Abstract
Drivers of successful introduction of exotic species remain a major headline in marine invasion biology. We ran two experiments aiming to disentangle the effects of abiotic factors like contaminants and the effect of predation on recruits’ survival of one native and one alien ascidian species. A feeding experiment allowed us to monitor microscale variation of generalist fish predation, which varied significantly within a marina. We also monitored the in situ survival of lab-grown ascidians at three locations within the marina, half with predator cage exclusion. The survival of the native Ciona intestinalis was conjointly highly influenced by location and caging. We were able to identify a link between predation intensity exerted by mobile generalist macropredators and C. intestinalis survival, whereas none of the measured contaminants accounted for site variability of survival. The non-indigenous Styela clava had significant higher survival and biomass when uncaged, suggesting a positive effect of predation for this species. The natural in situ recruits of C. intestinalis showed higher biomass when caged and may have competed with lab-grown S. clava. Our results suggest that generalist fish predation may play a crucial role in the success of non-indigenous species due to facilitation through competitive release.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With increasing globalization and interconnectivity of trade among countries, accidental and voluntary species introductions have multiplied with the use of trade ships, a trend that is predicted to further increase in coming years (Levine and D’Antonio 2003; Seebens et al. 2016; Carrasco et al. 2017). The ecological consequences of the spread of non-indigenous species (NIS) can be drastic, with some invaders completely restructuring ecosystems, potentially leading to the loss of biodiversity and ecosystem services (Pejchar and Mooney 2009; Blackburn et al. 2011). These potential impacts have led to a large body of literature focusing on the conditions of successful transport and establishment in the new environment of these species, identifying several filters that constitute strong selective barriers that only few species successfully overcome (Williamson and Fitter 1996; Jarić and Cvijanovic 2012).
Upon survival of the transport phase, the first major obstacle a NIS may encounter in a new environment are the environmental conditions. Abiotic factors have been shown to vastly influence species’ evolution and the local structure of ecosystems (Je et al. 2004; Benton 2009; Lewis et al. 2017). NIS seem to show higher resistance to abiotic stress and disturbance than their native counterparts (Gröner et al. 2011; Lejeusne et al. 2014; Marie et al. 2017; Kenworthy et al. 2018a), which is attributed to two different processes. First, in some habitats, pre-adaptation of the considered NIS may attenuate the effect of abiotic filters of its new environment (Schlaepfer et al. 2010; MacDougall et al. 2018). Second, some NIS can undergo rapid evolution within several generations due to strong selective forces acting on them (Huey 2000; Hejda et al. 2009; Jarić and Cvijanovic 2012; Elst et al. 2016).
Biotic interactions, through a complex interplay between NIS and native species, may also condition successful introductions and invasions, and are the focus of numerous theories in invasion ecology. As a major regulator of ecosystem functioning, top-down regulation often occupies a central role in many of these theories (Hunter and Price 1992; Shurin et al. 2002; Heck and Valentine 2007; Riginos and Grace 2008). In the Biotic Resistance Hypothesis (Elton 1958), negative interactions between species, including competition and predation, may allow species-rich communities to resist the establishment of invasive species through their more effective use of resources than species-poor communities. However, despite its popularity in the scientific community, the Biotic Resistance Hypothesis remains controversial (Jeschke et al. 2012). Another explanation for the success of NIS is provided by the Enemy Release Hypothesis (ERH; Keane and Crawley, 2002), which states that NIS may be released at least partially from their biotic regulators (predators, parasites and pathogens). NIS may thus perform better in introduced areas and gain competitive advantages over native species (Keane and Crawley 2002; Joshi and Vrieling 2005; Battini et al. 2021). As host-switching remains rare and as generalist predators expose a certain food selectivity potentially favoring native species, NIS may benefit from predators due to competitive release (Keane and Crawley 2002). These hypotheses provide a complex interplay of ideas in the context of predation and acknowledge the importance of it. Predation may be a key component in determining the success or failure of NIS (Keane and Crawley 2002; Cappuccino and Carpenter 2005; Joshi and Vrieling 2005; Skein et al. 2020; Battini et al. 2021). While these theories have predominantly been tested in terrestrial habitats, few studies have been conducted on marine invasions often with inconsistent results (Chan and Briski 2017).
Fouling communities and especially artificial harbor or marina communities hold many NIS due to the process of primary introduction via fouling on boat hulls and transport in ballast waters (Sylvester et al. 2011; Clarke Murray et al. 2012). The spread of NIS can lead to high economic and ecological costs, revealing the importance of better understanding the factors behind NIS success in these ecosystems (Ojaveer et al. 2015). Often related with human activities, permanent disturbances (press type disturbances) can have profound effects on communities and species (Nimmo et al. 2015). Fouling communities in marinas experience strong anthropogenic disturbances (Dafforn et al. 2015; Todd et al. 2019), which have been the focus of numerous studies showing their effect on local species. Artificial substrata are physically and chemically distinct from their natural counterparts and high concentrations of heavy metals and persistent organic pollutants (POPs) are present (Johnston et al. 2015; Saloni and Crowe 2015; Chase et al. 2016; Kinsella and Crowe 2016). The selection exerted by all these disturbances may even result in differential selection and local adaptation at a very small spatial scale (ca. 50 m) with distinct communities adapted to local conditions (Kawecki and Ebert 2004; Colautti and Lau 2015; Kenworthy et al. 2018b). Despite an apparent focus on abiotic factors, studies have also assessed the applicability of the above-mentioned ecological hypotheses in marine environments. Among these theories, the main focus has laid on biotic resistance to invasions (Kimbro et al. 2013). Biotic resistance due to interspecific competition seems to have a crucial importance on the success or failure of NIS (Fletcher et al. 2018; Gestoso et al. 2018), but interspecific competition may be modulated by predation (Oricchio et al. 2016a). Many studies have shown that predation strongly influences the population dynamics of several fouling species and contributes to the biotic resistance against NIS (Rogers et al. 2016; Rodemann and Brandl 2017; Yorisue et al. 2019). There is however no clear consensus on the importance of predation, with some studies indicating only minor effects masked by abiotic factors, and some others even indicating a facilitation of NIS due to predation on native competitors, probably due to generalist predators’ preference for native prey (Astudillo et al. 2016; Gestoso et al. 2018; Kincaid and de Rivera 2020).
Studies however rarely include the complex interplay of biotic and abiotic factors, revealing the necessity for further research on how the combination of disturbance and generalist predation affects the successful establishment of NIS. In the present study, we aimed to assess these respective influences on the survival of young recruits of two ascidian species, the sea vase tunicate Ciona intestinalis (Linnaeus, 1767) and the Asian clubbed tunicate Styela clava Herdman, 1881, respectively native and non-indigenous in the North East Atlantic. Here we mainly focused on predatory fish, which were considered as a generalist predator model in this study. The recruit stage of tunicates is particularly vulnerable to predation due to its small size and lower structural defense. Recruits may also be more sensitive to environmental stressors (Saloni and Crowe 2015). Based on previous research we expected environmental conditions to vary in space, within the same marina, with levels of disturbance most likely organized in a gradient (Je et al. 2004; Kenworthy et al. 2018b). We hypothesized that this organization results in spatial variation in recruit survival, being lower in the inner part of the marina, where pollution levels are likely maximal. NIS tend to better resist disturbances due to their selection during introduction (Gröner et al. 2011), which should result in better survival than native species in the marina. Predation intensity was predicted to vary in space and to negatively affect both species. However, we predicted that generalist predators prefer native prey, thus having a higher negative impact on the native species.
