Abstract
Biotic resistance from native consumers can reduce the abundance and impacts of introduced species. Previous studies documented the escape of the introduced alga Kappaphycus alvarezii from abandoned farms in Bocas del Toro, Panama. Both attached and unattached aggregations of this invasive alga accumulated on and smothered native corals and seagrasses. However, native urchins and parrotfish were also observed feeding on the alga in the field suggesting that native herbivores may act as agents of biotic resistance. In this study, we conducted an herbivore-exclusion experiment in the field to determine the effect of native herbivory on K. alvarezii and a laboratory experiment to measure the rate of herbivory on the introduced alga by two common native urchins (Lytechinus variegatus and Echinometra lucunter). Consistent with the biotic resistance hypothesis, native herbivores rapidly consumed algae in both field and lab studies. Loss of algal biomass was approximately nine times higher in herbivore-exposed treatments than in herbivore-exclusion treatments in the field. Lab experiments revealed L. variegatus ate 3.5 times more algae than E. lucunter. While K. alvarezii was abundant in surveys during 2014, we did not detect any remaining individuals in our field sites during a return visit one year later. Thus, both native urchins consume K. alvarezii and, along with other herbivores, are likely important agents of biotic resistance. However, longer-term studies are needed to test if native herbivores can control the introduced algae still escaping from active farms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Introduced algae can negatively affect native communities by fundamentally altering habitat structure and trophic dynamics (Schaffelke and Hewitt 2007; Williams and Smith 2007; Kelly et al. 2020). In the Mediterranean, invasive algae (Caulerpa racemosa and Womerseyella setacea) overgrew native foundation species (gorgonians, sponges) causing mortality, injury, and reduced growth and reproduction (Cebrian et al. 2012; de Caralt and Cebrian 2013). Numerous species of algae that were introduced to Kane‘ohe Bay, Hawai‘i for aquaculture now dominate the benthos in some sites, displacing native species and altering community composition (Smith et al. 2002; Fukunaga et al. 2014). Indeed, a meta-analysis of introduced algae impacts consistently found introduced seaweeds tend to monopolize space, thereby reducing abundances and biomass of native algae and altering the diversity of fish and invertebrates (Schaffelke and Hewitt 2007).
The red alga Kappaphycus alvarezii has been intentionally introduced throughout the tropics including Africa, Southeast Asia, Central America, and South America for carrageenan production (Ask et al. 2003). This alga has a rapid growth rate, disperses via fragmentation, and can double its biomass in 15–30 days (Trono 1992). This makes the alga a great candidate for cultivation, but a difficult species to remove and contain if it spreads beyond farms. Previous work in 2013 and 2014 revealed that introduced K. alvarezii drifted to adjacent marine habitats (seagrass beds, coral, and mangroves) up to 300 m away from two abandoned farms in Bocas del Toro, Panama (Sellers et al. 2015). The alga mostly accumulated on top of and smothered adjacent coral and seagrass beds, but some algal pieces attached to and overgrew coral. Native herbivores, particularly the urchins Lytechinus variegatus and Echinometra lucunter were observed feeding on the alga (Sellers et al. 2015). Echinometra spp. are typically limited in movement and graze on adjacent or drifting algae close to reef structures (Shulman 2020), while the larger L. variegatus is a more active wanderer and opportunistic grazer found in different habitats (open sand, seagrass beds, etc.; Parson et al. 2021). These two common urchins were frequently observed eating K. alvarezii in our field sites, which suggests they may act as agents of biotic resistance.
The biotic resistance hypothesis posits that interactions with natural enemies in the introduced range can limit the establishment of nonnative species. Despite the ubiquity of tropical algae invasions (Williams and Smith 2007; Kelly et al. 2020), relatively few studies have examined how consumption by native grazers may influence invasive algae in the tropics. Tropical herbivores are known to strongly limit algal growth and cover in shallow marine environments (Menge and Lubchenco 1981; Burkepile and Hay 2006) and may thus be effective agents of biotic resistance. In this study, we examined if native herbivores could reduce biomass of K. alvarezii using an herbivore exclusion experiment in the field and assessed the rate at which two common native urchins eat the introduced alga using a lab experiment. If native herbivores exert biotic resistance on introduced K. alvarezii, then we predicted that the loss of algal biomass would be lowest in herbivore exclusions compared to treatments exposed to herbivores. We also predicted that the larger of the two commonly occurring native urchins, Lytechinus variegatus, would consume more algae per capita than the smaller native urchin Echinometra lucunter.
