Abstract
Ecologists have long wondered how plants and algae persist under constant herbivory, and studies have shown that factors like chemical defense and morphology can protect these species from consumption. However, grazers are also highly diverse and exert varying top-down control over primary producers depending on traits such as body size. Moreover, susceptibility of plants and algae to herbivory may vary across life stages and size classes, with juveniles potentially the most vulnerable. Here, we focus on diverse grazing communities within giant kelp forests and compared consumption on two size classes of juvenile giant kelp (Macrocystis pyrifera) across four herbivore species ranging in size. We also integrated field and literature densities to estimate impacts on populations of juvenile kelp. We found that purple sea urchins, a species known for exerting strong control over adult M. pyrifera, had weak per capita impact on microscopic kelp, on par with a much smaller crustacean species. While urchin consumption increased with macroscopic juvenile kelp, it never surpassed the smaller brown turban snail, suggesting that feeding morphology, in addition to herbivore body size, is a predictor of consumption at these small size classes. The smaller herbivores also occurred in high densities in the field, increasing their predicted population-level impacts on juvenile kelp compared to urchins and perhaps other larger, but less abundant, herbivores. This study highlights the variation in species’ roles within an herbivore guild and the importance of age-related changes in grazing vulnerability to better understand herbivore control on plant and algae population dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Successful recruitment and growth are critical to species persistence. Understanding what drives variation in recruitment and growth is particularly important for primary producers, including plants and algae, that form the basis of food webs and play important roles in ecosystem processes, including nutrient and energy cycling that support higher trophic levels (Hooper and Vitousek 1997; Field et al. 1998). Many plants and algae also serve as foundation species, providing structural complexity and facilitating species within a community (Dayton 1972; Angelini et al. 2011). For example, the foliage of Douglas fir forests create microclimates and provide unique habitats for wildlife (Parker et al. 2004), seagrass beds provide nursery habitat for fish (Gullström et al. 2008), and giant kelp forests provide refuges for animals to escape benthic predation (Watanabe 1984).
While many factors influence the persistence of plants and algae, herbivores can exert strong top-down control (Huntly 1991; Valentine and Heck 1999; Silliman and Zieman 2001). Ecologists have long wondered how plants persist under constant grazing pressure, with one of the most well-known hypotheses being the green world hypothesis put forth by Hairston, Smith, and Slobodkin who argued that predators keep herbivores in check, thereby allowing the persistence of primary producers (Hairston et al. 1960). The alternative to top-down control of herbivores is that plants and algae have chemical compounds and structural defenses that deter herbivory (Hay and Fenical 1988; Hanley et al. 2007; Wilkinson and Sherratt 2016), though herbivores can develop behaviors and mouthparts to counter these defenses, such as specializing on different plant life stages and tissues (Cates 1980; Wittstock et al. 2004; Novotny et al. 2010). Important in this discussion is that not all herbivores are equal in their impacts on plant populations and understanding persistence in primary producers involves examining variation in herbivore consumption rates and preference.
Based on metabolic theory, herbivore biomass may be a good predictor of per capita rates of consumption (Gillooly et al. 2001; Brown et al. 2004), because temperature and body mass influence metabolic rates, and therefore processes like feeding. A meta-analysis of herbivory in freshwater, marine, and terrestrial systems showed consumption rates increased with body size over 11 orders of magnitude (Hillebrand et al. 2009). Therefore, we would expect that larger herbivores would consume proportionately more plant or algal biomass. However, an alternate hypothesis is that species feeding morphology, behavior, and attack method are also key characteristics determining consumption rates (Bernays 1998; Hochuli 2001; Novotny et al. 2010). For example, smaller herbivores that form fronts can lead to large impacts in systems such as salt marshes, where small snail grazers promote fungal growth in marsh grass, leading to tissue death (Silliman and Newell 2003). These front formations are common in marine and terrestrial systems (Silliman et al. 2013) where despite small body sizes, grazers can have disproportionately large negative impacts on plant and algal biomass. Therefore, under the latter hypothesis, grazing vulnerability is expected to depend on factors such as plant size and stage and herbivore characteristics such as preference for certain tissues or feeding apparatus constraints, which may also be related to phylogenetics (Parker 1985; Van Alstyne et al. 2001; Milchunas and Noy-Meir 2002).
While much of the research concerning top-down control by herbivores has focused on terrestrial ecosystems, many examples have been reported in marine ecosystems with one of the most well known being the control of kelps by sea urchins. In rocky reef kelp beds, kelp can persist when urchin populations are kept in check; however, when they occur in high abundances, kelp beds can be overgrazed, leading to urchin barrens (Estes et al. 1978; Sala et al. 1998). The formation of these barrens can lead to a behavioral change in urchins, where they switch from subsistence on drift algae to actively scraping the benthos (Harrold and Reed 1985), potentially leading to low algal recruitment and a sustained barren state. Therefore, understanding the mechanisms that allow for or constrain recovery of macroalgae, including kelps, is important. The survival and persistence of juveniles are especially critical for the establishment of adult kelp, many of which are foundation species like the widely distributed, highly productive giant kelp (Macrocystis pyrifera) (Graham et al. 2007). Within giant kelp forests, urchins are one of the larger benthic herbivores, and it is hypothesized that their ability to overgraze adult kelp may extend to juvenile stages as well (Mann 1977). However, the role of urchins and other smaller herbivores on early life stages is not well understood for marine algae in general (though see Sala and Graham 2002). Considering the potential vulnerability of juvenile stages to herbivory, including their small size and more delicate tissues (Van Alstyne et al. 2001), this is a critical area of study to add to the discussion about the impacts of herbivores on the persistence of plants and algae.
