ecol 12:105–115. https://doi.org/10.1046/j.1365-294x.2003.01701.x
Diamante NA, Fabrin TM, Silveira MJ, Oliveira AV, Thomaz SM, Priolli SMAP, Priolli AJ (2020) Molecular analysis of the invasive populations of Urochloa (Poaceae) in a large Neotropical reservoir. Aquat Bot. https://doi.org/10.1016/j.aquabot.2019.103183
Dietz H, Edwards PJ (2006) Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology 87(6):1359–1367. https://doi.org/10.1890/0012-9658(2006)87[1359:rtcpcd]2.0.co;2
Estoup A, Ravigné V, Hufbauer R, Vitalis R, Gautier M, Facon B (2016) Is there a genetic paradox of biological invasion? Annu Rev Ecol Evol Syst 47:51–72. https://doi.org/10.1146/annurev-ecolsys-121415-032116
Evangelista HB, Michelan TS, Thomaz SM (2016) Shade provided by riparian plants are biotic resistance by macrophytes reduce the establishment of an invasive Poaceae. J Appl Ecol 54:648–656. https://doi.org/10.1111/1365-2664.12791
Fares ALB, Nonato FAS, Michelan TS (2020a) New records of the invasive macrophyte, Urochloa arrecta extend its range to eastern Brazilian Amazon altered freshwater ecosystems. Biodivers Conserv. https://doi.org/10.1590/1809-4392201903831
Fares ALB, Nonato FAS, Michelin TS (2020b) New records of the invasive macrophyte, Urochloa arrecta extend its range to eastern Brazilian Amazon altered freshwater ecosystems. Acta Amaz 50:133–137. https://doi.org/10.1590/1809-4392201903831
GBIF.org (2021), GBIF Home Page. Disponível em: https://www.gbif.org/
Galvanese EF, Costa APL, Araújo ES, Falkievicz BC, de Melo GGV, Vitule JRS, Padial AA (2022) Community stability and seasonal biotic homogenisation emphasize the effect of the invasive tropical tanner grass on macrophytes from a highly dynamic neotropical tidal river. Aquat Sci. https://doi.org/10.1007/s00027-022-00858-3
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and ana- lysis program for Windows 95/98/ NT. Nucleic Acids Symp Ser. 41, 95–98. https://doi.org/10.14601/Phytopathol_Mediterr-14998u1.29
Hussner A, Heidbuchel P, Coetzee J, Gross EM (2021) From introduction to nuisance growth: a review of traits of alien aquatic plants which contribute to their invasiveness. Hydrobiologia 848:2119–2151. https://doi.org/10.1007/s10750-020-04463-z
Kaeppler SM, Kaeppler HF, Rhee Y (2000) Epigenetic aspects of somaclonal variation in plants. In: Matzke MA, Matzke AJM (eds) Plant gene silencing. Springer, pp 59–68
Kolada A, Kutyła S (2016) Elodea canadensis (Michx.) in Polish lakes: a non-aggressive addition to native flora. Biol Invasions 18:3251–3264. https://doi.org/10.1007/s10530-016-1212-4
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Lambertini C, Riis T, Olesen B, Clayton JS, Sorrell BK, Brix H (2010) Genetic diversity in three invasive clonal aquatic species in New Zealand. BMC Genet 11:52. https://doi.org/10.1186/1471-2156-11-52
Latini AO, Resende DC, Pombo VB, Coradin L (2016) Espécies exóticas invasoras de águas continentais no Brasil. MMA, Brasília
Leal RP, Silveira MJ, Petsch DK, Mormul RP, Thomaz SM (2022) The success of an invasive Poaceae explained by drought resilience but not by higher competitive ability. Environ Exp Bot 194:104717. https://doi.org/10.1016/j.envexpbot.2021.104717
Leigh JW, Bryant D (2015) PopART: full-feature software for haplotype network construction. Methods Ecol Evol 6(9):1110–1116. https://doi.org/10.1111/2041-210X.12410
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensives analysis of DNA polymorphism. Bioinformatics 25(11):1451–1452. https://doi.org/10.1093/bioinformatics/btp187
Lucio LC, Thomaz SM, Prioli SMAP, Boni TA, De Oliveira AV, Prioli AJ (2019) Molecular characterization of the invasive aquatic macrophyte Hydrilla verticillata (Hydrocharitaceae) in Brazil. An Acad Bras Cienc 91:e20180494. https://doi.org/10.1590/0001-3765201920180494