Methods
Timeline, site description, and follow-up survey
Kappaphycus alvarezii was first introduced for cultivation in Bocas del Toro, Panama sometime before 2009 (Sellers et al. 2015). Algae farms in Bocas del Toro appeared to use a floating line method for growing K. alvarezii; pieces of the alga are affixed to floating lines suspended above the ground inaccessible to benthic herbivores and left to grow (Ask and Azanza 2002; pers. obs.). In April 2013, we first encountered large abundances of the introduced alga K. alvarezii outside of two abandoned farms southeast of the town of Almirante (hereafter: Almirante) and near the island of Cristobal (hereafter: Cristobal) in Bocas del Toro (Sellers et al. 2015). The sites were adjacent to red mangrove cays (Rhizophora mangle) and largely composed of shallow seagrass beds (0–3 m deep) with scattered aggregations of sponges and coral (mainly Porites sp. and Millepora alcicornis). Both farms were in a state of disrepair and large aggregations of the alga, previously affixed to suspended lines, had fallen and drifted tens of meters to up to 300 m away, accumulating on adjacent coral reef habitat and seagrass beds. Most pieces of K. alvarezii laid unattached on top of seagrass beds, while some pieces attached to coral. We also observed several herbivores consuming the fallen alga, including the urchins L. variegatus and E. lucunter and the parrotfish Scarus iseri (Sellers et al. 2015). It is unclear when the farms were established, how long they had operated, or when they were abandoned.
We returned to the abandoned farms at Almirante and Cristobal at the end of September/early October 2013 to conduct surveys measuring the cover of the introduced alga and urchin densities (Sellers et al. 2015). Cover of K. alvarezii was substantially higher at Almirante (~ 37%) than Cristobal (~ 11%) and urchin densities were lower (0.15 urchins per m2 in Almirante vs. 0.67 per m2 in Cristobal). We also established an herbivore exclusion experiment at Almirante (this study, described below) during our initial survey (Sept/Oct 2013) due to the abundance of K. alvarezii at this site. We surveyed the sites again in March 2014 and found K. alvarezii cover had decreased to ~ 32% in Almirante and ~ 1% cover in Cristobal (Sellers et al. 2015). Finally, in March 2015, we returned a 3rd time to our sites and conducted a follow-up survey to assess the status of K. alvarezii. By this time, our qualitative surveys conducted by snorkel and boat did not detect any K. alvarezii fragments or clumps at either site or in the immediate surrounding areas.
Field experiment
To test the effects of local grazers on invasive K. alvarezii, we conducted an herbivore exclusion experiment using a randomized-block design (n = 14 blocks). We conducted the field experiment at Almirante (9° 17.35′ N; 82° 21.32′ W) from September 25–30, 2013 when K. alvarezii was still abundant. In each replicate block, we assigned tagged clumps of fresh K. alvarezii (50.7 ± 1.2 g; mean ± SE) into one of three treatments: (1) exclusion cage (-herbivores, + cage), (2) uncaged (+ herbivores, -cage), and (3) cage control (+ herbivores, + cage). We placed each treatment on sand substrate intermixed with seagrass and adjacent to coral patches. Exclusion and control cages were made from plastic Vexar ™ mesh (approximately 40 × 25 × 25 cm; 1.3-cm mesh size) and anchored to the sandy seafloor using rebar. Clumps of K. alvarezii were affixed to the bottom of the cages using cable ties. Our cages were designed to exclude urchin and fish herbivores observed feeding on K. alvarezii (e.g., L. variegatus, E. lucunter, S. iserti; Sellers et al. 2015), but smaller grazers (< 1 cm) potentially could have entered our cages. We observed urchins and fish feeding on algae inside coral patches and seagrass beds adjacent to our experiment. The cage control was similar to the exclusion cage but had two open ends to allow herbivores to enter. The uncaged treatment featured only a tagged clump of fresh K. alvarezii fastened to a piece of rebar embedded into the seafloor. The treatments were separated from each other by at least 30 cm in each block and each of the 14 blocks were evenly spaced along a 200-m transect placed parallel to the shoreline in 1–2 m deep water. We ensured all blocks were free of K. alvarezii (within 3-m) prior to experimentation and did not detect any new algae colonizing our blocks (or leaving our treatments) during the experiment. After five days, we retrieved the deployed algae and measured the final wet weight.