We focused on giant kelp forest communities due to the high diversity of grazing species, in addition to the ecological importance of M. pyrifera as a foundation species. M. pyrifera is a food source for many mesograzers that are known to consume adult fronds, such as amphipods and isopods (Bernstein and Jung 1979; Davenport and Anderson 2007) and, in the case of urchins, whole individuals (Pearse 2006). It is well documented that urchins exert population impacts on adult kelp, but we examined whether their grazing impacts extend to very small juvenile size classes (microscopic and barely macroscopic), where persistence is crucial, and how their impacts compare to herbivores of smaller sizes to test biomass–consumption relationships. Because juvenile kelp are small, herbivores of multiple sizes can presumably exert demographic impacts by consuming whole individuals within M. pyrifera populations. In this study, we focus on two size classes within the juvenile stage of M. pyrifera to measure grazing impacts made by smaller kelp forest mesograzers, which may readily consume these early stages and which small differences in M. pyrifera size may translate to large differences in their grazing rates. Therefore, we ask: (1) what is the vulnerability of giant kelp at two juvenile size classes (microscopic and barely macroscopic)? (2) does grazing impact change across those size classes? and (3) can consumption of juveniles be predicted by herbivore body size? We hypothesized that: (1) impacts depend on the species’ feeding morphology, since certain animals are constrained by their ability to consume juvenile kelps of very small sizes (microscopic), and (2) at a larger size (macroscopic juvenile kelp), the positive relationship between biomass and consumption would hold. We tested these hypotheses using controlled laboratory experiments with two size classes of juvenile M. pyrifera and common grazers in giant kelp forests that span a range of sizes.
Methods
Experimental setup
We conducted laboratory experiments in an aquaria facility at the Hopkins Marine Station (HMS) in Pacific Grove, CA (36°37′14.5″ N, 121°54′15.4″ W) using a 50-gallon insulated flow-through aquarium tank, which was fed by a header tank because pH of incoming seawater to HMS is oftentimes low (pH < 7.80). Therefore, we continuously aerated our header tank with an air pump to raise pH and oxygen levels. We then lowered pH and oxygen levels to our desired setpoints and maintained treatment tank conditions with an Arduino-microcontroller system (Low et al., in review), which monitors oxygen with a Vernier optical DO probe and pH levels with a Honeywell Durafet pH sensor. It opens or closes solenoid valves that deliver N2 (to lower oxygen) and CO2 (to lower pH) gas into the treatment tank to achieve stable oxygen and pH conditions. Dissolved oxygen was maintained at 7.50 ± 0.1 mg L−1 and pH at 7.90 ± 0.05, levels common in nearby coastal waters (Booth et al. 2012). Temperature was not directly controlled by the system but was measured every 15 min with an iButton (Maxim Integrated). Despite a range in temperature across rounds of experiments (12–16.5 °C), within rounds, temperature did not fluctuate more than 1.5 °C.
We chose four common, widespread kelp forest species of different sizes that are known to consume M. pyrifera (Leighton 1966; Watanabe 1984; Light 2007): the purple urchin (Strongylocentrotus purpuratus, 2.5–3.0 cm test diameter, 0.5235–2.0515 g ash-free dry mass (AFDM)), the brown turban snail (Tegula brunnea, 1.7–1.9 cm shell basal diameter, 0.1533–0.4009 g AFDM), the kelp isopod (Idotea resecata, 2.0–2.8 cm body length excluding antennae, 0.0248–0.0891 g AFDM), and the kelp curler amphipod (Peramphithoe humeralis, 1.1–1.5 cm body length excluding antennae, 0.0027–0.0327 g AFDM). We used average sizes for T. brunnea and I. resecata observed in the kelp forest next to HMS, S. purpuratus slightly smaller than the average to provide adequate room in experimental containers, and P. humeralis large enough to easily identify to species level and confirm that they were not brooding any young, which was close to the average size of all collected individuals. All species are found on the benthos on overlapping habitat where juvenile kelp are found. Urchins in this system are primarily confined to crevices due to the presence of otters (Lowry and Pearse 1973), though they are also found outside of crevices usually under low-lying algae (Ng, personal observations). Urchins have an Aristotle’s lantern, which is composed of five plates, to feed primarily on drift algae (Tegner et al. 1995; Harrold and Reed 1985). While T. brunnea uses adult M. pyrifera for refuge from predation, there are still abundant on the substrate (Watanabe 1984) and use a radula, a ribbon of chiton, to scrape food into their mouths. I. resecata and P. humeralis are generally found on adult M. pyrifera fronds closer to the surface, though they are also found in holdfasts and are particularly active at night, leaving for foraging bouts (Hammer and Zimmerman 1979) where they can potentially encounter juvenile M. pyrifera on the substrate. In this system, we also noted that P. humeralis can create nests in low-lying, small M. pyrifera (one lamina ~ 5 cm long) (Ng, personal observations).
