Abstract
Crabs are some of the most successful introduced species among marine organisms, and they can be an important structuring force in marine communities. Recently, the North American white-fingered mud crab, Rhithropanopeus harrisii, has invaded the Northern Baltic Sea. This is an area where no native crab species exist, and the addition of a novel functional species to the low species diversity of the Baltic Sea could have large community-level impacts i.e. modifying biotic interactions and/or altering ecosystem functioning. We examined the predatory behavior of introduced R. harrisii both in the laboratory and field focusing in shallow, hard bottom habitats dominated by the alga Fucus vesiculosus. In the laboratory environment, R. harrisii was an effective predator of littoral grazers, readily consuming both sessile fauna (Mytilus trossulus) and also mobile species such as isopods (Idotea balthica) and gammarid amphipods (Gammarus sp.). When studying the predation of different sized prey items, R. harrisii preyed upon small and medium sized prey of both mobile and sessile species. However, in the field experiment with the native faunal community associated with F. vesiculosus, R. harrisii negatively impacted only the abundance of the snail Theodoxus fluviatilis, possibly through indirect effects. Nevertheless, R. harrisii significantly decreased both the prey species richness and diversity but not the total number of potential prey individuals associated with F. vesiculosus. In conclusion, predatory behavior of this novel crab has the potential to impact the native macroinvertebrate littoral community, but the realized predation pressure in the field is lower than could be expected from laboratory experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among marine organisms, crabs are some of the most successful introduced species (Roche and Torchin 2007; Compton et al. 2010). While the ability to establish populations may be mostly due to the physiological and/or biological attributes of the invader, long-term success can depend on the ecological structure of the invaded habitat. Invasive crabs can be an important structuring force in marine communities by altering habitats and ecosystem functioning (i.e. primary production, decomposition, hydrology, nutrient cycling, disturbance regimes) and/or by modifying biotic interactions by changes in the abundance, distribution, and behaviors of other native or introduced species (Glude 1955; Hanks 1961; Lubchenco 1978; Grosholz and Ruiz 1995; Grosholz et al. 2000; Brousseau et al. 2001; Walton et al. 2002; Ross et al. 2004; Rudnick et al. 2005; Hollebone and Hay 2008; Kimbro et al. 2009). Generalist species tend to fare well as invaders (Lefebvre et al. 2004; Vazquez 2006; Weis 2010), and many successful invasive crab species have been shown to have opportunistic, broad diets that enable them to survive in various communities [e.g. Carcinus maenas (Cohen et al. 1995), Charybdis hellerii (Dineen et al. 2001), Eriocheir sinensis (Rudnick and Resh 2005), Hemigrapsus sanguinesus (Ledesma and O’Connor 2001), Charybdis japonica (Fowler, unpublished data)].
Simple low-diversity communities are more susceptible to invasions than more diverse communities (Elton 1958; Tilman 1999; Naeem et al. 2000; Stachowicz et al. 2002; Kennedy et al. 2002; but see Lonsdale 1999; Stohlgren et al. 1999) and provide a unique opportunity to detect the impacts of introduced species as well as to test the eco-evolutionary feedback mechanisms affecting community function (Strauss 2014). However, the predatory effects of introduced species on native species are often speculated but rarely empirically tested (but see Grosholz et al. 2000), even though species interactions in newly invaded habitats would provide an excellent natural experiment concerning both direct and indirect effects e.g. the top-down control of food webs. The impacts of introduced species on native community structure can be relatively pronounced, especially in areas, such as the Baltic Sea, with a depauperate species assemblage and simple food webs (Paavola et al. 2005; Strauss 2014). The northern Baltic Sea lacks any native crab species (Bonsdorff 2006), and therefore the introduction of a novel species can be assumed to form a new functional link in the food web and cause alterations in community structure and functioning that may expand into effects on ecosystem properties.
One such novel species is the North American white-fingered mud crab Rhithropanopeus harrisii, which arrived to southern parts of the Baltic Sea in 1936 (Schubert 1936) and rapidly spread along the southern Baltic coast (Demel 1953; Wolff 1954; Bacevičius and Gasiūnaitė 2008; Kotta and Ojaveer 2012). In 2009, it was discovered in the Archipelago Sea along the southwestern coast of Finland (Karhilahti 2010), and the population has continued to spread along the coast (Fowler et al. 2013). Little is known about the prey choice of R. harrisii in either native or introduced regions, and there is no information about the trophic position of R. harrisii in the northern Baltic Sea communities. The only studies on the feeding behavior of R. harrisii have been conducted in the crab’s introduced range in the southern Baltic (i.e. Poland). Stomach content analyses from the southern Baltic demonstrate that R. harrisii is omnivorous (Turoboyski 1973) and feeds on sessile and mobile macroinvertebrates such as mussels, ragworms, hydroids, gastropods, amphipods, on green algae (Turoboyski 1973; Hegele-Drywa and Normant 2009), and dead organic material (Czerniejewski and Rybczyk 2008). However, with such a broad diet, it could be hard to isolate the impact of this omnivorous species on the food web without knowing the prey choice of the predator.
Given that this information is vital to critically evaluate the effects of R. harrisii on food web structure in the northern Baltic Sea, we observed R. harrisii prey choice in laboratory and field experiments. While native and introduced populations of R. harrisii have been found in various types of habitats (e.g. soft bottom, mixed bottom, reed grass, oyster reefs, etc.), in the study area R. harrisii is also found among habitat forming Fucus vesiculosus (Fowler et al. 2013). F. vesiculosus is considered to be the keystone species in hard and rocky bottom subtidal habitats in the Baltic Sea, which has been designated as a national habitat type of importance in Finland due to its function as habitat for several mobile and sessile invertebrates, fish, and filamentous macroalgae species (Kautsky et al. 1992; Wikström and Kautsky 2007). Because of this designation and the possibility that R. harrisii could impact food webs associated with F. vesiculosus, we studied prey choice of R. harrisii among potential prey species and prey-size classes from F. vesiculosus habitats in the laboratory and then evaluated whether the results from the laboratory experiments were applicable to field conditions. To our knowledge, this is the first time that R. harrisii prey choice and community impacts have been studied in either a native or introduced population.