Lab experiment
We conducted a lab feeding experiment from March 27–30, 2015 using L. variegatus and E. lucunter to confirm that native urchins may be responsible for the patterns observed in the field experiment and to measure rates of herbivory. Urchins were haphazardly collected from Almirante on March 26, 2015 and placed in free-flowing aerated outdoor aquaria (~ 38 L) at the Smithsonian Tropical Research Institute Bocas del Toro Research Station in Panama. We starved the urchins for 24 h before experimentation, then measured their blotted wet weight. The mean wet weight (± SD) of L. variegatus was 67.4 ± 12.5 g (range: 45.2–86.6) and E. lucunter was 14.7 ± 6.0 g (range: 7.1–24.7), which are within the typical range of adults for these species (McPherson 1969; Beddingfield and McClintock 2000). In each aquarium, we placed an individual of either L. variegatus or E. lucunter (n = 15 for each species) and allowed them to acclimate for 8 h before introducing algae. The urchin species treatments were interspersed across two rows of 15 aquaria. We added approximately 125 g (± 2.0 g, SD) of freshly collected K. alvarezii to each aquarium in 2 to 7 pieces. There was not a significant difference in the mean numbers of pieces offered to L. variegatus (3.5 ± 1.8) vs. E. lucunter (4.5 ± 1.4; P = 0.11), and there was no significant relationship between the numbers of starting fragments and numbers of new fragments created for L. variegatus (r2 = 0.09, P = 0.27) or E. lucunter (r2 = 0.21, P = 0.09). Because we could not find K. alvarezii in our previous field sites of Cristobal and Almirante, we obtained specimens that had drifted outside of an active farm near Popa Island. After three days, we measured the total blotted wet weight of the algae and counted the number of K. alvarezii fragments in each tank. To prevent the loss of algal fragments, we placed 1-mm2 fiberglass mesh over the water exit tube of each aquarium. Even the smallest algal fragments were negatively buoyant, and thus were unable to escape through the screened exit tube at the water surface. The mean (± SD) temperature and salinity of the aquaria during the experiment were 27.9 ± 1.0 °C and 33.9 ± 0.4‰, respectively. Due to the limited numbers of aquaria available, we were unable to include a control treatment assessing algae growth and fragmentation without urchins. However, our lab experiment was short in duration (three days), and we would not expect any potential experimental artifacts to strongly influence our results.
Statistical analysis
We used a mixed-model ANOVA to test if the loss of algal weight in our field experiment varied with cage treatment (fixed factor), while accounting for variation among experimental blocks (random factor). Following a test of the main effect, we performed a Tukey Post-Hoc test to identify which treatments differed from each other. For the laboratory experiment, a two-sample t-test was used to compare the loss of algal weight consumed by the two urchin species. We tested how the numbers of new fragments created varied between urchin species using a Generalized Linear Model with a quasi-Poisson distribution. We used a quasi-Poisson distribution because our count data were overdispersed and the variance exceeded the mean. We used residual-, box-, and QQ-plots to examine the assumptions of the statistical tests. A rank transformation was applied to the field experiment data, but transformation was unnecessary for the lab data. All analyses were conducted using R (ver. 3.6.3; R Core Team 2020).
Results
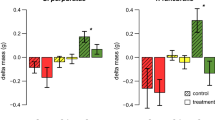
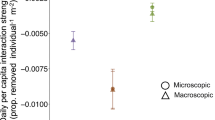
Loss of algal biomass was approximately nine times higher in the herbivore-exposed treatments than in the herbivore-exclusion treatments (F(2, 16) = 6.33, p = 0.009, Fig. 1). These effects were not due to the presence of the cage since the cage control and uncaged treatments did not differ. While some algal fragments in exclusion cages exhibited growth, most still lost biomass, suggesting that small grazers may have been able to access and feed on the algae. In the lab experiment, there were significant differences in the loss in the wet weight of algae (t = − 8.12) and number of new algal fragments (χ2 = 22.29) between urchin species (p < 0.001 for both, Fig. 2). Lytechnius variegatus ate 3.5 times more algae (5.1 ± 0.4 g per day) and created three times more fragments during feeding (4.0 ± 0.4 fragments per day) than E. lucunter (1.4 ± 0.3 g; 1.2 ± 0.4 fragments per day). However, the biomass of algae consumed (t = 0.92, p = 0.36) and the number of fragments generated (t = 1.03, p = 0.31) did not vary among species when these values were scaled to urchin weight.