We replicated 48-h experiments through time for a total of 9 rounds for the microscopic size of kelp from February to May 2018 (n = 16 total individuals species−1; Round 9 was run with just T. brunnea to increase the sample size due to mortality of T. brunnea in earlier rounds likely from disease) and 8 rounds for the macroscopic size from March 2017 to January 2018 (n = 16 individuals species−1). Prior to experiments, each species was fed adult giant kelp ad libitum and acclimated in flowing seawater tanks for at least two weeks. Forty-eight hours prior to each round, we acclimated grazers in individual partitioned containers in the treatment tank and fed them adult M. pyrifera fronds. We then replaced the fronds with a plastic tile of cultured juvenile kelp on each side of the partition. One side acted as the grazer side, and the other as a control side to account for any changes in kelp survival not due to grazing. S. purpuratus and T. brunnea were each placed in 22.5 × 13.5 × 6.5 cm containers with tiles 9.5 × 7.5 cm large, and I. resecata and P. humeralis were placed in 14 × 14 × 5 cm containers with tiles 5 × 7.5 cm large. For each round, there were eight total herbivore containers in the treatment tank (n = 2 species−1 round−1; exception was microscopic size Round 9 where n = 4 for T. brunnea). We quantified the density of juvenile kelp before and after each round by counting the number of individuals in 16 random fields of view for larger tiles and 8 random fields of view for smaller tiles using an inverted microscope at 100 × magnification.
Kelp culturing
Juvenile M. pyrifera sporophytes were grown in the lab at HMS to microscopic and barely macroscopic sizes. We focused on two juvenile sporophyte size classes, rather than comparing grazing rates on two different life stages, to avoid the potentially confounding effect of life stage preference on herbivore consumption rates and to focus our findings on grazer impacts on populations of M. pyrifera (examining viable sporophytes with the potential to grow into reproductive adults). We collected sporophylls from the kelp forest near the marine station (8–10 m depth) and kept them in flowing seawater up to 3 h before the spore release process. To prepare the sporophylls, we wiped them to remove any epibionts and rinsed them with a 10% iodine solution followed by filtered seawater. They were layered with wet paper towels, left to desiccate for 30 min in 10–12 °C, and transferred to a dish of filtered seawater to induce spore release. Spores were settled in seawater-filled trays (19.5 × 36 cm) lined with clear PVC tiles (9.5 × 7.5 cm and 5 × 7.5 cm large) at 5 spores mm−2. We placed trays in incubators at 10–12 °C with a 14:10 light:dark cycle, and water was replaced every week with new growth media (Provosoli’s enriched seawater) (Andersen 2005). For microscopic kelp, we waited until sporophytes were > 8 cells large (average ~ 160 μm total length), and the mean density of sporophytes (± SE) across tiles was 2.16 ± 0.17 mm−2. For macroscopic kelp, we waited until sporophytes were 1–2 mm in length, and the mean density of sporophytes across tiles was 3.28 ± 0.12 mm−2.
Estimates of maximum per capita interaction strength
Interaction strength is generally defined as the effect of a species on the population growth of another (MacArthur 1972). We calculated maximum daily per capita interaction strengths (PCIS) represented as the proportion of juveniles removed individual-1 m-2 day-1 (‘maximum’ because there was no competition, no influx of new M. pyrifera juveniles, and one prey species offered). We used the dynamic index, which estimates PCIS under negative exponential prey growth (Wootton 1997, Sala and Graham 2002) and is calculated as:
where Gt is the proportion of surviving juvenile sporophytes (prey) after time t in the presence of grazing consumers, Ct is the proportion of surviving juvenile sporophytes after time t in the absence of consumers, and D is the density of consumers m−2.
Estimates of mass-specific grazing rates
To obtain mass-specific grazing rates for each species, we calculated the proportion of kelp consumed and adjusted it for the mass of each grazing individual. The proportion of kelp consumed was calculated as:
where Gt and Ct are the same as in the PCIS equation. This was multiplied by the area of the tile in m2 (0.007125 m2 for the larger tiles offered to T. brunnea and S. purpuratus; 0.00375 m2 for the smaller tiles offered to I. resecata and P. humeralis) and divided by the AFDM of each individual grazer to get the proportion of juveniles removed m−2 g of grazer−1. To get AFDM, animals were euthanized, dried at 60 °C for 48 h, and weighed to obtain the dry mass. They were placed in a muffle furnace at 550 °C for two hours and weighed to obtain the ashed mass (AFDM is the difference between the dry and ashed masses). We did not properly obtain mass for the first two rounds of macroscopic experiments, so n = 12 for macroscopic mass data and n = 16 for microscopic mass data.
Estimates of consumer densities and consumer impacts
To extrapolate laboratory-derived PCIS to population-level impacts on juvenile kelp, we estimated the densities of grazers through field surveys and literature data. We multiplied the average densities (individuals m−2) by the average and upper and lower bounds of maximum daily PCIS (mean ± SE) to estimate impacts (proportion removed m−2 day−1) for each species and size class of juvenile M. pyrifera. When calculating average densities, we included densities that were zero (including literature-derived data, see below) to more accurately approximate impacts on juvenile kelp (reflecting instances in which grazing species do and do not occur).