Materials and methods
Study site
The Marina du Château in Brest, France, was chosen as our study site (48° 22′ 43.4″ N; 4° 29′ 22.1″ W; Fig. 1). This recreational marina is part of a larger marine urban zone with a commercial and military harbor leading to a highly anthropized environment. In this marina numerous artificial substrata like jetties, pillars or pontoons are present, contamination by elemental trace metals (such as copper and lead) and persistent organic pollutants (POPs) is high, and NIS are abundant and diverse (Kenworthy et al. 2018b). We focused on the completely shaded environment of the floating pontoons in our experimental setups because they are the most abundant substratum in the marina. This marina has significant variations in environmental conditions among locations (spaced < 100 m), driving different fouling communities at the entrance, the middle and inner parts of the marina (Kenworthy et al. 2018b). In accordance with Kenworthy et al. (2018b), the same three locations (inner, middle and entrance) were selected which were spaced 80–90 m apart. These locations show higher disturbance (pollution) at the inner part of the marina and a lower disturbance at the entrance due to higher water exchanges with the outer environment.
Study species
To assess the survival of ascidian recruits, we compared a native species and a NIS, being among the most abundant ascidian species in the whole marina. The native Ciona intestinalis (Linnaeus, 1767) is the dominant fouling animal in terms of abundance and biomass in most marinas in Brittany (NW France), including the present one (Bouchemousse et al. 2016). This species is characterized by rapid growth, a high reproduction rate and a short life cycle (Jackson 2008). The clubbed tunicate Styela clava (Herdman, 1881) was introduced from the NE Pacific into European waters, and was first recorded in Plymouth, UK in 1953. Since then, it has spread, constituting a potential pest on oyster and mussel farms (Carlisle 1954; Davis and Davis 2010). Both tunicate species are frequent in disturbed ecosystems such as harbors and marinas (Jackson 2008; Therriault and Herborg 2008; Davis and Davis 2010).
In situ assessment of recruit survival
Large adult individuals (> 15 cm) of both species were sampled in spring from the middle of the marina. Due to slower growth of S. clava, this species was sampled one month (April) before C. intestinalis (May). Both species were transferred into aquaria facilities with circulating sea water. After stabilization and fattening for two weeks to improve gamete production and quality, ca. 30 individuals of each species were randomly selected for reproduction. Individuals of S. clava were separated into two pools to cross-fertilize and dissected by cutting the stipe at the base and opening the branchial sac, revealing the male and female reproductive apparatus. To avoid self-fertilization, oocytes and sperm were separately extracted with a thin pipette. Oocytes were rinsed and isolated from tissue debris on an 80 µm mesh filter with filtered (1.3 µm) seawater. Oocytes were distributed on Petri dishes, using two individuals per dish to maintain variability between recruits. Sperm was assigned to a sperm pool to avoid fertilizing oocytes from the same individual and mixed with filtered seawater. One portion of the sperm pool was introduced into each Petri dish containing the oocytes from different individuals. Similarly, C. intestinalis individuals were dissected to harvest gametes. Oocytes were collected by concentrating them in the narrow part of the oviduct and puncturing them with a glass pipette. Oocytes from different individuals were separated as described above for S. clava. Sperm was sampled similarly to the oocytes, by concentrating the mass in one side of the sacs and puncturing them with a pipette. As described for S. clava, C. intestinalis individuals were separated into different genitor pools and mixed accordingly. For each species, 40 petri dishes with fertilized gametes were created. Cell divisions started at 1 h post fertilization, and larvae appeared after 24 h (seawater at constant 18 °C). Recruits of both species were fed with a mix of Isochrysis affinis galbana Tahiti (T-iso RCC 1349) and Chaetoceros calcitrans (so called ‘Argenton’ strain) strains cultured at the Roscoff Culture Collection (RCC, https://roscoff-culture-collection.org/). They were maintained for 15 days in the dark in a temperature-regulated environment (18 °C). After this period, the Petri dishes were transferred into temperature-regulated aquaria that were brought to match the varying outside water temperature of approx. 19 °C progressively over one week.
The number of C. intestinalis or S. clava individuals successfully attached on each Petri dish was counted and the position of the recruits was marked on the underside of each dish. We selected the 30 dishes with the highest abundance of recruits for each species. If the number of individuals exceeded 50 on a given Petri dish, we reduced it to this threshold by randomly removing individuals to avoid recruits overcrowding. Later, all dishes were individually fixed on 20 cm × 20 cm black PP panels to prevent from direct sunlight and were photographed (Olympus Tough TG5). For each location in the marina, 10 such panels were randomly selected for each species. Half of them were caged with plastic coated iron wire mesh with a 10 mm mesh size to exclude macro-predators, mostly fish (Dumont et al. 2011; Giachetti et al. 2019). Micro-predators and meso-predators such as small crustaceans and gastropods would still have access to caged treatments. The 20 randomly chosen panels (5 caged and 5 uncaged for each species) were suspended under the pontoon at each of the three locations (ca. 1 m depth; > 3 m away from the seafloor; 25 June 2019). After 20 and 28 days, cages and panels (but not the dishes) were cleaned to limit the effects of fouling on the recruits. Dishes were photographed to count the remaining individuals. Although fouling did occur on dishes, individuals stayed visible and could be identified on the dish and by their significantly larger size compared to additional natural recruits of the same species. The experiment ended 50 days after deployment. Dishes were recovered and individuals were counted. At this stage, heavy fouling on the dishes, especially due to marina-recruited C. intestinalis, made it impossible to analyze photos for both in vitro recruited species. Thus, all S. clava individuals were manually counted and collected for drying (1 week at 60 °C) and weighing. All S. clava individuals present at the end of the experiment were lab-grown, as their positions were marked. While at 28 days after deployment of the recruits it was easy to discriminate lab-grown recruits of C. intestinalis from marina-recruited ones due the size difference, it was not possible anymore 50 days after deployment, especially considering that lab-grown recruits’ mortality was high—likely even 100% since only few individuals were recorded at 28 days. We thus chose to consider the survival observations for this species after 28 days rather than at 50 days to avoid a potential underestimation of survival. Statistical analyses were conducted taking this into account. Nevertheless, the biomass of natural C. intestinalis recruits was compared between treatments, as these individuals are also influenced by environmental conditions and predation. The mass of potentially remaining lab-grown recruits can be neglected here due to their very low contribution to the total mass. For each dish, all recruits were collected and dried (1 week at 60 °C) for weighing.
Contaminant assessment
Three sediment samples (ca. 0.4 kg) were taken at ca. 3 to 7 m depth by divers at each of the locations for testing the concentration of Persistent Organic Pollutants (POP) including Polycyclic Aromatic Hydrocarbons (PAHs), Polychlorinated Biphenyls (PCBs) and the most frequent pesticides. Additionally, for each location, five sediment samples (ca. 0.4 kg) were taken to quantify Metallic Trace Elements (MTEs).