Materials and methods
Laboratory prey choice assays
Diet composition
Individual R. harrisii were collected between June and July 2011 from three sites (N60°23′ E22°02′, N60°22′ E22°03′, and N60°24′ E22°07′) in the Archipelago Sea using artificial collectors (30 × 30 × 30 cm plastic crates filled with dead, autoclaved oyster shells from Maryland, USA). The collectors have been used successfully in previous studies (Roche et al. 2009; Fowler et al. 2013). R. harrisii individuals were brought to the Archipelago Research Institute in Seili, Nauvo (N60°14′ E21°60′) and maintained in aerated, natural, unfiltered seawater (6 ‰ salinity) at a room temperature of 15 °C under a light regime of 8L:16D. R. harrisii were fed daily with Chironomidae larvae, which they consumed readily. During this period, we avoided feeding R. harrisii with the prey species later used in the experiments as that might affect their feeding behavior.
In order to compare the relative predation pressure on mobile and sessile prey species, we conducted the first experiment on July 26, 2011 using 29 crabs (3 females and 26 males, average carapace width ± SD = 16.2 ± 3.38 mm, range 6.5–22.25 mm). Four prey species were chosen as potential food items based on known prey species of R. harrisii according to other studies and prey prevalence in F. vesiculosus dominated littoral habitat that R. harrisii has invaded in southwestern Finland. The prey species included the blue mussel Mytilus trossulus, gastropod snail Theodoxus fluviatilis, gammarid amphipods, and juvenile gobies (Gobiidae), all of which were collected from the waters around Seili. For the experiment, we visually chose prey items of the same size within a species, with the exception of blue mussels. Blue mussel sizes were chosen to correspond to the carapace width of R. harrisii (i.e. smaller crabs were presented with smaller mussels) so that the mussel size did not exceed the maximum size that R. harrisii can open (e.g. Milke and Kennedy 2001). All of the chosen prey items were smaller than the maximum carapace width of R. harrisii individuals used in the experiment.
Before the start of the experiment, R. harrisii were not fed for 12 h. This time was kept relatively short to increase the probability of actual prey choice and prevent crabs from consuming the first encountered prey item due to starvation. Individual R. harrisii were placed in plastic aquaria (23 × 13 × 14 cm) containing 2.7–3.0 liters of seawater at 15 °C and 6 ‰ salinity with a piece of a clay pot for shelter. They were allowed to acclimate for 7 h, which was part of the 12 h starvation period, before the start of the experiment. After acclimation, we added four prey individuals to each aquarium (1 M. trossulus, 1 T. fluviatilis, 1 gammarid amphipod, 1 Gobiidae sp.). All replicates (n = 29 crabs) were run simultaneously overnight in the dark, with one R. harrisii individual per aquarium. The experiment was concluded after 12 h. At that time, R. harrisii were removed, and the remaining prey species were counted. Prey species were considered consumed if the prey item was not found in the tank or if remnants of the prey were found (i.e. pieces of mussel shell).
In order to compare the relative predation pressure on various mobile littoral invertebrates that occupy a similar niche, we conducted another experiment on July 28, 2011 using 27 crabs from the experiment described above (average carapace width ± SD = 16.2 ± 3.38 mm, range 6.5–22.25 mm). The prey species used in this experiment, with exactly the same experimental set up as described above, were juvenile shrimp (Palaemon elegans or P. adspersus), juvenile isopods (Idotea sp.), and juvenile amphipods (Gammarus sp.). All individuals of the same prey species were approximately the same size as determined by visual inspection. After R. harrisii had been added to the experimental aquarium and allowed to acclimate for 7 h, we added the three prey items (1 Palaemon sp., 1 Idotea sp., 1 Gammarus sp.). The experiment was concluded after 12 h, and R. harrisii were removed. The remaining prey individuals were counted. If the prey item was not found in the tank or if remnants of the prey were found (i.e. pieces of mussel shell), the individual was considered consumed.
To control for natural mortality of the prey species in both experiments, we ran control aquaria with the same set up as described above but without the crab (n = 30 for each prey item). All prey items were alive and present after the conclusion of the experiment.
Prey choice dependent on species and size
In order to determine whether R. harrisii has the same impact on the prey’s different life stages (i.e. juvenile and adult), we examined whether R. harrisii exhibited any prey size choice. We used three prey species (M. trossulus blue mussels, gammarid amphipods, and Idotea balthica isopods). These species were chosen based on the prey choice of R. harrisii from the previous experiments. However, for this experiment we used multiple, different-sized individuals of each prey species to include both juvenile and adult life stages. Within the prey species, size variation was determined from individuals collected from the vicinity of Seili Island, and prey items were divided into three different size classes: small, medium, and large. Blue mussels were measured (maximum length) with electronic calipers and divided as follows: small 5–10 mm, medium 12–15 mm, and large 17–27 mm. The gammarid amphipods were weighed with an electronic balance after drying the animals quickly between paper tissues and divided as follows: small < 20.2 mg, medium 21.0–34.0 mg, and large > 35.5 mg. The isopods were also dried quickly, weighed and divided as follows: small < 29.9 mg, medium 30.6–49.7 mg, and large > 50.0 mg. Weighed and measured individuals of all prey species were used for the experiment within 6 h of the measurement process.
This experiment was conducted on September 13, 2011 using 30 crabs (8 females and 22 males, average carapace width ± SD = 16.74 ± 2.49 mm, range 10.8–19.95 mm). Individual R. harrisii were collected in September 2011 from the same sites as for the experiments above and treated as described above. Before the start of the experiment, R. harrisii were not fed for 24 h to standardize the hunger levels. Individual R. harrisii were placed into plastic aquaria (23 × 13 × 14 cm) containing 2.7–3 liters of seawater with a layer of sand on the bottom, and a piece of F. vesiculosus was also provided for shelter for prey species. R. harrisii were allowed to acclimate for 20 h at 15 °C under a light regime of 8L:16D, after which two live individuals from each size class of all three prey species were added simultaneously to each aquarium (n for each prey species was 6, total n = 18 of all prey items in each aquarium). All replicates (n = 30 crabs, one individual per aquarium) were run simultaneously, and thus each crab was only used once.