Machado SA, Oliveira AV, Fabrin TMC, Prioli SMAP, Prioli AJ (2016) Molecular characterization of the species Salvinia (Salviniaceae) from the upper Paraná River floodplain. Genet Med Res. https://doi.org/10.4238/gmr.15038575
Michelan TS, Dainez Filho MS, Thomaz SM (2018) Aquatic macrophyte mats as dispersers of one invasive plant species. Braz J Biol 78:169–171. https://doi.org/10.2307/2426651
Michelan TS, Thomaz SM, Carvalho P, Rodrigues RB, Silveira MJ (2010b) Regeneration and colonization of an invasive macrophyte grass in response to desiccation. Nat Cons 8:133–139. https://doi.org/10.4322/natcon.00802005
Michelan TS, Thomaz SM, Mormul RP, Carvalho P (2010a) Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Fresh Biol 55:1315–1326. https://doi.org/10.1111/j.1365-2427.2009.02355.x
Moore MJ, Soltis PS, Bell CD, Burleigh JG, Soltis DE (2010) Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proc Natl Acad Sci U S A 107:4623–4628. https://doi.org/10.1073/pnas.0907801107
Nakamura T, Kadono Y (2000) Genetic diversity of the submerged macrophyte Hydrilla verticillata (L. f.) Royle in a river system in Japan. Limnology 1:27–31. https://doi.org/10.1007/s102010070026
Naugzemys D, Lambertini C, Patamsyte J, Butkuviene J, Khasdan V, Zvingila D (2021) Genetic diversity patterns in Phragmites australis populations in straightened and in natural river sites in Lithuania. Hydrobiologia 848:3317–3330. https://doi.org/10.1007/s10750-021-04606-w
Pearson DE, Hierro JL, Chiuffo M, Villarreal D (2014) Rodent seed predation as a biotic filter influencing exotic plant abundance and distribution. Biol Invasions 16:1185–1196. https://doi.org/10.1007/s10530-013-0573-1
Pessoa-Filho M, Martins AM, Ferreira ME (2017) Molecular dating of phylogenetic divergence between Urochloa species based on complete chloroplast genomes. BMC Genomics. https://doi.org/10.1186/s12864-017-3904-2
Pieterse AH, Murphy KJ (1990) Aquatic weeds. The ecology and management of nuisance aquatic vegetation. Oxford Science Publication
Pitelli RA, Martins D, Velini ED (2008) Interferências e controle de macrófitas aquáticas. In: Vargas L, Roman ES (eds) Manual de manejo e controle de plantas daninhas. Embrapa Trigo, Passo Fundo, pp 299–328
Poaceae in Flora do Brasil (2020) Jardim Botânico do Rio de Janeiro. Disponível em: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB26027
Pott VJ, Pott A, Lima LCP, Moreira SN, Oliveira AKM (2011) Aquatic macrophyte diversity of the Pantanal wetland and upper basin. Braz J Biol 71:255–263. https://doi.org/10.1590/S1519-69842011000200004
Reid JD, Plunkett GM, Peters GA (2006) Phylogenetic relationships in the hetero- sporous fern genus Azolla (Azollaceae) based on DNA sequence data from three noncoding regions. Int J Plant Sci 167:529–538. https://doi.org/10.1086/501071
Richardson DM, Pysek P (2006) Plant invasions: merging the concepts of species invasiveness and community invasibility. Prog Phys Geog 30:409–431. https://doi.org/10.1191/0309133306pp490pr
Rosenthal A, Coutelle O, Craxton M (1993) Large-scale production of DNA sequencing templates by microtitre format PCR. Nucleic Acids Res 21(1):173174. https://doi.org/10.1093/nar/21.1.173
Salariato DL, Zuloaga FO, Giussani LM, Morrone O (2010) Molecular phylogeny of the subtribe Melinidinae (Poaceae: Panicoideae: Paniceae) and evolutionary trends in the homogenization of inflorescence. Mol Phylogenet Evol 56:355–369. https://doi.org/10.1016/j.ympev.2010.02.009
Santamaría L (2002) Why are most aquatic plants widely distribuited? Dispersal clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecol 23:137–154. https://doi.org/10.1016/S1146-609X(02)01146-3