For surveys, we laid six 30 m transects each season—summer (August), fall (November), winter (February–early March), and spring (May)—starting summer 2016 and continuing through summer 2018 in the kelp forest next to HMS (Ng and Micheli 2020). Transects were laid across rocky reef, and we placed 0.25 m2 quadrats on alternating sides of the transect and counted all T. brunnea and S. purpuratus. To calculate transect densities per m2, we multiplied each quadrat number by four (to get individuals m−2) and divided by 30. Literature-derived densities were obtained for I. resecata and P. humeralis from searches using Google Scholar, Web of Science, and all theses stored at the Miller A. Library at HMS using the current species name or previous name. For I. resecata, we searched for “Idotea resecata” and “Pentidotea resecata”, and for P. humeralis, we searched for “Peramphithoe humeralis”, “Perampithoe humeralis”, “Amphithoe humeralis”, and “Ampithoe humeralis”. Papers specifically listing the species names and numbers of individuals per m2 were kept, yielding three papers for I. resecata and P. humeralis (Andrews 1945; Hardy 1973; Sala and Graham 2002). One paper comprised surveys done at several time points and sites around the Monterey Peninsula (Andrews 1945), so each survey was included in our approximations of average densities.
Statistical analyses
We tested whether PCIS and mass-specific grazing rates differed across species and kelp size class. First, we tested for the effect of round for all four datasets (PCIS and mass-specific grazing rates with microscopic and macroscopic kelp) and found it to be non-significant, so the term was dropped from each model. We then tested for normality and homogeneity of variance using the Shapiro–Wilk and Levene’s tests, respectively. Because the homogeneity of variance assumption was violated for each dataset (p < 0.05), we ran non-parametric Welch’s ANOVAs with a multiple comparisons test (Games–Howell) to test for the difference in grazing across species (Games and Howell 1976). To test for a difference in grazing across kelp size classes, we ran a Welch’s t-test for each species. All analyses were run in R v 3.3.2.
Results
Maximum per capita interaction strengths
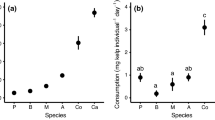
Interaction strengths of grazers on juvenile kelp varied greatly among species, and consumption rate did not scale with grazer biomass for the species examined. For both juvenile kelp size classes, the smallest (P. humeralis) and intermediate (T. brunnea) herbivore species had the lowest and greatest consumption rates, respectively. For the microscopic size class, daily maximum PCIS ranged from an average of − 0.001 for P. humeralis to − 0.009 for T. brunnea (proportion of kelp removed individual−1 m−2), representing a ninefold difference in consumption across the four herbivore species. This pattern of interaction strengths was also similar for the macroscopic size class (− 0.0006 for P. humeralis and − 0.009 for T. brunnea) (Fig. 1), further showing that T. brunnea is a dominant grazer on very small juvenile M. pyrifera. On the other hand, the largest species examined, the purple urchin, consumed an amount on par with the smallest species, P. humeralis (− 0.002 on average for the urchin, − 0.001 on average for the amphipod; Games–Howell post hoc test showed no significant difference p > 0.05), further suggesting that size alone does not predict consumption across these species. Overall, there was no significant effect of temperature on grazing for any species except P. humeralis at the macroscopic size class (P. humeralis temperature estimate = − 0.0019 ± 0.0001 SE, t = − 4.05, p = 0.001). Temperature can increase metabolic rate, and therefore grazing rates, though this was not explicitly tested in our study, and despite a range of temperatures, differences in PCIS among species were still prominent.
Daily maximum per capita interaction strength of four grazing species (left to right: S. purpuratus, T. brunnea, I. resecata, P. humeralis) on the microscopic (circles) and macroscopic (triangles) size classes of juvenile giant kelp. Data show means ± SE (n = 16 for microscopic and macroscopic kelp). More negative values indicate greater interaction strengths. A color version of this figure is available online
For the purple urchin, there was a noticeable change in interaction strength between the microscopic and macroscopic sizes of juvenile kelp. While each of the other three species showed no significant change in consumption across the two size classes (Welch’s t-test: I resecatap = 0.43, T. bunneap = 0.96, P. humeralisp = 0.10), the urchin consumed much more of the macroscopic size, tripling the amount it ate compared to microscopic M. pyrifera (average of − 0.002 for microscopic and − 0.006 for macroscopic kelp, Welch’s t-test p < 0.001) (Fig. 1). With this increase, the urchin far surpassed the amphipod but remained an intermediary consumer after the brown turban snail. This marked increase in consumption across the two size classes suggests that another factor, such as feeding morphology, likely influences urchin consumption on very small size classes of M. pyrifera.
Mass-specific grazing rates
By scaling consumption rate by body mass, the pattern across species largely reflected predictions based on metabolic theory, though urchins still consumed less than expected for mass–consumption relationships. The observed general pattern of higher to lower mass-specific grazing rate for smaller to larger species reflects metabolic theory, where mass-specific metabolic rates that affect processes like feeding are inversely related to body mass (Taylor et al. 1981). Per gram of grazer, the amphipod consumed the most, potentially removing on average 11.4% m−2 of microscopic kelp and 8.8% m−2 of macroscopic kelp. Urchins removed the least amount of kelp per gram of grazer (0.1% m−2 and 0.2% m−2 of microscopic and macroscopic kelp, respectively) (Fig. 2). Further, a post hoc test showed that all species’ mass-specific consumption rates were significantly different from one another for both the microscopic and macroscopic datasets.