After 24 h, R. harrisii were removed from the aquaria, and the prey items consumed were recorded. Prey species were considered consumed as previously described. During the experiment R. harrisii did not consume or damage any F. vesiculosus in the aquaria. To determine natural mortality over the course of the experiment, we ran five control aquaria with the same numbers of prey species introduced to replicate tanks without R. harrisii. All prey items were alive after 24 h.
Field experiment
We studied the ecological impact of R. harrisii on the natural littoral invertebrate community associated with F. vesiculosus in a sheltered bay near the Archipelago Research Institute in Seili, Nauvo (N60°14′ E21°60) where R. harrisii are readily observed (TF, AEF, OV pers. obs.). Due to the unethical transfer of introduced species for experimental purposes, we chose a site for the field experiment that was already colonized by R. harrisii. We conducted an enclosure experiment by placing 0.5 × 0.5 cm mesh cages around small bushes of F. vesiculosus. The experiment had three treatments: (1) “no predation”, (2) “natural predation” and (3) “crab predation”. (1) The “no predation” treatment included the natural faunal community associated with F. vesiculosus and excluded all predators (i.e. fish, birds, crabs) with 0.5 × 0.5 cm mesh cages, (2) the “natural predation” treatment included all predators as it had no cages, and (3) the “crab predation only” treatment included R. harrisii as the only predator and excluded other predators with mesh cages. The presence of the crab was ensured by tethering one experimental R. harrisii with clear fishing line from the back of their carapace to the mesh cage. Due to R. harrisii being an introduced species, we tethered crabs to the cages to ensure that they stayed within the enclosure for the duration of the experiment and did not escape. Tethering has been used previously in many field experiments without affecting crab behavior (e.g. Holdredge et al. 2009; Coverdale et al. 2013; Bishop and Byers 2015). The “no predation” treatment controlled for the possibility of predation not affecting the community and also allowed us to determine the effect of the enclosure itself. Individual R. harrisii for the “crab predation” treatment were collected from vicinity of the Seili using the artificial collectors described previously.
On June 4, 2013, F. vesiculosus was collected from the vicinity of Seili and cleaned of all other algae and invertebrates. To obtain bushes of a standard size between 9 and 12 grams, F. vesiculosus wet weights were measured after shaking the algae for 5 s. F. vesiculosus were then attached to bricks with cable ties, and floats were attached to the stalk to keep the algae in a vertical position in the water column. Bricks and algae were placed into flow-through seawater tanks until the start of the experiment. On June 10, 2013, bricks were placed to the sea approximately 1 m from each other along five horizontal lines with 20 bricks in each line, at a depth of 85–120 cm. The experimental lines of bricks were separated with lines of bricks with non-experimental F. vesiculosus to encourage the natural recruitment of littoral invertebrates to the area. F. vesiculosus were allowed to accumulate natural densities of macroinvertebrates until July 5 (3.5 weeks), whereupon we added the mesh cages (mesh size 0.5 × 0.5 cm) around the experimental bushes of F. vesiculosus. This was done by lifting the branches of the F. vesiculosus carefully up from the brick, adding the cage around it, and closing the bottom of the cage around the stipe of the F. vesiculosus. This prevented the immigration of R. harrisii into the experimental cages from the natural environment. For the no cage treatment, we lifted the algae up to mimic the handling of the other treatments. There were 20 replicates of each treatment (total n = 60), arranged systematically so that similar treatments were not next to each other.
The experiment was concluded on July 9, 2013, after 4 days of enclosure. The bricks and F. vesiculosus were carefully lifted into net bags to retain all of the macroinvertebrates. All R. harrisii were retrieved alive and still attached to the cage by their tether. All net bags with bricks were brought into the laboratory, and all associated macroinvertebrates were identified and counted. Epiphytic algae were removed, and dry weights of the epiphytes were measured for each experimental F. vesiculosus. The depth below the surface for each F. vesiculosus was also measured. During the experiment R. harrisii did not consume or damage any of the experimental F. vesiculosus. When concluding the experiment, one non-experimental R. harrisii was found hiding in a hole in a brick outside of a “crab predation” experimental cage, thereby confirming that R. harrisii was naturally present in this system over the course of the field experiment.
Statistical analyses
Laboratory prey choice assays
In all laboratory experiments, prey items were considered either alive or consumed by R. harrisii at the end of the trial. In all three experiments, the binary response variable was the fate of the prey item (consumed or alive). The binomial data were statistically analyzed as a mixed model using the Generalized Estimating Equations with GENMOD procedure (SAS Institute 9.2) to examine how prey species, prey size class, R. harrisii size, or their interactions contributed to prey survival. Because the predation risk of an individual prey item depended on how many prey items were present in the aquarium, we used the aquarium as a repeated factor to control for the dependency of the fate of the prey items within each aquarium. This analysis examines the relative fate of the prey items while also taking into account that their fates are interdependent. In all analyses, the best-fit model was found by simplifying the full model, which initially included the main factors (including covariates) and all the possible interactions (between main factors), on the basis of Akaike’s Information Criterion (AICs values; the final model with the smallest AIC value (Δ > 2) was selected). In the first two experiments, the final models included only the prey species and the size of R. harrisii, which acted as a covariate. In the third experiment of the consumption of different sized prey items, the explanatory variables in the final model were prey species, size class of the prey, weight of the F. vesiculosus and size of R. harrisii. The latter two were considered covariates of the model. Interactions in the final model were prey species x size class of the prey and the size of R. harrisii × size class of the prey. These models met the assumption of homogeneity of variances based on visual examination of diagnostic plots of residuals for all analyses. All pairwise comparisons were made using the ESTIMATE statement in the GENMOD procedure, and, as there were multiple comparisons of the same data, the results were Bonferroni corrected by multiplying the achieved P values with the number of pairwise comparisons. We used a P value of 0.05 as the statistical significance level in all of the analyses.