Field exclusion experiment for native herbivores feeding on the introduced macroalga Kappaphycus alvarezii. The closed circles represent the mean (± SE) loss of algae biomass in herbivore exclusion cages (- herbivore, + cage), cage controls (+ herbivore, + cage), and uncaged treatments (+ herbivore, − cage). Open circles are the raw data points. Negative values represent algae growth. Different letters denote a statistical difference between treatment levels (p < 0.05)
Lab herbivory experiment comparing the loss of biomass of the introduced macroalga Kappaphycus alvarezii and number of new algal fragments created by two native sea urchins (Lytechinus variegatus and Echinometra lucunter). Symbols are as described in Fig. 1
Discussion
Introduced algae exposed to native herbivores were rapidly consumed in both field and lab experiments, which supports our hypothesis that native herbivores may be acting as agents of biotic resistance to the invasive K. alvarezii at our field sites. In the field experiment, algae exposed to herbivores decreased approximately nine times faster (1.66 g and 3.5% biomass loss per day) than algae excluded from herbivores (0.19 g and 0.4% loss per day) and featured bite marks consistent with urchins. Similarly, we observed two native urchins (L. variegatus and E. lucunter) rapidly feeding on K. alvarezii during lab experiments, consuming an average of 5.1 g (4.1% loss/day) and 1.4 g (1.1% loss/day) of algae per day, respectively. These measurements of biomass loss are likely conservative because individuals of K. alvarezii were also growing during the experiment (Fig. 1). While K. alvarezii can grow rapidly in some environments (Trono 1992), our results demonstrate that herbivores can remove algal biomass faster than it can grow, suggesting that herbivores might influence algal establishment. Given the feeding rates, observations of urchins feeding on algae in the field, and prevalence of urchins observed in algae-impacted sites (Sellers et al. 2015), we posit that urchins, especially L. variegatus, are among the herbivores affecting this introduced alga at our sites. However, the urchin densities in our study sites in Panama (0.15–0.67 urchins per m2, Sellers et al. 2015) were low relative to other tropical systems (Beddingfield and McClintock 2000; Shulman 2020), so other herbivores such as the parrotfish Scarus iseri (common in our sites) likely play an important role as well. Indeed, based on the per capita feeding rates (above) and urchin densities presented in Sellers et al. (2015), we estimate urchin herbivory may only account for ~ 10% of the observed biomass loss measured in the field experiment in Almirante (although urchins may account for 94% in Cristobal, where urchin densities were higher; see Online Resource 1). Thus, future studies should investigate the effects of different herbivores on K. alvarezii abundance.
Our results are also consistent with other studies of grazing herbivores on introduced algae. Grazing by native herbivores can reduce the cover of invasive algae on reefs, including Kappaphycus spp. in Hawai‘i (Conklin and Smith 2005), and reduce growth of introduced algae inside farms (Ask and Azanza 2002). In the Mediterranean, the native sea urchin Paracentrotus lividus limited seasonal increases of the algal invader Lophocladia lallemandii, while Strongylocentrotus droebachiensis slowed the spread of invasive Codium fragile spp. tomentosoides in temperate Nova Scotia (Lyons and Scheibling 2008). Indeed, researchers have successfully used native sea urchins (Tripneustes gratilla) as biocontrol agents for introduced algae in Hawai‘i (Westbrook et al. 2015); urchins decreased introduced algae cover by 85% in two years when combined with manual removal (Neilson et al. 2018). Numerous studies have demonstrated that introduced algae and plants experience biotic resistance (Kimbro et al. 2013; Parker and Hay 2005; Conklin and Smith 2005), but few studies show that native consumers can completely remove introduced species in their novel range (Williams and Smith 2007). Thus, larger, longer term studies and monitoring are needed to determine if native herbivores can prevent the establishment of K. alvarezii from active farms in Bocas del Toro, Panama.
Feeding by native herbivores on K. alvarezii also created large numbers of fragments, as we observed in our lab experiments, which may facilitate dispersal. Most fragments created during the lab feeding experiment ranged from 5 to 70 mm long and appeared healthy, although some fragments appeared pale and chlorotic (pers. obs.). Previous studies demonstrate fragments of K. alvarezii as small as 10 mm can persist and regrow (Bulboa and de Paula 2005), and some algal species can survive or may even be stimulated when passed through herbivore guts, similar to dispersal strategies of some plants (Vermeij et al. 2013; Santelices and Ugarte 1987). Furthermore, Kappaphycus spp. is able to regrow from minute pieces of tissue remaining after manual removal (Conklin and Smith 2005), further suggesting that algal regrowth is possible from small propagules. Thus, caution must be used in any management strategies or programs aiming to control this species to avoid further dispersal (Kamalakannan et al. 2014).