Daily mass-specific grazing rate of four grazing species (left to right: S. purpuratus, T. brunnea, I. resecata, P. humeralis) on the microscopic (circles) and macroscopic (triangles) size classes of juvenile giant kelp. Data show means ± SE (n = 16 for microscopic kelp, n = 12 for macroscopic datasets). More positive values indicate greater consumption rate per gram of grazer. A color version of this figure is available online
While urchins were the largest herbivore examined and had the lowest mass-specific grazing rate, their grazing rates were strikingly low and did not follow the pattern of the other three species. Under metabolic theory, we would expect that with a larger proportional difference in body mass, there would be a larger proportional difference in mass-specific grazing rates. This held true when comparing P. humeralis and I. resecata, in addition to comparing I. resecata and T. brunnea. The larger proportional difference in mass (I. resecata weighed on average 7 × more) between P. humeralis and I. resecata led to larger proportional differences in grazing rates (P. humeralis consumed 5 × more) compared to I. resecata and T. brunnea, which were more closely matched in size and consumption (T brunnea weighed only 5 × more and ate 1/3 less on average). However, this was not true when comparing T. brunnea and S. purpuratus. Considering the difference in mass was the least of any other pairing, their difference in grazing rate was on average higher than any other pairing (Online Resource 1), meaning urchins were eating much less than what would be expected based on their body size. Additionally, when comparing the average percent removed by all individuals in the microscopic juvenile kelp experiments with the average percent removed by gram, it is clear that the urchins consumed an incredibly small of amount of kelp per gram (0.13% m−2) despite consuming an estimated total amount of kelp (2.01% m−2) on par with P. humeralis (1.18% m−2) and I. resecata (2.31% m−2) (Table 1). These findings further suggest that body mass does not always predict consumption rates on juvenile M. pyrifera and that S. purpuratus of this size range may not be able to efficiently consume kelp below a certain size.
Species’ impacts on juvenile M. pyrifera
With field density data incorporated, we estimated that the four grazers collectively have the potential to remove similar amounts of microscopic and macroscopic kelp (13.1% and 12.2% m−2 day−1, respectively). However, there were highly variable predicted impacts on juvenile kelp populations across the species (Fig. 3). In particular, incorporating densities highlighted the large effects of abundant, smaller grazers like T. brunnea and P. humeralis. The smallest species, P. humeralis, had a weak PCIS but had the highest estimated impact on microscopic kelp, potentially removing 6.4% m−2 day−1 due to its high densities (average 65.0 individuals m−2). With its high consumption rate and moderate densities (average 5.7 individuals m−2), T. brunnea also had a relatively high estimated impact (5% m−2 day−1 on both microscopic and macroscopic populations). Conversely, the purple urchin had the lowest estimated impact at the microscopic size class and was similar to I. resecata for the lowest impact at the macroscopic size. This was due to its low consumption and low densities during the survey period (average 2.7 individuals m−2), though depending on location, this impact can increase, especially in urchin barrens where urchins are found at much higher densities than in this study (e.g., Ling et al. 2015).
Daily grazing impact of four species (left to right: S. purpuratus, T. brunnea, I. resecata, P. humeralis) on the microscopic (circles) and macroscopic (triangles) size classes of juvenile giant kelp. Impact was calculated by multiplying the average densities (individuals m−2) by the average and upper and lower bounds of maximum daily PCIS (mean ± SE) from Fig. 1. Data show means ± SE (n = 16 for microscopic and macroscopic kelp). Density data are from transect surveys (T. brunnea and S. purpuratus) and the literature (I. resecata and P. humeralis). A color version of this figure is available online
Discussion
The four herbivore species examined had vastly different consumption rates, highlighting the different roles that species can play on the persistence of primary producers. Although grazing rates varied by body size and mass within a species, differences in herbivory between species were even greater. The ninefold range in interaction strengths reflects the diversity of grazer impacts within giant kelp forest communities, which is important for gaining a more nuanced picture of persistence in this foundation species. We should consider species-specific differences in impacts as crucial driving factors influencing the ability for algae to persist (Burkepile and Hay 2008; Poore et al. 2012), especially in the vulnerable early stages. Studies focusing on urchins because of their strong control on adult stages potentially overlook the impacts of other species within this herbivore guild (but see Graham 2004) that might be equally or potentially more influential than urchins on the persistence of juvenile kelp, such as the brown turban snail. Sala and Graham (2002) found similar results where several kelp forest gastropod species had similar PCIS on microscopic kelp compared to purple urchins.
Even within more closely related herbivores, species can exert different per capita effects, as in the case of the crustaceans in our study. One would expect phylogeny to play a role in contributing to similar grazing rates among closely related species, but in this study we found that there is still an effect of body size on grazing rates for P. humeralis and I. resecata. For example, isopods consumed triple the amount of juvenile kelp per capita compared to amphipods. Therefore, impacts of one species within a guild do not always translate to others, and quantifying variation in herbivore impacts can allow for more detailed understanding of interaction strengths and functional roles within communities. While not explicitly tested in our experiments, one potential interesting avenue of research would be examining the effects of phylogeny in combination with body size in driving consumption rates.
We show that herbivore impacts on a population are highly dependent on densities, especially for plants and algae that cannot move and are subject to what herbivore species are present and in what abundances in a particular location. For example, crustaceans like P. humeralis can have large population fluctuations and occur in very dense aggregations (Graham 2002), leading to potentially higher grazing impacts than larger herbivores even though their per capita consumption is low. Therefore, smaller species in high abundances can overtake larger species in moderate abundances, especially if their per capita consumption is low, as with the case of purple urchins in this study. Though, this is location dependent, so while urchins in our surveys were not very abundant, they can occur in much higher densities on rocky reefs and graze openly in areas where key predators like otters are absent. This highlights an often neglected variable in laboratory-based grazing experiments—incorporating the abundances of herbivores can alter the view of a particular species’ impact on a prey population in the field. While there are caveats to measuring grazing in the lab, for example the lack of competition and predation, not considering the composition and densities of herbivore species can under- or overestimate population-level impacts. Therefore, studying the natural history of a system and factoring this in is necessary for more accurately comparing herbivore effects on a particular primary producer.