Field experiment
We extracted seven response variables from the field data to study different aspects of the F. vesiculosus communities: the total number of species, total number of all macroinvertebrate individuals, Shannon-Wiener diversity index, number of Theodoxus snails, number of Idotea isopods, number of gammarid amphipods, and number of blue mussels. The Shannon-Wiener diversity index (H’) was calculated using the following equation: H′ = ∑Pi*ln(Pi), where Pi is the relative abundance of species i (Magurran 2004). All seven response variables were analyzed separately. All of the final models described below were simplified by reducing non-significant interactions from the full model containing all the possible interaction effects on the basis of Akaike’s Information Criterion (AICs values; the final models with smaller AIC values (Δ > 2) were selected). We used the treatment (“natural predation”, “crab predation”, or “no predation”) as explanatory variables in all final models. The depth of the bricks and the dry weight of epiphyte algae, both continuous variables, could possibly affect the number of species and individuals in each F. vesiculosus bush, so they were included in the final models as covariates. The interaction of the covariates depth and algae weight were also included in the final models that analyzed the total number of macroinvertebrate individuals and the number of gammarid amphipods. Because the enclosures were placed in three lines, we added the line number into the final models as a random factor that takes into account spatial variation in communities. The variation in macroinvertebrate community composition was statistically analyzed using a generalized linear mixed model and normal distribution with identity link function (Shannon-Wiener diversity index and number of species) and negative binomial distribution with log link function (the number of individuals of isopods, amphipods, Theodoxus snails and blue mussels). Model assumptions of normality and/or homogeneity of variances were assessed by visual examination of diagnostic plots of residuals for all analyses. Pairwise comparisons were conducted using the Tukey–Kramer method, and the adjusted P values are shown. All analyses were conducted with GLMM procedure (SAS Institute 9.2).
Results
Prey choices for species and size-classes in laboratory assays
Predation risk varied between the four different prey species when presented together (χ2 = 21.97, df = 3, P = <0.001). R. harrisii consumed more blue mussels (χ2 = 11.06, P = 0.003) and gammarid amphipods (χ2 = 11.24, P = 0.003) than Theodoxus snails or gobies (Fig. 1a; blue mussels vs gobies χ2 = 14.85, P = <0.001; gammarid amphipods vs gobies χ2 = 16.88, P = 0.001; gammarid amphipods vs blue mussels χ2 = 0.39, P = 0.53), but the total number of consumed snails and gobies did not differ significantly from each other (χ2 = 4.41, P = 0.07). The size of R. harrisii did not affect which prey species was consumed or how many individuals were preyed upon (χ2 = 0.43, df = 1, P = 0.5). Also in the second prey choice experiment with three crustacean prey species, predation risk varied among the species (χ2 = 21.00, df = 2, P = <0.001). Isopods were preyed upon more heavily than the other species (isopods vs gammarid amphipod χ2 = 7.00, P = 0.008; isopods vs shrimp χ2 = 32.74, P = <0.001) and gammarid amphipods more heavily than the shrimp (Fig. 1b; χ2 = 19.05, P = <0.001). Although statistically non-significant, smaller R. harrisii consumed slightly more of these small juvenile prey items than larger R. harrisii individuals. The size of R. harrisii did not affect its food choice (χ2 = 3.46, df = 1, P = 0.06).
The average percent (±95 % CI) of prey consumed within each prey species by a single R. harrisii (n = 29 for a and 27 for b) over a 12 h period. Different letters above the bars indicate significantly different values (P < 0.05). The total number of individuals eaten for each species is noted in parentheses
When presented with three different size classes of blue mussels, isopods, and gammarid amphipods, the prey size class had a significant impact on whether it was consumed or not (prey size class χ2 = 7.50, df = 2, P = 0.024). R. harrisii consumed mainly small and medium sized individuals from each of the different prey species (Fig. 2; χ2 = 5.65, df = 4, P = 0.227). Also, the prey species had a significant effect on whether it was consumed (χ2 = 20.00, df = 2, P = <0.001). R. harrisii consumed isopods (χ2 = 21.84, P = <0.001) and gammarid amphipods (χ2 = 27.61, P = <0.001) over blue mussels, but the total number of consumed isopods and gammarid amphipods did not differ significantly from each other (χ2 = 1.49 P = 0.222). R. harrisii consumed prey items at the same rate regardless of their own size (χ2 = 1.01, df = 1, P = 0.3), but there was a marginally non-significant but biologically interesting interaction between the size class of the prey item and the size of R. harrisii (χ2 = 5.25, df = 2, P = 0.072). Large R. harrisii consumed slightly larger prey items than small R. harrisii. The weight of the algae in the aquarium did not affect consumption (χ2 = 2.10, df = 1, P = 0.1).
The average percent (±95 % CI) of size classes (small, medium, large) consumed within each prey species (isopod Idotea sp., amphipods of the Gammarus sp., blue mussel M. trossulus) by a single R. harrisii (n = 30) over a 24 h period. The total number of individuals eaten for each species is noted in parentheses (total n = 60 per size class per species)
Effects of mud crabs on natural prey communities
In the field experiment on macroinvertebrate communities on F. vesiculosus, the treatment affected the mean number of prey species found occupying F. vesiculosus (Table 1), with a lower mean number of prey species in the “crab predation” treatment (4.9 SE ± 0.5; t53 = −2.39, P = 0.05) and the “natural predation” treatment (4.6 ± 0.5; t53 = −2.53, P = 0.038) compared to “no predation”(6.0 ± 0.5), but the predation treatments did not differ from each other (t53 = 0.55, P = 0.8). Depth or the dry weight of the epiphyte algae on the F. vesiculosus did not affect the mean number of prey species (Table 1). There was no difference in the mean total number of individuals of all prey species combined among treatments, and the depth of the experimental cage did not affect the number of prey individuals (Table 1). However the dry weight of the epiphyte algae on the F. vesiculosus and the interaction of depth x algae weight did have an effect on the number of prey individuals (Table 1).