The analytical method for PCBs and pesticides is further detailed in our supplementary material and is fully described by Wafo et al. (2006). PCB determination focused on 33 individual congeners (Sup. 4) including target congeners proposed by the International Council for the Exploration of the Sea (ICES) as indicators of PCB contamination, complemented by congeners with high environmental prevalence (Webster et al. 2013). The list of the 16 quantified pesticides can be seen in Table 1. PAHs were determined following Sarrazin et al. (2006), Ratier et al. (2018) and Dron et al. (2019), which is detailed in the supplementary material. We focused on 16 PAH congeners defined by the US Environmental Protection Agency (USEPA) priority list (Table 1, US EPA 2014). Each targeted PAH was identified based on the retention time and the mass spectrum from the chromatogram of standard solutions acquired in full scan mode. Quantification was then performed in the SIM mode for better selectivity.
For the quantification of MTEs the sediment samples were dissolved in a three-acid solution (HCL, HN03, HF) and were then analyzed using High Resolution Inductively Coupled Plasma Mass Spectrometry (HR-ICP-MS; Jacquet et al. 2021). The spectrometer was calibrated via an external calibration method adding In as an internal standard.
Environmental variables
Temperature was monitored at each location with a HOBO® (Onset®) TidbiT v2 temperature logger during the whole duration of the experiment. Light intensity was monitored with a HOBO® (Onset®) Light-Temperature logger.
Feeding assay
In addition to the exclusion cages, predation intensity was directly measured with a feeding assay. This assay consists of 25 dried squid baits tied on fishing line, attached to fiberglass stakes on a 25 m long transect (Duffy et al. 2015). Squid, due to its firm consistence is particularly adapted to long feeding experiments since it does not detach without a strong attack from a larger predator (> 10 cm) and can only be detached as one piece. The experiment thus targets almost exclusively fish (Duffy et al. 2015). However, native velvet swimming crabs Necora puber (Linnaeus, 1767) could also feed on them. This assay has been used in various environments, including artificial habitats (Duffy et al. 2015; Rodemann and Brandl 2017). Here, a modified version of this assay was used and 25 × 3 locations × 3 dates = 225 baits were deployed. A snorkeler counted the remaining baits 1 h, 3 h, 6 h and 24 h after deployment, allowing to use survival analysis rather than comparisons of means, thus providing detail on the bait consumption dynamics (Gauff et al. 2018). The bait was not deployed on the seafloor, because the height of the water column varied under each pontoon, but was suspended 1 m below the pontoons, close to the ascidian recruits. The assay was performed 17, 24 and 31 days after the deployment of ascidian recruits at the inner, middle and entrance locations respectively, with one transect per location and date. Surveillance cameras (2 per transect, GoPro HD4) were installed.
Statistical methods
The survival data resulting from the feeding assay and collected from the ascidian recruits was analyzed using the ‘survival’ package (version 2.41–3, Therneau and Lumley 2017) in R (CRAN, version 3.6.1). The Kaplan–Meier curves for each treatment were established and compared using a log-rank pairwise comparisons with Bonferroni correction to avoid false positives due to multiple tests (Pyke and Thompson 1986; Bretz et al. 2011). The dry mass per S. clava individual as well as the log of the total dry mass of C. intestinalis were compared between caged and uncaged treatments with a Wilcoxon test, and individual differences of dry mass between locations were identified using a Kruskal–Wallis multiple comparison test with the ‘pgirmess’ package (version 1.6.2, Giraudoux et al., 2018). Mean value and standard deviation were calculated for each contaminant. Differences between locations were identified via a Kruskal–Wallis test. Differences in predation intensity between sites were assessed via a Log-Rank test.
Since sediment samples (contaminants), feeding experiments (predation intensity) and the survival rates of ascidian recruits could not be paired unambiguously, we chose to use R to randomly attribute samples according to the locations they are linked to. The generated table was submitted to a nested Cox regression from the ‘nested cohort’ package in R (version 1.3, Katki and Mark 2013). The two models, one for each species of ascidians, tested for the effect of all contaminants for which significative differences between locations were previously identified, the effect of caging and predation intensity (squid bait consumption after 24 h) as well as their interaction, on the survival of ascidian recruits. Predation intensity was here treated as an environmental variable. The interaction term here is required since predation cannot apply to caged individuals. Both models were nested within the Petri dishes which contained several ascidian recruits and location, as well as their interaction term. This process of random pairing and modelization was repeated 105 times and p values and coefficients were saved in a separate table. For each model factor, we calculated the percentage of runs in which it resulted significant (p < 0.05) as well as the mean hazard ratio (the risk increase/decrease caused by the factor). Factors significant in > 25% of runs were considered as worth investigating (factor of interest) and those significant in > 50% as factors with a clear link to survival.
Results
Recruit survival
The Kaplan–Meier survival curve of the recruits showed mortality for both species (Fig. 2). Mortality for S. clava ranged from 15 to 30% within 50 days and from 60 to 100% for C. intestinalis within 28 days. A log-rank multiple comparison showed that survival for C. intestinalis (Fig. 2a) was consistently lower than for S. clava (Fig. 2b; χ2 > 255, p < 0.001). Location had a significant effect on the survival of caged C. intestinalis. Survival was the highest at the inner location, intermediate at the middle and the lowest at the entrance of the marina (χ2 > 32.5, p < 0.001). Caging had a significant influence on C. intestinalis, increasing the survival at the inner and the middle part of the marina compared with the uncaged treatment (χ2 > 13.4, p < 0.01). At the marina entrance, mortality was 100% for the caged and uncaged treatments for this species. No significant effect of caging or location was observed in the log-rank test for S. clava.
Kaplan–Meier survival curves for Ciona intestinalis (a) and Styela clava (b) for the inner, middle and entrance location of the marina. Dashed lines indicate uncaged (U) treatments; solid lines indicate caged (c) treatments. C. intestinalis Entrance C and C. intestinalis Entrance U overlap. S. clava Inner U and S. clava Middle U overlap
Dry mass
After 50 days, the total dry mass of natural C. intestinalis recruits and the dry mass per lab-grown S. clava individual varied between treatments (Fig. 3). The Wilcoxon tests revealed a significant caging effect for both species, but with opposite effects on species’ biomass: higher biomass for C. intestinalis in caged treatments than in uncaged treatments and lower biomass for S. clava in caged treatments than in uncaged treatments (p < 0.05 and p < 0.01, respectively; Fig. 3). Differences in biomass according to site were only identified for uncaged C. intestinalis between the inner and middle locations (Kruskal–Wallis multiple comparisons; adj. p < 0.05).