Our results demonstrated that body mass was not the sole predictor of consumption rates and that feeding morphology is also important to consider. The smaller crustacean species and T. brunnea consumed both size classes of juvenile M. pyrifera in amounts according to their body size; however, S. purpuratus, the largest herbivore examined, surprisingly ate very little kelp and was not the most dominant consumer. This likely reflects limitations in S. purpuratus’ feeding apparatus. Their Aristotle’s lantern is adept at chewing through algae (Tegner et al. 1995), scraping rocks (Ma et al. 2008), and, in some cases, biting conspecifics (Shulman 1990). In experiments, urchins attempted to graze on tiles and left star-shaped scars. This was likely due to an inability to grab on to kelp blades rather than chemical defense production in juvenile M. pyrifera, since they do not produce a lot of compounds like tannins (Ng, unpublished data). When urchins did consume juveniles, they left half a blade, suggesting that their coarser feeding apparatus may limit their consumption on very small kelp size classes; however, it remains to be seen whether smaller urchins would be better able to consume juvenile kelp. In contrast, the brown turban snail, the most dominant consumer, has a radula, which is more ideal for scraping the substrate. The structure of rhipidoglossan (e.g., Tegula) radulae allows for wide grazing strokes much like a broom (Steneck and Watling 1982). This feeding mode allowed T. brunnea to efficiently remove whole individuals of juvenile kelp from experimental tiles. While T. brunnea may not always be targeting juvenile M. pyrifera in the field, they may consume and therefore kill juveniles as they move across substrate and sweep food into their mouths. Our results point to an important consideration in the grazing literature: predicting consumption rates depends not only on factors such as body size, but also grazer morphology in relation to prey morphology. Grazer mass may be a general predictor, but for particular prey types, such as very short plants or biofilms, feeding morphology can constrain consumption.
Changes in vulnerability to herbivory across ontogeny are consistent with age-related changes in grazing risk among plants and algae (Boege and Marquis 2005). Several factors influence vulnerability through ontogeny such as size, chemical defenses, and herbivore feeding preferences (Barker and Chapman 1990; Van Alstyne et al. 2001; Barton and Koricheva 2010). We show that in this foundation species, demographic impacts of specific herbivores change as kelp gets older, even over very small differences in kelp size. Collectively, the impact of the four grazers was similar across the two size classes (13.1% and 12.3% m−2 day−1 for microscopic and macroscopic kelp, respectively), but certain grazers become more or less dominant depending on kelp size. For example, P. humeralis decreased its per capita consumption, and S. purpuratus tripled its consumption from the microscopic to the macroscopic size class. Based on our findings, we would expect herbivores like crustaceans and gastropods to quickly drop off in their ability to kill individuals of M. pyrifera that have even a small number of fronds. Conversely, smaller M. pyrifera juveniles that potentially escape predation from purple urchins would quickly become vulnerable as they attain more biomass and height. At that point, grazers would likely fall within expected mass–consumption relationships. This highlights the nuances in examining persistence in primary producers, since smaller grazers like gastropods and crustaceans that are assumed to not exert demographic impacts on adult stages can certainly exert impacts on juveniles (Lubchenco 1983; Norton and Manley 1990). Furthermore, research on persistence should include more studies that explore how herbivore impacts change over the life cycle of their prey. This is still relatively unexplored, especially within marine systems, but is crucial for understanding how ontogeny influences vulnerability to herbivory in plants and algae.
While we found that the potential for grazer impacts on juvenile M. pyrifera can be quite sizeable, it is also imperative to consider other factors that can contribute to the persistence and recovery of giant kelp populations to place these findings in the broader context of giant kelp forest ecology. While here we focus on top-down control of M. pyrifera by herbivores, the green world hypothesis put forth an important ecological dynamic, predator control of herbivores, which can allow giant kelp to persist. For example, amphipods, which we found to be highly abundant, are important prey items for a variety of kelp forest fishes (Bray and Ebeling 1975), and their populations could be kept in check through predation. Additionally, differences in behavior, distribution, and population cycles among grazer species can contribute to variation in giant kelp survival on local scales. For urchins, large recruitment events that lead to higher densities can initiate a change in feeding where drift algae becomes unavailable and urchins switch to actively scraping the substrate (Harrold and Reed 1985). While individually urchins may not consume large amounts of juvenile M. pyrifera, high densities in combination with a behavioral switch in feeding can have large consequences for M. pyrifera recruitment. Beyond the effects of grazers, abiotic conditions and density dependence have been shown to be important drivers in giant kelp survival (Graham et al. 2007), though here we shed light on an often overlooked and understudied contributor to juvenile giant kelp survival. While grazers may not determine large scale distributions in M. pyrifera, on local scales, grazers can present a more persistent force that should be more explicitly addressed in giant kelp forest research.