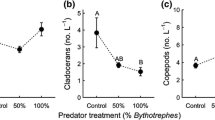
The mean diversity of the invertebrate communities varied among treatments (Fig. 3a; the Shannon-Wiener diversity index; Table 1). The mean diversity index declined from 1.49 (SE ± 0.09) in the “no predation” treatment to 1.22 (±0.09) in the “crab predation” treatment (t53 = −2.46, P = 0.044) and to 1.17 (±0.1) in the “natural predation” treatment (t53 = −2.42, P = 0.048) but there was no difference between the two predation treatments (t530.38, P = 0.9). Neither the depth of the experimental cage nor the dry weight of epiphyte algae on F. vesiculosus had an effect on the diversity index (Table 1). The presence of predators in both the “crab predation” and “natural predation” treatments had the greatest impact on the mean number of T. fluviatilis snails (Fig. 3b; Table 1). On average, there were 40 % less Theodoxus snails in the “crab predation” treatment (2.5 ± 0.9) than in the “no predation” treatment (4.3 ± 1.5; t53 = −2.54, P = 0.037) and almost 60 % less in the “natural predation” treatment (1.8 ± 0.7) than “no predation” treatment (t53 = −3.1, P = 0.009). The two predation treatments did not have a significant difference in the number of Theodoxus snails (t53 = 1.14, P = 0.5). Neither the depth of the experimental cage nor the dry weight of epiphyte algae on F. vesiculosus had an effect on the number of Theodoxus snails (Table 1). The experimental treatments also impacted the mean number of Idotea isopods present in the experimental cages (Table 1). The “natural predation” treatment had a lower number of isopods (2.1 ± 0.5) than did the “no predation” treatment (4.8 ± 0.9; t53 = −2.56, P = 0.038), but there was no difference between the two predation treatments (t53 = 1.27, P = 0.4) or between “crab predation” (3.2 ± 0.7) and “no predation” treatments (t53 = −1.55, P = 0.3). Overall, the dry weight of epiphyte algae on F. vesiculosus explained the differences in the number of Idotea isopods, but the depth of the experimental cage did not have an effect on the number of isopods (Table 1). The numbers of gammarid amphipods (Table 1) and blue mussels (Table 1) did not differ among the treatment levels or other variables (Table 1).
Box plot of the average a Shannon–Wiener diversity index and b number of T. fluviatilis individuals in the different experimental field treatments (“crab predation”—experimental single R. harrisii addition, cage added; “natural predation”—no experimental crab addition, no cage addition; “no predation”—no experimental crab addition, cage added) of F. vesiculosus (n = 20 bushes for each treatment). Different letters above the boxes indicate significantly different values (P < 0.05)
Discussion
In the laboratory environment, the North American white-fingered mud crab R. harrisii is an effective predator of the most common littoral grazers of F. vesiculosus dominated littoral habitats in the Archipelago Sea of southwestern Finland. R. harrisii consumed at least one individual from all presented prey species but also showed a clear choice for some species, such as isopods, gammarid amphipods, and blue mussels. Feeding choices depended on the availability of prey when R. harrisii was allowed to feed on multiple individuals of the same species. R. harrisii still consumed mainly isopods and gammarid amphipods but consumed substantially fewer blue mussels than expected based on the results from the first experiment where 66 % of blue mussels were consumed. As observed with other crab species in other regions (e.g. Juanes 1992; Mascaro et al. 2003; Smallegange et al. 2008), R. harrisii also preyed upon small and medium sized prey of both mobile (isopods and amphipods) and immobile (mussels) species. This suggests that R. harrisii could alter the prey population size-structure through size selective predation on the smaller individuals, as observed with other predators and their prey species (Ojeda and Dearborn 1991).
The ability of R. harrisii to prey on mobile species such as isopods and gammarid amphipods has been questioned (Hegele-Drywa and Normant 2009), but this study shows that R. harrisii can catch these fast moving species in an enclosed environment. In our second experiment, R. harrisii even preyed upon these mobile species more readily than the sessile mussels. In this case, R. harrisii may just be avoiding the risk of claw damage when opening hard shelled mussels (Juanes and Hartwick 1990), but other species of crabs have also shown a preference for consuming softer shelled crustaceans rather than hard shelled snails (Buck et al. 2003). In these experimental conditions, the consumption rate of R. harrisii on isopods and gammarid amphipods was high (up to 90 and 70 % respectively) and could be due to the naivety of these prey species to a novel predator and the prey’s lack of anti-predation behavior (Cox and Lima 2006; Sih et al. 2010). There is evidence that the isopods from this geographical area do not show the same kind of anti-predation behavior (immobility under the olfactory cue of a predator) towards R. harrisii as they do toward a native fish predator (Yli-Renko et al., unpubl. data). It is also possible that in the confined space of an aquarium in the laboratory environment, the mortality rates of the prey were elevated due to an increase in the number of interactions with R. harrisii and the lack of escape possibilities and/or hiding places for the prey.
In the field experiment with the macroinvertebrate community associated with F. vesiculosus, the sole presence of one R. harrisii in an enclosure decreased both the number of prey species and the Shannon-Wiener diversity index in a similar manner as in the treatment that had no enclosure and was open to natural predation (fish, crabs, birds etc.). This shows that R. harrisii impacts the native macroinvertebrate rocky littoral community on F. vesiculosus dominated habitats. Whether it is caused by direct predation of R. harrisii, prey species avoiding the cages containing R. harrisii, or the combination of both, remains uncertain. However, we did observe that the presence of R. harrisii was enough to decrease the number of prey species but not the total number of potential prey individuals associated with F. vesiculosus. Therefore, the decrease in the number of particular species in some enclosures allowed other species to increase their abundance so that the total number of individuals did not change between treatments. For example, the number of Idotea isopods was lower in the predation treatments than no predation treatment, while Jaera albifrons showed an opposite pattern, with higher abundances in the predation treatments than no predation treatment. This may be due to the body size difference between the two isopod species; J. albifrons grows to a maximum of 5 mm (Haahtela 1965), while Idotea can reach 25 mm (Segerstråle 1944). In that sense, species that R. harrisii does not directly prey upon might benefit from the presence of R. harrisii.