Logarithm of total dry mass of naturally recruited Ciona intestinalis (a) and dry mass per lab grown Styela clava individual (b) at 50 days. Significant differences between caged and uncaged treatments: * p < 0.05 and ** p < 0.01 (Wilcoxon test). Significant differences within the uncaged C. intestinalis (a-b) p < 0.05 (Dunn test)
Contaminants
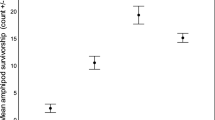
All tested PAHs concentrated in the sediments of the studied marina exceeded Canadian sediment quality guidelines and as well as concentrations at which 20% of sediments tested by the US Environmental Protection Agency would become toxic to model amphipods (Table 1; CCME 1999; US EPA 2005). Chrysene, phenanthrene, fluoranthene and fluorene largely exceeded values at which adverse effect on fauna are highly likely (Table 1). Total PCBs as well as two pesticides, lindane and pp’-DDD, also exceeded most guidelines used for comparison. Two PAHs, tPCB as well as seven pesticides had significant differences in concentration between locations (Kruskal–Wallis, p < 0.05; Table 1).
Almost all MTE were distributed as a gradient from the inner location (max.) to the entrance location (min.). Cu, Pb and Zn showed significant differences between locations (Kruskal–Wallis, p < 0.05). Cu and Zn concentrations in sediment were significantly higher at the inner location compared to the entrance location (Kruskal–Wallis multiple comparisons, adj. p < 0.05). Most of the MTE concentrations were slightly above the Canadian sediment quality guideline and above the concentration of 20% probability of toxicity, falling within a sediment quality category of good or moderate for Cu and Pb (Table 2; CCME 1999; US EPA 2005; Guerra-García et al. 2021).
Environmental variables
Temperature variation between locations was lower than logger precision (< 0.2 °C). Mean light intensity ranged from 7.6 and 8.5 Lux at the entrance and middle respectively to 45.4 Lux at the inner location.
Feeding assay
During the 60 h of video footage, no fish or other predators feeding on the bait were recorded but the black seabream Spondyliosoma cantharus (Linnaeus, 1758) was observed feeding on fouling communities close to the baits. A total of 42 out of 225 baits (18.67%) were consumed in the experiment. Predation intensity was relatively low at all locations, but varied in space, ranging from 10 and 16% at inner and entrance location respectively to 30% (middle location) consumption after 24 h. Using survival analysis on the baits, a log-rank comparison showed a significant difference (p < 0.01) between the middle and the inner location. The entrance location had an intermediate predation intensity not significatively different to either of the two other sites.
Effect of contaminants and predation intensity on recruit survival
The results of the 105 iterations of Cox models after random pairing of samples from the same location showed that while none of the tested contaminants influenced ascidian recruit survival; caging and predation intensity had an influence on C. intestinalis (Table 3). Caging reduced the mortality risk by 22% for C. intestinalis (p < 0.05 in 100% of iterations) while it increased it by 33% for S. clava (p < 0.05 in 86% of iterations). While predation intensity explained only a small fraction of the survival of C. intestinalis (p < 0.05 in 25% of iterations but risk increase of 0%), the interaction between predation intensity and caging seemed to have a stronger association with its survival (p < 0.05 in 52% of iterations, risk increase 1%).
Discussion
Our experiments yielded insights on the survival dynamics of the two species during their juvenile phase. They showed that the native C. intestinalis was strongly affected by environmental heterogeneity and predation (significant differences according to location variability and caged/uncaged treatments, respectively), but that the alien S. clava was less affected by either factor.
The survival of C. intestinalis varied significantly among locations within the marina, following an increasing gradient from the entrance to the inner-most part. Interestingly, the survival gradient was organized in the opposite direction of our prediction. We based this prediction on a sampling of three MTE (Cu, Pb and Zn) in 2016, which revealed a pollution gradient with maximal values in the innermost location of the marina, structuring the community along this gradient (Kenworthy et al. 2018b). In the present study, we confirm significant gradient of Cu, Pb and Zn among locations. Additionally, Benzo[g,h,i]perylene, Fluorene and total PCBs showed significant differences in concentrations among locations, but not as gradient. They had minimal values in the middle location of the marina. Additionally, seven pesticides had significant differences between locations with varying distribution profiles. It is notable that the studied marina sediment largely exceeds adverse effect levels indicated by Canadian (PEL) and USA (50% probability of toxicity) sediment quality guidelines for Chrysene, Fluorene, Fluoranthene and Phenanthrene (PAH) as well as for total PCBs (CCME 1999; US EPA 2005). On the contrary MTE pollution, even if it is above some of these sediment quality guidelines, falls within quality categories described as good or moderate (Guerra-García et al. 2021). Despite the significant effect of location variability on C. intestinalis as shown by the Log-Rank test, none of the contaminants varying significantly between locations could explain the survival profile of any of both tested species in the Cox models. It may be that the sediment contaminant concentrations, which integrate long term tendencies, might not have reflected the very variable water contaminant concentration at the time of our experiment. This also might indicate that another, not measured confounding factor, varied between locations and influenced the survival of this species. Factors like salinity, physical disturbance and hydrodynamics, modulated by the artificial structures could impact the survival of the recruits (Walters and Wethey 1996; Clark and Johnston 2009; Saloni and Crowe 2015; Kenworthy et al. 2018a; Ferrario et al. 2020). Additionally, mesopredators or micropredators like nudibranchs and caprellids, which have been shown to have strong influence on benthic communities, would still have access to the inside of the cages and could thus affect recruit survival independently of caging (Osman and Whitlach 1995; Lavender et al. 2014; Leclerc et al. 2019). If these mesopredators are impacted by contaminants, their abundance could vary in space and be maximal at the entrance of the marina, which would explain the observed survival pattern of C. intestinalis recruits. To disentangle the effect of mesopredators and abiotic factors, future experiments should integrate a way of identifying the effect of smaller predators on survival and integrate more environmental factors (Lavender et al. 2014). The systematically higher survival of S. clava compared to C. intestinalis suggests that this species is more resistant to the environmental variables impacting the survival of C. intestinalis. However, its abundance in marinas of the studied region is much lower than that of C. intestinalis, indicating this survival advantage may only occur at the recruit stage or indicate that C. intestinalis dominates due to its high fecundity and growth rate and not survival (Jackson 2008). The higher survival of S. clava in its recruit stage supports the idea that NIS are more resistant to abiotic stress and is consistent with studies indicating that NIS in marina environments show higher abundance in anti-fouling polluted environments (Dafforn et al. 2008; Piola and Johnston 2008; Piola et al. 2009).