Previous surveys in 2013 and 2014 revealed that K. alvarezii was abundant in our field sites in Almirante (average of ~ 35% cover) and to a lesser extent in Cristobal (~ 6% cover; Sellers et al. 2015) but was completely absent by March 2015 (this study). Initially, the alga was suspended on long lines inaccessible to benthic herbivores, as observed on active algae farms (Ask and Azanza 2002; pers. obs.). However, after the farms in our field sites were apparently abandoned, the algae fell to the seafloor and became accessible to benthic herbivores (Sellers et al. 2015). Thus, our data are consistent with the hypothesis that native grazers removed algae and exert biotic resistance on K. alvarezii. An alternative non-mutually exclusive hypothesis is that the algae (most of which was unattached, Sellers et al. 2015) drifted to other locations and/or that humans removed the algae. However, we did not observe K. alvarezii in the deeper areas surrounding the abandoned farms when we returned to these sites in 2015, suggesting that the alga had not moved to the surrounding areas. We also find it unlikely that humans completely removed algae from our sites because K. alvarezii is difficult to manually remove and missed fragments or those attached to the benthos can rapidly regrow (Conklin and Smith 2005). While the alga was not detected during follow-up visits to our study sites in Almirante and Cristobal in 2015 (this study), active farms with K. alvarezii were present elsewhere in Bocas del Toro in 2018, and loose algal fragments were observed in the seagrass beds under and adjacent to the cultivation lines in August 2017 (A. Cannon, per. comm.). Thus, these new farms may act as a source for future dispersal of algae to other habitats. High growth rates and ease of reproduction and dispersal through fragmentation are important invasive species traits (Smith et al. 2002), but also make species such as K. alvarezii a popular candidate for cultivation. As algae introductions for aquaculture continue to increase globally and over time (Thomsen et al. 2016), the risk of the introduction and spread of non-indigenous algae to native habitats continues.
Data availability
The data for this study are available by contacting the corresponding author.
References
Ask EI, Azanza RZ (2002) Advances in cultivation technology of commercial eucheumatoid species: a review with suggestions for future research. Aquaculture 206:257–277
Ask EI, Batibasaga A, Zertuche-González JA, de San M (2003) Three decades of Kappaphycus alvarezii (Rhodophyta) introduction to non-endemic locations. In: Chapman ARO, Anderson RJ, Vreeland VJ, Davison IR (eds), Proceedings of the 17th international seaweed symposium. Cape Town, South Africa, pp 49–57.
Beddingfield SD, McClintock JB (2000) Demographic characteristics of Lytechinus variegatus (Echinoidea: Echinodermata) from three habitats in a North Florida Bay, Gulf of Mexico. Mar Ecol 21:17–40
Bulboa CR, de Paula EJ (2005) Introduction of non-native species of Kappaphycus (Rhodophyta, Gigartinales) in subtropical waters: Comparative analysis of growth rates of Kappaphycus alvarezii and Kappaphycus striatum in vitro and in the sea in south-eastern Brazil. Phycol Research 53:183–188
Burkepile DE, Hay ME (2006) Herbivore vs. nutrient control of marine primary producers: Context-dependent effects. Ecology 87:3128–3139
Cebrian E, Linares C, Marschal C, Garrabou J (2012) Exploring the effects of invasive algae on the persistence of gorgonian populations. Biol Invasions 14:2647–2656
Conklin EJ, Smith JE (2005) Abundance and spread of the invasive red algae, Kappaphycus spp., in Kane‘ohe Bay, Hawai‘i and an experimental assessment of management options. Biol Invasions 7:1029–1039
de Caralt S, Cebrian E (2013) Impact of an invasive alga (Womersleyella setacea) on sponge assemblages: compromising the viability of future populations. Biol Invasions 15:1591–1600
Fukunaga A, Peyton KA, Thomas FI (2014) Epifaunal community structure and ammonium uptake compared for the invasive algae, Gracilaria salicornia and Acanthophora specifera, and the native alga, Padina thivyi. J Exp Mar Biol Ecol 456:78–86
Kamalakannan B, Jeevamani JJJ, Nagendran A, Padiaraja D, Chandrasekaran S (2014) Impact of removal of invasive species Kappaphycus alvarezii from coral reef ecosystem in Gulf of Mannar. India Curr Sci 106(10):1401–1408