Of the individual species, only the abundance of the snail T. fluviatilis was negatively impacted by R. harrisii in the field experiment. However, as R. harrisii only consumed an average of 28 % of the snails presented in the laboratory experiments, the shortage of snails in the “crab predation” and “natural predation” treatments would suggest an indirect effect of the predator rather than strong direct predation on the snails. The presence of R. harrisii could alter the behavior and habitat choice of the snails so that they would avoid crab-invaded F. vesiculosus. This type of predator avoidance behavior, triggered by the scent of a predator feeding on their own kind, has been seen with different snail species in previous studies (Marko and Palmer 1991; Jacobsen and Stabell 1999, 2004; Mach and Bourdeau 2011). Also, during the laboratory experiment T. fluviatilis were found climbing in the walls of the aquaria, possibly due to a predator escape response or just natural inclination. Another explanation could be that in the laboratory environment R. harrisii can prey on mobile species more easily as the prey cannot escape, but in the field R. harrisii prey on more sedentary species as the mobile ones escape or hide.
Unlike the results from the laboratory experiments, the results from the field experiment implied that R. harrisii did not impact the number of isopods or gammarid amphipods that colonized the F. vesiculosus. As this study shows, laboratory experiments conducted in confined spaces with less available refuge than in nature can magnify predation estimates, leading to an exaggerated hypothesized impact of an introduced species. On the other hand, it is possible that the impact of R. harrisii in the field experiment remained hidden due to the ongoing colonization of the prey species, the relatively short experimental duration, and/or the low natural predation pressure of a single R. harrisii.
Conclusion
The abundance and distribution range of R. harrisii along the southwestern coast of Finland has increased since the first report of their presence in 2009 (Fowler et al. 2013), suggesting that R. harrisii could have an increasing impact on the local communities. In particular, previous studies on other species have linked an increasing introduced crab population with a decrease in gammarid amphipod populations (Van Dolah 1978; Grosholz et al. 2000). The effects of R. harrisii predation on macroinvertebrates in littoral F. vesiculosus dominated areas could also cascade to other trophic levels, as it has been found with other predatory crab species and their grazer prey (Silliman and Bertness 2002; Trussell et al. 2002). In the northern Baltic Sea, microalgae and macroalgae both benefit from the cascade effect created by fish predation on littoral grazers (Engkvist et al. 2000; Korpinen et al. 2007). An additive predation effect created by both native fish species and R. harrisii has the potential to actually increase the abundance of F. vesiculosus by decreasing the number of grazers consuming F. vesiculosus (Engkvist et al. 2000). On the other hand, if Theodoxus snails are removed from F. vesiculous habitat, by either direct or indirect means, F. vesiculosus could also decrease in abundance as T. fluviatilis feed on fouling organisms that would otherwise completely cover the F. vesiculosus (Honkanen and Jormalainen 2005). However, top-down effects by R. harrisii are likely being modified by top-down effects by fish, as some fish species have included R. harrisii in their diet (Fowler et al. 2013). Also seabirds such as goldeneyes (Bucephala clangula) and great cormorants (Phalacrocorax carbo sinensis) have been shown to prey on R. harrisii (J. Salmi, pers. comm.), thereby creating a new link between primary and secondary (now tertiary) consumers.
With any novel predator, the fundamental question is how the predator modifies the local food web. Due to the simplicity of the northern Baltic food web as compared to other marine systems and the lack of native crab species, the introduction of R. harrisii to this system provides an opportunity to study how novel species are assimilated into historically isolated species interactions.
References
Bacevičius E, Gasiūnaitė ZR (2008) Two crab species-Chinese mitten crab (Eriocheir sinensis Edw.) and mud crab (Rhithropanopeus harrisii (Gould) ssp. tridentatus (Maitland) in the Lithuanian coastal waters, Baltic Sea. Transit Waters Bull 2:63–68. doi:10.1285/i1825229Xv2n2p63
Bishop MJ, Byers JE (2015) Predation risk predicts use of a novel habitat. Oikos. doi:10.1111/oik.01967
Bonsdorff E (2006) Zoobenthic diversity-gradients in the Baltic Sea: continuous post-glacial succession in a stressed ecosystem. J Exp Mar Biol Ecol 330:383–391. doi:10.1016/j.jembe.2005.12.041
Brousseau DJ, Filipowicz A, Baglivo JA (2001) Laboratory investigations of the effects of predator sex and size on prey selection by the Asian crab, Hemigrapsus sanguineus. J Exp Mar Biol Ecol 262:199–210. doi:10.1016/S0022-0981(01)00290-8
Buck TL, Breed GA, Pennings SC, Chase ME, Zimmer M, Carefoot TH (2003) Diet choice in an omnivorous salt-marsh crab: different food types, body size, and habitat complexity. J Exp Mar Biol Ecol 292:103–116. doi:10.1016/S0022-0981(03)00146-1
Cohen AN, Carlton JT, Fountain MC (1995) Introduction, dispersal and potential impacts of the green crab Carcinus maenas in San Francisco Bay, California. Mar Biol 122:225–237
Compton TJ, Leathwick JR, Inglis GJ (2010) Thermogeography predicts the potential global range of the invasive European green crab (Carcinus maenas). Divers Distrib 16:243–255. doi:10.1111/j.1472-4642.2010.00644.x
Coverdale TC, Axelman EE, Brisson CP, Young EW, Altieri AH, Bertness MD (2013) New England salt marsh recovery: opportunistic colonization of an invasive species and its non-consumptive effects. PLoS ONE 8:e73823. doi:10.1371/journal.pone.0073823