In addition to the location, caging also seemed to strongly affect the survival of C. intestinalis recruits, their survival increased when they were caged. At the entrance location, this effect was however not observable in the Log-Rank test due to the high mortality in both caged and uncaged treatments. This probably also leads to an underestimation of the risk decrease in the Cox model. Predation intensity estimated by the feeding experiment varied in space with maximal predation pressure in the middle of the marina and a minimal pressure at the inner part of the marina. When treated as an explanatory variable, the spatially heterogenous predation intensity may be linked to the survival of C. intestinalis recruits in the Cox models since it slightly reduces their survival in 25% of iterations. The interaction of caging and predation intensity further reveals a potential link between the increased survival in caged treatments and the impact of predators partaking in the feeding experiment. As previously highlighted, the squid-bait feeding experiment targets mostly fish with a probable contribution of swimming crabs. Everything considered, we suggest that a significant part of predation on ascidians may thus be exerted by highly mobile generalist predators. The surveillance cameras installed around the feeding assay observed the black seabream Spondyliosoma cantharus (Linnaeus, 1758) feeding on fouling communities. This species is a plausible predator on ascidian recruits, as multiple Sparidae species can significantly influence marina communities, and also feed on squid bait (Oricchio et al. 2016b; Rodemann and Brandl 2017). Studies frequently demonstrated that C. intestinalis (see Astudillo et al. 2016) and its congener Ciona robusta (Hoshino & Tokioka, 1967) are vulnerable to predation in their respective introduced ranges, highlighting that predators may participate in biotic resistance against them (Dumont et al. 2011; Astudillo et al. 2016; Leclerc et al. 2019; Giachetti et al. 2020). Our results are consistent with observations of the predation on Ciona spp., although in the present case, C. intestinalis was studied in its native range. The most abundant solitary non-indigenous ascidian in the Brest marina, S. clava, was not affected by predation intensity and had a higher mortality risk if caged. Considering the invasive status of S. clava, biotic resistance – as exerted by predators – does not seem to occur in the context of the present study. Styela clava, especially adults are an unappealing food item for predators due to their tough tunic (Clarke and Thomas 2007). Moreover, its congener Styela plicata (Lesueur, 1823) has been shown to be unpalatable to fish due the accumulation of chemically deterrent secondary metabolites in their gonads (Pisut and Pawlik 2002; Koplovitz and McClintock 2011). We suggest that this may also be the case for S. clava as adults or recruits. On the other hand, C. intestinalis has no chemical deterrents with regard to palatability and is readily consumed by crabs and fishes (Teo and Ryland 1994; Koplovitz and McClintock 2011). Our results thus may provide support for the Novel Weapons Hypothesis (NWH), as in the absence of co-evolution of the native species, predator avoidance strategies of a NIS can indirectly result in competitive superiority (Hay et al. 1994; Callaway and Ridenour 2004; Cappuccino and Carpenter 2005). The non-indigenous S. clava may possess a strong predator avoidance system, whereas the native C. intestinalis may be comparatively heavily affected by predation.
The total dry mass of naturally recruited C. intestinalis was significantly lower in uncaged treatments than in caged ones. This difference in dry mass was either due to lower abundance or smaller individual size since predation would cause mortality before recruits could grow in uncaged treatments. In both cases, this difference can be attributed to higher mortality due to predation. Interestingly, no natural S. clava recruits were observed in this study. Having strong differences in reproductive season according to locality, this species seems to reproduce and recruit in late summer/autumn, explaining the absence of natural recruits (Clarke and Thomas 2007). For S. clava, the individual dry mass was significantly higher in uncaged treatments than in caged treatments, suggesting a negative effect of caging on S. clava. This result is further supported by the Cox model indicating a 33% increased risk of mortality when caged. This may appear intriguing because predators cannot directly have a positive influence on S. clava. A cage control treatment could have helped to understand if these results were linked to the presence of the cage itself or due to the influence of predation (Giachetti et al. 2019). Here we were however not able to integrate a cage control treatment as we were not able to produce enough dishes with a sufficient number of recruits of both species in the laboratory for three treatments per location. It might be, that the cage acted as a refuge for meso- and micro-predators, which could feed on ascidians, here especially S. clava, as devoid of predation by macro-predators (Lavender et al. 2014). However, such an effect of mesopredation would uniquely affect survival and not the mean individual mass as it has been affected in our experiment. Furthermore, a previous study conducted in the same marina has shown no effect of caging (protection from large predators) on small mobile fauna (mesopredators) assemblage and abundance (Leclerc and Viard 2018). It thus seems unlikely that the cages acted as refuge for mesopredators and that they may be responsible for the negative effect of caging on S. clava. Another mechanism seems more likely to explain the observed results. When observing photos of the S. clava dishes, it is striking that uncaged S. clava were prominent, while they were smothered by naturally recruited C. intestinalis in caged treatments. We thus think that an indirect positive interaction might be induced by the predation on natural C. intestinalis recruits in uncaged S. clava panels, leading to a decrease in the spatial and trophic competition exerted on S. clava by C. intestinalis (Fig. 4). Considering this potential competition our study case seems to indicate that generalist predators might facilitate the NIS S. clava through competitive release, a specific mechanism of the Enemy Release Hypothesis (ERH; Keane and Crawley 2002). This hypothesis could be confirmed by conducting a similar experiment to ours, but mixing both species’ recruits on the dishes from the beginning.
Caged and uncaged Petri dish of Styela clava recruits after 50 days in the field. Both panels originating from the inner location of the marina. Natural recruits of Ciona intestinalis are more visible (larger, more abundant) in the caged treatment. Individual number refers to final S. clava at 50 days
In the present study, we showed that the survival of the native C. intestinalis was highly influenced by both location and predation, itself variable in space. To date, the very few studies that have focused on the very small-scale spatial variability of communities in marina environments have not considered the possibility of spatially varying predation. For the studied ascidians, we observed a preference of predators for the native prey similar to Cuthbert et al. (2018) and Kincaid and de Rivera (2020). The present results support the idea that generalist predation may play a crucial role in the success of NIS due to facilitation through competitive release (Keane and Crawley 2002; Kincaid and de Rivera 2020). The results also provide a new rationale for how generalist predators may or may not contribute to biotic resistance. Many studies involving Ciona spp. in its introduced range, conclude that various predators exert biotic resistance against this species (Dumont et al. 2011; Leclerc et al. 2019; Giachetti et al. 2020). Here, we showed that in the native range of C. intestinalis, predation exerted by mobile generalist predators potentially leads to the facilitation of a NIS. Thus, the question if biotic resistance against NIS is exerted by predators may depend more on the identity and characteristics of the considered species (native, NIS and predator), rather than on their status (NIS vs native; Skein et al. 2020). Predictions on whether a NIS encounters resistance or facilitation by local predators in its introduced range could thus be formulated by looking at how various types of predation affect it in its native environment.
Data accessibility
All data tables generated or analyzed during this study are included in this published article and its supplementary material files. Photos analyzed shall be shared upon request (corresponding author).
Code availability
Code for the program ‘R’ can be requested upon the corresponding author.