Kelly EL, Cannon AL, Smith JE (2020) Environmental impacts and implications of tropical carrageenophyte seaweed farming. Conserv Biol 34:326–337
Kimbro DL, Cheng BS, Grosholz ED (2013) Biotic resistance in marine environments. Ecol Lett 16:821–833
Lyons DA, Scheibling RE (2008) Context-dependent survival of the invasive seaweed Codium fragile ssp. tomentosoides in kelp bed and urchin barren habitats off Nova Scotia. Aquat Biol 2:17–27
McPherson BF (1969) Studies on the biology of the tropical sea urchins, Echinometra lucunter and Echinometra viridis. Bull Mar Sci 19:194–213
Menge BA, Lubchenco J (1981) Community organization in temperate and tropical rocky intertidal habitats: prey refuges in relation to consumer pressure gradients. Ecol Monogr 51:429–450
Neilson BJ, Wall CB, Mancini FT, Gewecke CA (2018) Herbivore biocontrol and manual removal successfully reduce invasive macroalgae on coral reefs. PeerJ 6:e5332
Parker JD, Hay ME (2005) Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecol Lett 8:959–967
Parson A, Dirnberger JM, Mutchler T (2021) Patterns of dispersion, movement and feeding of the sea urchin Lytechinus variegatus, and the potential implications for grazing impact on live seagrass. Gulf Carib Res 32:8–18
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Santelices B, Ugarte R (1987) Algal life-history strategies and resistance to digestion. Mar Ecol Prog Ser 35:267–275
Schaffelke B, Hewitt CL (2007) Impacts of introduced seaweeds. Bot Mar 50:397–417
Sellers AJ, Saltonstall K, Davidson TM (2015) The introduced alga Kappaphycus alvarezii (Doty ex P.C. Silva, 1996) in abandoned cultivation sites in Bocas del Toro. Panama Bioinvasions Rec 4:1–7
Shulman MJ (2020) Echinometra sea urchins on Caribbean coral reefs: Diel and lunar cycles of movement and feeding, densities, and morphology. J Exp Mar Biol Ecol 530–531:151430
Smith JE, Hunter CL, Smith CM (2002) Distribution and reproductive characteristics of nonindigenous and invasive marine algae in the Hawaiian Islands. Pac Sci 56:299–315
Thomsen MS, Wernberg T, South PM, Schiel DR (2016) Non-native seaweeds drive changes in marine coastal communities around the world. Seaweed phylogeography. Springer, Dordrecht, pp 147–185
Trono GC (1992) Eucheuma and Kappaphycus: Taxonomy and cultivation. Bull Mar Sci and Fish, Kochi University 12:51–65
Vermeij MJA, van der Heijden RA, Olthuis JG, Marhaver KL, Smith JE, Visser PM (2013) Survival and dispersal of turf algae and macroalgae consumed by herbivorous coral reef fishes. Oecologia 171:417–425
Westbrook CE, Ringang RR, Cantero SMA, HDAR, TNCA Urchin Team, Toonen RJ (2015) Survivorship and feeding preferences among size classes of outplanted sea urchins, Tripneustes gratilla, and possible use as biocontrol for invasive alien algae. PeerJ 3:e1235
Williams SL, Smith JE (2007) A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu Rev Ecol Evo Syst 38:327–359
Acknowledgements
We thank Carmen Schloeder, Samantha Flounders, and Arcadio Castillo for field and lab assistance. Abigail Cannon provided information and a helpful discussion of the more recent status of K. alverezii in Bocas del Toro, Panama. Mark Torchin and the staff at STRI provided logistical support. We appreciate the comments and critiques by our anonymous reviewers, the associate editor Ladd Johnson, and Amanda MF Davidson. Funding was provided by the National Science Foundation (OCE-1323429 to TMD).
Funding
This study was funded by the National Science Foundation (OCE-1323429 to TMD).
Author information
Authors and Affiliations
Contributions
AS and TD designed the study and collected the data. SA and TD conducted the data analysis. SA wrote the first draft, and all authors contributed substantially to additional drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors approve of this submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Albright, S.L., Sellers, A.J. & Davidson, T.M. Native urchins as potential agents of biotic resistance to the introduced alga Kappaphycus alvarezii in a tropical lagoon. Biol Invasions 24, 345–351 (2022). https://doi.org/10.1007/s10530-021-02651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02651-z