This study is a detailed, comparative quantification of grazing impacts on giant kelp. We demonstrate high variation in consumption rates and bring attention to smaller herbivores that can exert demographic impacts on the juvenile stage of this coastal foundation species. This highlights priority areas for research, particularly the importance of quantifying consumption rates of herbivores that are less well-studied and incorporating those species’ impacts into demographic models. Large dominant species that are known to specialize on one life stage may not exert strong control on early life stages that can escape predation, which has implications for understanding community dynamics and management decisions. For example, restoration techniques based on transplanting or protecting juveniles should bear in mind that predators may be different at those life stages and choose locations to minimize predation events by potentially overlooked species or instead transplant larger individuals (Lubchenco 1983; Kearsley and Whitham 1989; Viejo et al. 1999). A common technique for restoring giant kelp forests in a barren state is culling urchins (e.g., Tracey et al. 2015); however, in light of this study’s findings, urchins may not be the most dominant or efficient consumer of small algal recruits and restoration techniques should take this into consideration. Overall, this work fills a major gap in knowledge about the role of herbivores on the persistence of early life stages of algae, contributing to a broader understanding of herbivore control on primary producers. Moving forward, more research is needed, especially in marine systems, to understand not only the influence of herbivores on plant and algae persistence, but also how their impacts can change through the ontogeny of their prey.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Andersen RA (2005) Algal culturing techniques. Elsevier, Burlington
Andrews HL (1945) The kelp beds of the Monterey region. Ecology 26:24–37. https://doi.org/10.2307/1931912
Angelini C, Altieri AH, Silliman BR, Bertness MD (2011) Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. Bioscience 61:782–789. https://doi.org/10.1525/bio.2011.61.10.8
Barker KM, Chapman ARO (1990) Feeding preferences of periwinkles among four species of Fucus. Mar Biol 106:113–118. https://doi.org/10.1007/bf02114681
Barton KE, Koricheva J (2010) The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. Am Nat 175:481–493. https://doi.org/10.1086/650722
Bernays EA (1998) Evolution of feeding behavior in insect herbivores. Bioscience 48:35–44. https://doi.org/10.2307/1313226
Bernstein BB, Jung N (1979) Selective pressures and coevolution in a kelp canopy community in southern California. Ecol Monogr 49:335–355. https://doi.org/10.2307/1942488
Boege K, Marquis RJ (2005) Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol Evol 20:441–448. https://doi.org/10.1016/j.tree.2005.05.001
Booth JAT, McPhee-Shaw EE, Chua P, Kingsley E, Denny M, Phillips R, Bograd SJ, Zeidberg LD, Gilly WF (2012) Natural intrusions of hypoxic, low pH water into nearshore marine environments on the California coast. Cont Shelf Res 45:108–115. https://doi.org/10.1016/j.csr.2012.06.009
Bray RN, Ebeling AW (1975) Food, activity, and habitat of 3 “picker-type” microcarnivorous fishes in kelp forests off Santa Barbara, California. Fish Bull 73:815–829
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. https://doi.org/10.1890/03-9000
Burkepile DE, Hay ME (2008) Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Natl Acad Sci 105:16201–16206. https://doi.org/10.1073/pnas.0801946105
Cates RG (1980) Feeding patterns of monophagous, oligophagous, and polyphagous insect herbivores: the effect of resource abundance and plant chemistry. Oecologia 46:22–31. https://doi.org/10.1007/bf00346961
Davenport AC, Anderson TW (2007) Positive indirect effects of reef fishes on kelp performance: the importance of mesograzers. Ecology 88:1548–1561. https://doi.org/10.1890/06-0880
Dayton PK (1972) Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica Proceedings of the Colloquium on Conservation Problems in Antarctica. Allen Press Lawrence, Kansas, USA, pp 81–96
Estes JE, Smith NS, Palmisano JF (1978) Sea otter predation and community organization in the western Aleutian Islands, Alaska. Ecology 59:822–833. https://doi.org/10.2307/1938786
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240. https://doi.org/10.1126/science.281.5374.237
Games PA, Howell JF (1976) Pairwise multiple comparison procedures with unequal n’s and/or variances: a Monte Carlo study. J Educ Stat 1:113–125. https://doi.org/10.2307/1164979
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251. https://doi.org/10.1126/science.1061967
Graham MH (2002) Prolonged reproductive consequences of short-term biomass loss in seaweeds. Mar Biol 140:901–911. https://doi.org/10.1007/s00227-001-0761-x
Graham MH (2004) Effects of local deforestation on the diversity and structure of southern California giant kelp forest food webs. Ecosystems 7:341–357. https://doi.org/10.1007/s10021-003-0245-6
Graham MH, Vasquez JA, Buschmann AH (2007) Global ecology of the giant kelp Macrocystis: from ecotypes to ecosystems. Oceanogr Mar Biol Annu Rev 45:39
Gullström M, Bodin M, Nilsson PG, Öhman MC (2008) Seagrass structural complexity and landscape configuration as determinants of tropical fish assemblage composition. Mar Ecol Prog Ser 363:241–255. https://doi.org/10.3354/meps07427