References
Astudillo JC, Leung KMY, Bonebrake TC (2016) Seasonal heterogeneity provides a niche opportunity for ascidian invasion in subtropical marine communities. Mar Environ Res 122:1–10. https://doi.org/10.1016/j.marenvres.2016.09.001
Battini N, Giachetti CB, Castro KL, Bortolus A, Schwindt E (2021) Predator–prey interactions as key drivers for the invasion success of a potentially neurotoxic sea slug. Biol Invasions 23:1207–1229. https://doi.org/10.1007/S10530-020-02431-1
Benton MJ (2009) The Red Queen and the Court Jester: Species diversity and the role of biotic and abiotic factors through time. Science (80-) 323:728–732. https://doi.org/10.1126/science.1157719
Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, Wilson JRU, Richardson DM (2011) A proposed unified framework for biological invasions. Trends Ecol Evol 26:333–339. https://doi.org/10.1016/j.tree.2011.03.023
Bouchemousse S, Bishop JDD, Viard F (2016) Contrasting global genetic patterns in two biologically similar, widespread and invasive Ciona species (Tunicata, Ascidiacea). Sci Rep. https://doi.org/10.1038/srep24875
Bretz P, Hothorn T, Westfall P (2011) Multiple comparisons using R. Taylor & Francis Group, New York
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443. https://doi.org/10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2
Cappuccino N, Carpenter D (2005) Invasive exotic plants suffer less herbivory than non-invasive exotic plants. Biol Lett 1:435–438. https://doi.org/10.1098/rsbl.2005.0341
Carlisle DB (1954) Styela mammiculata n.sp., a new species of ascidian from the plymouth area. J Mar Biol Assoc United Kingdom 33:329–334. https://doi.org/10.1017/S0025315400008365
Carrasco LR, Chan J, McGrath FL, Nghiem LTP (2017) Biodiversity conservation in a telecoupled world. Ecol Soc 22:24. https://doi.org/10.5751/ES-09448-220324
CCME (1999) Protocol for the derivation of canadian sediment quality guidelines for the protection of aquatic life. In: CCME EPC-98E
Chan FT, Briski E (2017) An overview of recent research in marine biological invasions. Mar Biol 164:121. https://doi.org/10.1007/s00227-017-3155-4
Chase AL, Dijkstra JA, Harris LG (2016) The influence of substrate material on ascidian larval settlement. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2016.03.049
Clark GF, Johnston EL (2009) Propagule pressure and disturbance interact to overcome biotic resistance of marine invertebrate communities. Oikos 118:1679–1686. https://doi.org/10.1111/J.1600-0706.2009.17564.X
Clarke CM, Thomas WT (2007) Biological Synopsis of the Invasive Tunicate Styela clava ( Herdman 1881 ) Canadian Manuscript Report of Fisheries and Aquatic Sciences 2807. Fish. Ocean. Canada 2807
Clarke Murray C, Therriault TW, Martone PT (2012) Adapted for invasion? Comparing attachment, drag and dislodgment of native and nonindigenous hull fouling species. Biol Invasions 14:1651–1663. https://doi.org/10.1007/s10530-012-0178-0
Colautti RI, Lau JA (2015) Contemporary evolution during invasion: evidence for differentiation, natural selection, and local adaptation. Mol Ecol 49:1999–2017. https://doi.org/10.1111/mec.13162
Cuthbert RN, Dickey JWE, McMorrow C, Laverty C, Dick JTA (2018) Resistance is futile: lack of predator switching and a preference for native prey predict the success of an invasive prey species. R Soc Open Sci. https://doi.org/10.1098/rsos.180339
Dafforn KA, Glasby TM, Johnston EL (2008) Differential effects of tributyltin and copper antifoulants on recruitment of non-indigenous species. Biofouling 24:23–33. https://doi.org/10.1080/08927010701730329
Dafforn KA, Glasby TM, Airoldi L, Rivero NK, Mayer-pinto M (2015) Marine urbanization: an ecological framework for designing multifunctional artificial structures. Front Ecol Environ. https://doi.org/10.1890/140050
Davis MH, Davis ME (2010) The impact of the ascidian Styela clava Herdman on shellfish farming in the Bassin de Thau. France J Appl Ichthyol 26:12–18. https://doi.org/10.1111/j.1439-0426.2010.01496.x
Dron J, Revenko G, Chamaret P, Chaspoul F, Wafo E, Harmelin-Vivien M (2019) Contaminant signatures and stable isotope values qualify European conger (Conger conger) as a pertinent bioindicator to identify marine contaminant sources and pathways. Ecol Indic 107:105562. https://doi.org/10.1016/j.ecolind.2019.105562
Duffy JE, Ziegler SL, Campbell JE, Bippus PM, Lefcheck JS (2015) Squidpops: a simple tool to crowdsource a global map of marine predation intensity. PLoS ONE 10:e0142994. https://doi.org/10.1371/journal.pone.0142994
Dumont CP, Gaymer CF, Thiel M (2011) Predation contributes to invasion resistance of benthic communities against the non-indigenous tunicate Ciona intestinalis. Biol Invasions 13:2023–2034. https://doi.org/10.1007/s10530-011-0018-7
Elst EM, Acharya KP, Dar PA, Reshi ZA, Tufto J, Nijs I, Graae BJ (2016) Pre-adaptation or genetic shift after introduction in the invasive species impatiens glandulifera? Acta Oecologica 70:60–66. https://doi.org/10.1016/j.actao.2015.12.002
Elton CS (1958) The Ecology of Invasions by Animals and Plants. Springer, US
US EPA (2005) Predicting toxicity to amphipods from sediment chemistry. Natl. Cent. Environ. Assessment, Washington, DC Epa/600/R-
US EPA (2014) EPA priority pollutant list. In AIChE Symp. Ser., Water
Ferrario J, Gestoso I, Ramalhosa P, Cacabelos E, Duarte B, Caçador I, Canning-Clode J (2020) Marine fouling communities from artificial and natural habitats: comparison of resistance to chemical and physical disturbances. Aquat Invasions 15:196–216. https://doi.org/10.3391/AI.2020.15.2.01
Fletcher LM, Atalah J, Forrest BM (2018) Effect of substrate deployment timing and reproductive strategy on patterns in invasiveness of the colonial ascidian Didemnum vexillum. Mar Environ Res. https://doi.org/10.1016/j.marenvres.2018.08.006
Gauff RPM, Bejarano S, Madduppa HH, Subhan B, Dugény EMA, Perdana YA, Ferse SCA (2018) Influence of predation risk on the sheltering behaviour of the coral-dwelling damselfish. Pomacentrus Moluccensis Environ Biol Fishes 101:639–651. https://doi.org/10.1007/s10641-018-0725-3
Gestoso I, Ramalhosa P, Canning-Clode J (2018) Biotic effects during the settlement process of non-indigenous species in marine benthic communities. Aquat Invasions 13:247–259. https://doi.org/10.3391/ai.2018.13.2.06
Giachetti CB, Battini N, Bortolus A, Tatián M, Schwindt E (2019) Macropredators as shapers of invaded fouling communities in a cold temperate port. J Exp Mar Bio Ecol 518:151177. https://doi.org/10.1016/j.jembe.2019.151177
Giachetti CB, Battini N, Castro KL, Schwindt E (2020) Invasive ascidians: how predators reduce their dominance in artificial structures in cold temperate areas. J Exp Mar Bio Ecol 533:151459. https://doi.org/10.1016/j.jembe.2020.151459
Giraudoux P, Antonietti J-P, Beale C, Pleydell D, Treglia M (2018) Package “pgirmess” Title Spatial Analysis and Data Mining for Field Ecologists
Gröner F, Lenz M, Wahl M, Jenkins SR (2011) Stress resistance in two colonial ascidians from the Irish Sea: the recent invader Didemnum vexillum is more tolerant to low salinity than the cosmopolitan Diplosoma listerianum. J Exp Mar Bio Ecol 409:48–52. https://doi.org/10.1016/J.JEMBE.2011.08.002
Guerra-García JM, Navarro-Barranco C, Martínez-Laiz G et al (2021) Assessing environmental pollution levels in marinas. Sci Total Environ 762:144169. https://doi.org/10.1016/j.scitotenv.2020.144169
Hay ME, Kappel QE, Fenical W (1994) Synergisms in plant defenses against herbivores: interactions of chemistry, calcification, and plant quality. Ecology 75:1714–1726. https://doi.org/10.2307/1939631
Heck KL, Valentine JF (2007) The primacy of top-down effects in shallow benthic ecosystems. Estuaries Coasts 30:371–381. https://doi.org/10.1007/BF02819384
Hejda M, Pyšek P, Pergl J, Sádlo J, Chytrý M, Jarošík V (2009) Invasion success of alien plants: do habitat affinities in the native distribution range matter? Glob Ecol Biogeogr 18:372–382. https://doi.org/10.1111/j.1466-8238.2009.00445.x
Huey RB (2000) Rapid evolution of a geographic cline in size in an introduced fly. Science 287:308–309. https://doi.org/10.1126/science.287.5451.308
Hunter MD, Price PW (1992) Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73:724–732. https://doi.org/10.2307/1940152
Jackson A (2008) A sea squirt (Ciona intestinalis). Mar Life Inf Netw Biol Sensit Key Inf Rev. https://doi.org/10.17031/marlinsp.1369.1
Jacquet S, Monnin C, Herlory O et al (2021) Characterization of the submarine disposal of a Bayer effluent (Gardanne alumina plant, southern France): I. Size distribution, chemical composition and settling rate of particles forming at the outfall. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.127695
Jarić I, Cvijanovic G (2012) The tens rule in invasion biology: Measure of a true impact or our lack of knowledge and understanding? Environ Manage 50:979–981. https://doi.org/10.1007/s00267-012-9951-1
Je, J. G., T. Belan, C. Levings, and B. J. Koo. 2004. Changes in benthic communities along a presumed pollution gradient in Vancouver Harbour. Mar Environ Res. 121–135.