Cox JG, Lima SL (2006) Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol Evol 21:674–680. doi:10.1016/j.tree.2006.07.011
Czerniejewski P, Rybczyk A (2008) Body weight, morphometry, and diet of the mud crab, Rhithropanopeus harrisii tridentatus (Maitland, 1874) in the Odra estuary, Poland. Crustaceana 81:1289–1299. doi:10.1163/156854008X369483
Demel K (1953) Nowy gatunek w faunie Baltyku. Kosmos 2:105–106
Dineen JF, Clark PF, Hines AH, Reed SA, Walton HP (2001) Life history larval description and natural history of Charybdis hellerii (Decapoda:Brachyura:Portunidae) and invasive crab in the Western Atlantic. J Crustacean Biol 21:774–805. doi:10.1651/0278-0372(2001)021[0774:LHLDAN]2.0.CO;2
Elton CS (1958) The ecology of invasions by animals and plants. Methuen, London
Engkvist R, Malm T, Tobiasson S (2000) Density dependent grazing effects of the isopod Idotea baltica Pallas on Fucus vesiculosus L in the Baltic Sea. Aquat Ecol 34:253–260. doi:10.1023/A:1009919526259
Fowler AE, Forsström T, von Numers M, Vesakoski O (2013) The North American mud crab Rhithropanopeus harrisii (Gould, 1841) in newly colonized Northern Baltic Sea: distribution and ecology. Aquat Invasion 8:89–96. doi:10.3391/ai.2013.8.1.10
Glude JB (1955) The effects of temperature and predators on the abundance of the softshell clam Mya arenaria in New England. Trans Am Fish Soc 84:13–26
Grosholz ED, Ruiz GM (1995) Spread and potential impact of the recently introduced European green crab Carcinus maenas, in central California. Mar Biol 122:239–247
Grosholz ED, Ruiz GM, Dean CA, Shirley KA, Maron JL, Connors PG (2000) The impacts of a nonindigenous marine predator in a California Bay. Ecology 81:1206–1224. doi:10.1890/0012-9658(2000)081[1206:TIOANM]2.0.CO;2
Haahtela I (1965) Morphology, habitats and distribution of species of the Jaera albifrons group (Isopoda, Janiridae) in Finland. Ann Zool Fennici 2:309–314
Hanks RW (1961) Chemical control of the green crab Carcinus maenas (L.). Proc Natl Shellfish Assoc 52:75–86
Hegele-Drywa J, Normant M (2009) Feeding ecology of the American crab Rhithropanopeus harrisii (Crustacea, Decapoda) in the coastal waters of the Baltic Sea. Oceanologia 51:361–375. doi:10.5697/oc.51-3.361
Holdredge C, Bertness MD, Altieri AH (2009) Role of crab herbivory in die-off of New England salt marshes. Conserv Biol 23:672–679. doi:10.1111/j.1523-1739.2008.01137.x
Hollebone AL, Hay ME (2008) An invasive crab alters interaction webs in a marine community. Biol Invasions 10:347–358. doi:10.1007/s10530-007-9134-9
Honkanen T, Jormalainen V (2005) Genotypic variation in tolerance and resistance to fouling in the brown alga Fucus vesiculosus. Oecologia 144:196–205. doi:10.1007/s00442-005-0053-0
Jacobsen HP, Stabell OB (1999) Predator-induced alarm responses in the common periwinkle, Littorina littorea: dependence on season, light conditions, and chemical labelling of predators. Mar Biol 134:551–557. doi:10.1007/s002270050570
Jacobsen HP, Stabell OB (2004) Source antipredator behaviour mediated by chemical cues: the role of conspecific alarm signaling and predator labeling in the avoidance response of a marine gastropod. Oikos 104:43–50. doi:10.1111/j.0030-1299.2004.12369.x
Juanes F (1992) Why do decapod crustaceans prefer small-sized molluscan prey? Mar Ecol Prog Ser 87:239–249
Juanes F, Hartwick EB (1990) Prey size selection in Dungeness crabs: the effect of claw damage. Ecology 71:744–758
Karhilahti A (2010) Taskurapu tarttui pyydykseen. Suomen luonto 4:12–13
Kautsky H, Kautsky L, Kautsky N, Kautsky U, Lindblad C (1992) Studies on the Fucus vesiculosus community in the Baltic Sea. Acta Phytogeogr Suec 78:33–48
Kennedy TA, Naeem S, Howe KM, Knops JMH, Tilman D, Reich P (2002) Biodiversity as a barrier to ecological invasion. Nature 417:636–638. doi:10.1038/nature00776
Kimbro DL, Grosholz ED, Baukus AJ, Nesbitt NJ, Travis NM, Attoe S, Coleman-Hulbert C (2009) Invasive species cause large-scale loss of native California oyster habitat by disrupting trophic cascades. Oecologia 160:563–575. doi:10.1007/s00442-009-1322-0
Korpinen S, Jormalainen V, Honkanen T (2007) Bottom-up and cascading top-down control of macroalgae along a depth gradient. J Exp Mar Biol Ecol 343:52–63. doi:10.1016/j.jembe.2006.11.012
Kotta J, Ojaveer H (2012) Rapid establishment of the alien crab Rhithropanopeus harrisii (Gould) in the Gulf of Riga. Est J Ecol 61:293–298. doi:10.3176/eco.2012.4.04
Ledesma ME, O’Connor NJ (2001) Habitat and diet of the non-native crab Hemigrapsus sanguineus in southeastern New England. Northeast Nat 8:63–78. doi:10.2307/3858263
Lefebvre L, Reader SM, Sol D (2004) Brains, innovations and evolution in birds and primates. Brain Behav Evol 63:233–246. doi:10.1159/000076784
Lonsdale WM (1999) Global patterns of plant invasions and the concept of invisibility. Ecology 80:1522–1536. doi:10.1890/0012-9658(1999)080[1522:GPOPIA]2.0.CO;2
Lubchenco J (1978) Plant species diversity in a marine intertidal community: importance of herbivore food preference and algal competitive abilities. Am Nat 112:23–39
Mach ME, Bourdeau PE (2011) To flee or not to flee? Risk assessment by a marine snail in multiple cue environments. J Exp Mar Biol Ecol 409:166–171. doi:10.1016/j.jembe.2011.08.018
Magurran AE (2004) Measuring biological diversity. Blackwell Science Ltd, UK
Marko PB, Palmer AR (1991) Responses of a rocky shore gastropod to the effluents of predatory and non-predatory crabs: avoidance and attraction. Biol Bull 181:363–370. doi:10.2307/1542356
Mascaro M, Hidalgo LE, Chiappa-Carrara X, Simoes N (2003) Size-selective foraging behaviour of blue crabs, Callinectes sapidus (Rathbun), when feeding on mobile prey: active and passive components of predation. Mar Freshw Behav Physiol 36:143–159. doi:10.1080/10236240310001603224