Santini R, de Lima JP, Gratão PL, Camargo AFM (2022) Evaluation of growth and oxidative stress as indicative of salinity tolerance by the invasive tropical aquatic macrophyte tanner grass. Hydrobiologia 849:1261–1271. https://doi.org/10.1007/s10750-021-04787-4
Sato RY, Costa APL, Padial AA (2021) The invasive tropical tanner grass decreases diversity of the native aquatic macrophyte community at two scales in a subtropical tidal river. Acta Bot Brasilica 35(1):140–150. https://doi.org/10.1590/0102-33062020abb0360
Silva C, Snak C, Schnadelbach AS, Van Den Berg C, Oliveira RP (2015) Phylogenetic relationships of Echinolaenaand Ichnanthus within Panicoideae (Poaceae) reveal two new genera of tropical grasses. Mol Phyl Evol 93:212–233. https://doi.org/10.1016/j.ympev.2015.07.015
Spencer W, Bowes G (1990) Ecophysiology of the world’s most troublesome aquatic weeds. In: Pieterse AH, Murphy KJ (eds) Aquatic weeds. The Ecology and management of Nuisance Aquatic Vegetation, New York
Thiebaut G, Tarayre M, Jambon O, Le Bris N, Colinet H, Renault D (2021) Variation of thermal plasticity for functional traits between populations of an invasive aquatic plant from two climatic regions. Hydrobiologia 848:2077–2091. https://doi.org/10.1007/s10750-020-04452-2
Thomaz SM, Carvalho P, Mormul RP, Ferreira FA, Silveira MJ, Michelan TS (2009) Temporal trends and effects of diversity on occurrence of exotic macrophytes in a large reservoir. Acta Oecol 35:614–620. https://doi.org/10.1016/j.actao.2009.05.008
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequencing weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(1):4673–4680. https://doi.org/10.1093/nar/22.22.4673
Torres González AM, Morton CM (2005) Molecular and morphological phylogenetic analysis of Brachiaria and Urochloa (Poaceae). Mol Phylogenet Evol 37:36–44. https://doi.org/10.1016/j.ympev.2005.06.003
Wahl C, Kaller M, Diaz R (2021) Invasion of floating fern alters freshwater macroinvertebrate community structure with implications for bottom-up processes. Hydrobiologia 848:2523–2537. https://doi.org/10.1007/s10750-021-04571-4
Wang A, Gopurenko D, Wu H, Lepschi B (2017) Evaluation of six candidate DNA barcode loci for identification of five important invasive grasses in eastern Australia. PLoS ONE. https://doi.org/10.1371/journal.pone.0175338
Acknowledgements
SM Thomaz thanks the National Council for Scientific and Technological Development (CNPq) for continuous funding through a Research Productivity Grant, MJ Silveira and B Scorsim thanks this same agency for providing a post-doc and master’s scholarship, respectively. We also acknowledge the Itaipu Binacional for funding part of this research and Thaísa Sala Michelan (Universidade Federal do Pará) who furnished the sample collected in the State of Mato Grosso.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Molecular analyzes, the general structure of the manuscript, and discussion of results was performed by BS. Molecular analyzes and discussion of results was also performed by NAD and TMCF. Sample collection, discussion of results, and revision of the manuscript were performed by MJS and SMT. Molecular analyzes, discussion of results, and revision of the manuscript was performed by AVO. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No competing financial interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scorsim, B., Diamante, N.A., Fabrin, T.M.C. et al. Urochloa arrecta: an African invasive Poaceae in Brazil with low genetic diversity. Biol Invasions 25, 863–872 (2023). https://doi.org/10.1007/s10530-022-02952-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02952-x