Jeschke J, Gómez Aparicio L, Haider S, Heger T, Lortie C, Pyšek P, Strayer D (2012) Support for major hypotheses in invasion biology is uneven and declining. NeoBiota 14:1–20. https://doi.org/10.3897/neobiota.14.3435
Johnston EL, Mayer-Pinto M, Crowe TP (2015) Chemical contaminant effects on marine ecosystem functioning. J Appl Ecol 52:140–149. https://doi.org/10.1111/1365-2664.12355
Joshi J, Vrieling K (2005) The enemy release and EICA hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett 8:704–714. https://doi.org/10.1111/j.1461-0248.2005.00769.x
Katki HA, Mark SD (2013) Survival Analysis of Studies Nested within Cohorts using the Nested Cohort Package.
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241. https://doi.org/10.1111/j.1461-0248.2004.00684.x
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. https://doi.org/10.1016/S0169-5347(02)02499-0
Kenworthy JM, Davoult D, Lejeusne C (2018a) Compared stress tolerance to short-term exposure in native and invasive tunicates from the NE Atlantic: when the invader performs better. Mar Biol 165:1–11. https://doi.org/10.1007/s00227-018-3420-1
Kenworthy JM, Rolland G, Samadi S, Lejeusne C (2018b) Local variation within marinas: effects of pollutants and implications for invasive species. Mar Pollut Bull 133:96–106. https://doi.org/10.1016/j.marpolbul.2018.05.001
Kimbro DL, Cheng BS, Grosholz ED (2013) Biotic resistance in marine environments. Ecol Lett 16:821–833. https://doi.org/10.1111/ele.12106
Kincaid ES, de Rivera CE (2020) Predators associated with marinas consume indigenous over non-indigenous ascidians. Estuaries Coasts. https://doi.org/10.1007/s12237-020-00793-2
Kinsella CM, Crowe TP (2016) Separate and combined effects of copper and freshwater on the biodiversity and functioning of fouling assemblages. MPB 107:136–143. https://doi.org/10.1016/j.marpolbul.2016.04.008
Koplovitz G, McClintock JB (2011) An evaluation of chemical and physical defenses against fish predation in a suite of seagrass-associated ascidians. J Exp Mar Bio Ecol 407:48–53. https://doi.org/10.1016/j.jembe.2011.06.038
Lavender JT, Dafforn KA, Johnston EL (2014) Meso-predators: a confounding variable in consumer exclusion studies. J Exp Mar Bio Ecol 456:26–33. https://doi.org/10.1016/j.jembe.2014.03.008
Leclerc J-C, Viard F (2018) Habitat formation prevails over predation in influencing fouling communities. Ecol Evol 8:477. https://doi.org/10.1002/ece3.3654
Leclerc J-C, Viard F, Brante A (2019) Experimental and survey-based evidences for effective biotic resistance by predators in ports. Biol Invasions. https://doi.org/10.1007/s10530-019-02092-9
Lejeusne C, Latchere O, Petit N, Rico C, Green AJ (2014) Do invaders always perform better? Comparing the response of native and invasive shrimps to temperature and salinity gradients in south-west Spain. Estuar Coast Shelf Sci 136:102–111. https://doi.org/10.1016/j.ecss.2013.11.014
Levine JM, D’Antonio CM (2003) Forecasting Biological Invasions with Increasing International Trade. Conserv Biol 17:322–326. https://doi.org/10.1109/CEIT.2015.7233181
Lewis JS, Farnsworth ML, Burdett CL, Theobald DM, Gray M, Miller RS (2017) Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci Rep. https://doi.org/10.1038/srep44152
MacDougall AS, McCune JL, Eriksson O, Cousins SAO, Pärtel M, Firn J, Hierro JL (2018) The Neolithic Plant Invasion Hypothesis: the role of preadaptation and disturbance in grassland invasion. New Phytol 220:94–103. https://doi.org/10.1111/nph.15285
Marie AD, Smith S, Green AJ, Rico C, Lejeusne C (2017) Transcriptomic response to thermal and salinity stress in introduced and native sympatric Palaemon caridean shrimps. Sci Rep. https://doi.org/10.1038/s41598-017-13631-6
Nimmo DG, Mac Nally R, Cunningham SC, Haslem A, Bennett AF (2015) Vive la résistance: reviving resistance for 21st century conservation. Trends Ecol Evol 30:516–523. https://doi.org/10.1016/j.tree.2015.07.008
Ojaveer H, Galil BS, Campbell ML et al (2015) Classification of non-indigenous species based on their impacts: considerations for application in marine management. PLoS Biol 13:1–13. https://doi.org/10.1371/journal.pbio.1002130
Oricchio FT, Flores AAV, Dias GM (2016a) The importance of predation and predator size on the development and structure of a subtropical fouling community. Hydrobiologia 776:209–219. https://doi.org/10.1007/s10750-016-2752-4
Oricchio FT, Pastro G, Vieira EA, Flores AAV, Gibran FZ, Dias GM (2016b) Distinct community dynamics at two artificial habitats in a recreational marina. Mar Environ Res 122:85–92. https://doi.org/10.1016/j.marenvres.2016.09.010
Osman RW, Whitlach RB (1995) Predation on early ontogenetic life stages and its effect on recruitment into a marine epifaunal community. Mar Ecol Prog Ser 117:111–126. https://doi.org/10.3354/meps117111
Pejchar L, Mooney HA (2009) Invasive species, ecosystem services and human well-being. Trends Ecol Evol 24:497–504. https://doi.org/10.1016/j.tree.2009.03.016
Piola RF, Johnston EL (2008) Pollution reduces native diversity and increases invader dominance in marine hard-substrate communities. Divers Distrib 14:329–342. https://doi.org/10.1111/j.1472-4642.2007.00430.x
Piola RF, Dafforn KA, Johnston EL (2009) The influence of antifouling practices on marine invasions. Biofouling 25:633–644. https://doi.org/10.1080/08927010903063065
Pisut DP, Pawlik JR (2002) Anti-predatory chemical defenses of ascidians: Secondary metabolites or inorganic acids? J Exp Mar Bio Ecol 270:203–214. https://doi.org/10.1016/S0022-0981(02)00023-0
Pyke DA, Thompson JN (1986) Statistical Analysis of Survival and Removal Rate Experiments. Ecology 67:240–245. https://doi.org/10.2307/1938523