Milke LM, Kennedy VS (2001) Mud crabs (Xanthidae) in Chesapeake Bay: claw characteristics and predation on epifaunal bivalves. Invertebr Biol 120:67–77
Naeem S, Knops JMH, Tilman D, Howe KM, Kennedy T, Gale S (2000) Plant diversity increases resistance to invasion in the absence of covarying extrinsic factors. Oikos 91:97–108. doi:10.1034/j.1600-0706.2000.910108.x
Ojeda FP, Dearborn JH (1991) Feeding ecology of benthic mobile predators: experimental analyses of their influence in rocky subtidal communities of the Gulf of Maine. J Exp Mar Biol Ecol 149:13–44
Paavola M, Olenin S, Leppäkoski E (2005) Are invasive species most successful in habitats of low native species richness across European brackish water seas? Estuar Coast Shelf Sci 64:738–750. doi:10.1016/j.ecss.2005.03.021
Roche DG, Torchin ME (2007) Established population of the North American Harris mud crab, Rhithropanopeus harrisii (Gould 1841) (Crustacea: Brachyura: Xanthidae) in the Panama Canal. Aquat Invasion 2:155–161. doi:10.3391/ai.2007.2.3.1
Roche DG, Torchin ME, Leung B, Binning SA (2009) Localized invasion of the North American Harris mud crab, Rhithropanopeus harrisii, in the Panama Canal: implications for eradication and spread. Biol Invasions 11:983–993. doi:10.1007/s10530-008-9310-6
Ross DJ, Johnson CR, Hewitt CL, Ruiz GM (2004) Interaction and impacts of two introduced species on a soft-sediment marine assemblage in SE Tasmania. Mar Biol 144:747–756. doi:10.1007/s00227-003-1223-4
Rudnick D, Resh V (2005) Stable isotopes, mesocosms and gut content analysis demonstrate trophic differences in two invasive decapod crustaceans. Freshw Biol 50:1323–1336. doi:10.1111/j.1365-2427.2005.01398.x
Rudnick DA, Chan V, Resh VH (2005) Morphology and impacts of the burrows of the Chinese Mitten crab, Eriocheir sinensis H. Milne Edwards (Decapoda, Grapsoidea), in south San Francisco Bay, California, U.S.A. Crustaceana 78:787–807. doi:10.1163/156854005774445500
Schubert K (1936) Pilumnopeus tridentatus Maitland, eine neue Rundkrabbe in Deutschland. Zool Anz 116:320–323
Segerstråle SG (1944) Über die Verbreitung der Idotea-Arten im baltischen Meeresgebiet Finnlands. Comment Biol 9:1–6
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator-prey naiveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621. doi:10.1111/j.1600-0706.2009.18039.x
Silliman BR, Bertness MD (2002) A trophic cascade regulates salt marsh primary production. Proc Natl Acad Sci USA 99:10500–10505. doi:10.1073/pnas.162366599
Smallegange IM, Hidding B, Eppenga JMA, van der Meer J (2008) Optimal foraging and risk of claw damage: How flexible are shore crabs in their prey size selectivity? J Exp Mar Biol Ecol 367:157–163. doi:10.1016/j.jembe.2008.09.011
Stachowicz JJ, Fried H, Osman RW, Whitlatch RB (2002) Biodiversity, invasion resistance, and marine ecosystem function: reconciling pattern and process. Ecology 83:2575–2590
Stohlgren TJ, Binkley D, Chong GW, Kalkhan MA, Schell LD, Bull KA, Otsuki Y, Newman G, Bashkin M, Son Y (1999) Exotic plant species invade hot spots of native plant diversity. Ecol Monogr 69:25–46. doi:10.1890/0012-9615(1999)069[0025:EPSIHS]2.0.CO;2
Strauss SY (2014) Ecological and evolutionary responses in complex communities: implications for invasions and eco-evolutionary feedbacks. Oikos 123:257–266. doi:10.1111/j.1600-0706.2013.01093.x
Tilman D (1999) The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80:1455–1474. doi:10.1890/0012-9658(1999)080[1455:TECOCI]2.0.CO;2
Trussell GC, Ewanchuk PJ, Bertness MD (2002) Field evidence of trait-mediated indirect interactions in a rocky intertidal food web. Ecol Lett 5:241–245. doi:10.1046/j.1461-0248.2002.00304.x
Turoboyski K (1973) Biology and ecology of the crab Rhithropanopeus harrisii ssp. tridentatus. Mar Biol 23:303–313. doi:10.1007/BF00389338
Van Dolah RF (1978) Factors regulating the distribution and population dynamics of the amphipod Gammarus palustris in an intertidal salt marsh community. Ecol Monogr 48:191–217
Vazquez D (2006) Exploring the relationship between niche breadth and invasion success. In: Cadotte MW, McMahon SM, Fukami T (eds) Conceptual ecology and invasions biology: reciprocal approaches to nature. Springer, Berlin, pp 307–322. doi:10.1007/1-4020-4925-0_14
Walton WC, MacKinnon C, Rodriguez LF, Proctor C, Ruiz GM (2002) Effect of an invasive crab upon a marine fishery: green crab, Carcinus maenas, predation upon a venerid clam, Katelysia scalarina, in Tasmania (Australia). J Exp Mar Biol Ecol 272:171–189. doi:10.1016/S0022-0981(02)00127-2
Weis JS (2010) The role of behavior in the success of invasive crustaceans. Mar Freshw Behav Physiol 43:83–98. doi:10.1080/10236244.2010.480838
Wikström SA, Kautsky L (2007) Structure and diversity of invertebrate communities in the presence and absence of canopy-forming Fucus vesiculosus in the Baltic Sea. Estuar Coast Shelf Sci 72:168–176. doi:10.1016/j.ecss.2006.10.009
Wolff T (1954) Occurrence of two East American species of crabs in European waters. Nature 174:188–189
Acknowledgments
The study was financed by the Nottbäck foundation (AF), University of Turku graduate school (TF), and Suomen luonnonsuojelun säätiö. We would like to thank Henry Hellström for providing us with Rhithropanopeus harrisii in 2011. We are grateful for the comments from Veijo Jormalainen and two anonymous reviewers that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forsström, T., Fowler, A.E., Manninen, I. et al. An introduced species meets the local fauna: predatory behavior of the crab Rhithropanopeus harrisii in the Northern Baltic Sea. Biol Invasions 17, 2729–2741 (2015). https://doi.org/10.1007/s10530-015-0909-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-0909-0