Ratier A, Dron J, Revenko G, Austruy A, Dauphin CE, Chaspoul F, Wafo E (2018) Characterization of atmospheric emission sources in lichen from metal and organic contaminant patterns. Environ Sci Pollut Res 25:8364–8376. https://doi.org/10.1007/s11356-017-1173-x
Riginos C, Grace JB (2008) Savanna tree density, herbivores, and the herbaceous community: Bottom-up vs. top-down effects. Ecology 89:2228–2238. https://doi.org/10.1890/07-1250.1
Rodemann JR, Brandl SJ (2017) Consumption pressure in coastal marine environments decreases with latitude and in artificial vs. natural habitats. Mar Ecol Prog Ser 574:167–179. https://doi.org/10.3354/meps12170
Rogers TL, Byrnes JE, Stachowicz JJ (2016) Native predators limit invasion of benthic invertebrate communities in Bodega Harbor, California, USA. Mar Ecol Prog Ser. https://doi.org/10.3354/meps11611
Saloni S, Crowe TP (2015) Impacts of multiple stressors during the establishment of fouling assemblages. Mar Pollut Bull 91:211–221. https://doi.org/10.1016/j.marpolbul.2014.12.003
Sarrazin L, Diana C, Wafo E, Pichard-Lagadec V, Schembri T, Monod JL (2006) Determination of polycyclic aromatic hydrocarbons (PAHs) in marine, brackish, and river sediments by HPLC, following ultrasonic extraction. J Liq Chromatogr Relat Technol 29:69–85. https://doi.org/10.1080/10826070500362987
Schlaepfer DR, Glättli M, Fischer M, van Kleunen M (2010) A multi-species experiment in their native range indicates pre-adaptation of invasive alien plant species. New Phytol 185:1087–1099. https://doi.org/10.1111/j.1469-8137.2009.03114.x
Seebens H, Schwartz N, Schupp PJ, Blasius B (2016) Predicting the spread of marine species introduced by global shipping. Proc Natl Acad Sci USA 113:5646–5651. https://doi.org/10.1073/pnas.1524427113
Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS (2002) A cross-ecosystem comparison of the strength of trophic cascades. Ecol Lett 5:785–791. https://doi.org/10.1046/j.1461-0248.2002.00381.x
Siegel S, Castellan-Jr NJ (1988) Nonparametric statistics for the behavioral sciences, International. McGraw-Hill Book Company, New York
Skein L, Alexander ME, Robinson TB (2020) Characteristics of native predators are more important than those of alien prey in determining the success of biotic resistance in marine systems. Aquat Ecol. https://doi.org/10.1007/s10452-020-09814-5
Sylvester F, Kalaci O, Leung B et al (2011) Hull fouling as an invasion vector: Can simple models explain a complex problem? J Appl Ecol 48:415–423. https://doi.org/10.1111/j.1365-2664.2011.01957.x
Teo L-M, Ryland JS (1994) Toxicity and palatability of some British ascidians. Springer, Berlin
Therneau TM, Lumley T (2017) Package “survival.”
Therriault TW, Herborg L-M (2008) A qualitative biological risk assessment for vase tunicate Ciona intestinalis in Canadian waters: using expert knowledge. ICES J Mar Sci 65:781–787. https://doi.org/10.1093/icesjms/fsn059
Todd PA, Heery EC, Loke LHL, Thurstan RH, Kotze DJ, Swan C (2019) Towards an urban marine ecology: characterizing the drivers, patterns and processes of marine ecosystems in coastal cities. Oikos 128:1215–1242. https://doi.org/10.1111/oik.05946
Wafo E, Sarrazin L, Diana C, Schembri T, Lagadec V, Monod JL (2006) Polychlorinated biphenyls and DDT residues distribution in sediments of Cortiou (Marseille, France). Mar Pollut Bull 52:104–107. https://doi.org/10.1016/j.marpolbul.2005.09.041
Walters LJ, Wethey DS (1996) Settlement and early post-settlement survival of sessile marine invertebrates on topographically complex surfaces: the importance of refuge dimensions and adult morphology. Mar Ecol Prog Ser 137:161–171. https://doi.org/10.3354/meps137161
Webster L, Roose P, Bersuder B, Kotterman M, Haarich M, Vorkamp K (2013) Determination of polychlorinated biphenyls (PCBs) in sediment and biota. ICES Tech Mar Environ Sci 53:18. https://doi.org/10.25607/OBP-237
Williamson M, Fitter A (1996) The varying success of invaders. Ecology 77:1661–1666
Yorisue T, Ellrich JA, Momota K (2019) Mechanisms underlying predator-driven biotic resistance against introduced barnacles on the Pacific coast of Hokkaido. Japan Biol Invasions. https://doi.org/10.1007/s10530-019-01980-4
Acknowledgements
This work benefited from access to the Station Biologique de Roscoff, an EMBRC-France and EMBRC-ERIC Site. We warmly thank the Centre de Ressources Biologiques Marines (CRBM) at the Station Biologique de Roscoff for its technical support and the reproduction of ascidian recruits. We also want to thank Bastien Taormina and Elyne Dugény for their support during the feeding experiment and Noémie Coulon and Brian Sevin for helping to count surviving recruits. We thank Carolyn Engel-Gautier for English proofreading and Stéphanie Jacquet for analysing MTE contaminants in sediment samples. We also want to thank the reviewers of this article for their valuable contributions.
Funding
Funding for this project has been provided through a PhD grant from the Sorbonne Université—Museum National d’Histoire Naturelle (Ecole Doctorale 227) and a field mission grant from the Société Française d’Ecologie et Evolution to Robin Gauff. Financial support was also provided by the INEE-CNRS’ PEPS Ecomob grant ‘InPor’ (PIs: C. Lejeusne and D. Davoult).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no potential conflicts of interest with any organization or entity with financial or non-financial interest in the subject discussed in this manuscript.
Ethical standards
We followed national and international ethical guidelines whenever applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gauff, R.P.M., Lejeusne, C., Arsenieff, L. et al. Alien vs. predator: influence of environmental variability and predation on the survival of ascidian recruits of a native and alien species. Biol Invasions 24, 1327–1344 (2022). https://doi.org/10.1007/s10530-021-02720-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02720-3