Hairston NG, Smith FE, Slobodkin LB (1960) Community structure, population control, and competition. Am Nat 94:421–425. https://doi.org/10.1086/282146
Hammer RM, Zimmerman RC (1979) Species of demersal zooplankton inhabiting a kelp forest ecosystem off Santa Catalina Island, California. Bull South Calif Acad Sci 78:199–206
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM (2007) Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst 8:157–178. https://doi.org/10.1016/j.ppees.2007.01.001
Hardy RA (1973) A survey of the marine environment near the city of Monterey ocean outfall. Marine Resources Administrative Report California Department of Fish and Game, Long Beach
Harrold C, Reed DC (1985) Food availability, sea urchin grazing, and kelp forest community structure. Ecology 66:1160–1169. https://doi.org/10.2307/1939168
Hay ME, Fenical W (1988) Marine plant-herbivore interactions: the ecology of chemical defense. Annu Rev Ecol Syst 19:111–145. https://doi.org/10.1146/annurev.ecolsys.19.1.111
Hillebrand H et al (2009) Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol Lett 12:516–527. https://doi.org/10.1111/j.1461-0248.2009.01304.x
Hochuli DF (2001) Insect herbivory and ontogeny: how do growth and development influence feeding behaviour, morphology and host use? Austral Ecol 26:563–570. https://doi.org/10.1111/j.1442-9993.2001.tb00137.x
Hooper DU, Vitousek PM (1997) The effects of plant composition and diversity on ecosystem processes. Science 277:1302–1305. https://doi.org/10.1126/science.277.5330.1302
Huntly N (1991) Herbivores and the dynamics of communities and ecosystems. Annu Rev Ecol Syst 22:477–503. https://doi.org/10.1146/annurev.ecolsys.22.1.477
Kearsley MJ, Whitham TG (1989) Developmental changes in resistance to herbivory: implications for individuals and populations. Ecology 70:422–434. https://doi.org/10.2307/1937547
Leighton DL (1966) Studies of food preference in algivorous invertebrates of southern California kelp beds. Pac Sci 20:104–113
Light SF (2007) The Light and Smith manual: intertidal invertebrates from central California to Oregon. University of California Press, Berkeley
Ling SD et al (2015) Global regime shift dynamics of catastrophic sea urchin overgrazing. Philos Trans Royal Soc B 370:20130269. https://doi.org/10.1098/rstb.2013.0269
Lowry L, Pearse JS (1973) Abalones and sea urchins in an area inhabited by sea otters. Mar Biol 23:213–219. https://doi.org/10.1007/bf00389487
Lubchenco J (1983) Littorina and Fucus: effects of herbivores, substratum heterogeneity, and plant escapes during succession. Ecology 64:1116–1123. https://doi.org/10.2307/1937822
Ma Y, Cohen SR, Addadi L, Weiner S (2008) Sea urchin tooth design: an “All-Calcite” polycrystalline reinforced fiber composite for grinding rocks. Adv Mater 20:1555–1559. https://doi.org/10.1002/adma.200702842
MacArthur RH (1972) Strong, or weak, interactions. Trans Conn Acad Arts Sci 44:177–188
Mann KH (1977) Destruction of kelp-beds by sea-urchins: a cyclical phenomenon or irreversible degradation? Helgoländer Wissenschaftliche Meeresuntersuchungen 30:455. https://doi.org/10.1007/bf02207854
Milchunas DG, Noy-Meir I (2002) Grazing refuges, external avoidance of herbivory and plant diversity. Oikos 99:113–130. https://doi.org/10.1034/j.1600-0706.2002.990112.x
Ng CA, Micheli F (2020) Short-term effects of hypoxia are more important than effects of ocean acidification on grazing interactions with juvenile giant kelp (Macrocystis pyrifera). Sci Rep 10:5403. https://doi.org/10.1038/s41598-020-62294-3
Norton TA, Manley NL (1990) The characteristics of algae in relation to their vulnerability to grazing snails. Behavioural mechanisms of food selection. Springer, New York, pp 461–478
Novotny V et al (2010) Guild-specific patterns of species richness and host specialization in plant–herbivore food webs from a tropical forest. J Anim Ecol 79:1193–1203. https://doi.org/10.1111/j.1365-2656.2010.01728.x
Parker MA (1985) Size-dependent herbivore attack and the demography of an arid grassland shrub. Ecology 66:850–860. https://doi.org/10.2307/1940547
Parker GG et al (2004) Three-dimensional structure of an old-growth Pseudotsuga-Tsuga canopy and its implications for radiation balance, microclimate, and gas exchange. Ecosystems 7:440–453. https://doi.org/10.1007/s10021-004-0136-5
Pearse JS (2006) Ecological role of purple sea urchins. Science 314:940–941. https://doi.org/10.1126/science.1131888
Poore AGB et al (2012) Global patterns in the impact of marine herbivores on benthic primary producers. Ecol Lett 15:912–922. https://doi.org/10.1111/j.1461-0248.2012.01804.x
Sala E, Boudouresque C, Harmelin-Vivien M (1998) Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos. https://doi.org/10.2307/3546364
Sala E, Graham MH (2002) Community-wide distribution of predator–prey interaction strength in kelp forests. Proc Natl Acad Sci 99:3678–3683. https://doi.org/10.1073/pnas.052028499
Shulman MJ (1990) Aggression among sea urchins on Caribbean coral reefs. J Exp Mar Biol Ecol 140:197–207. https://doi.org/10.1016/0022-0981(90)90127-x
Silliman BR, McCoy MW, Angelini C, Holt RD, Griffin JN, van de Koppel J (2013) Consumer fronts, global change, and runaway collapse in ecosystems. Annu Rev Ecol Evol Syst 44:503–538. https://doi.org/10.1146/annurev-ecolsys-110512-135753
Silliman BR, Newell SY (2003) Fungal farming in a snail. Proc Natl Acad Sci 100:15643–15648. https://doi.org/10.1073/pnas.2535227100
Silliman BR, Zieman JC (2001) Top-down control of Spartina alterniflora production by periwinkle grazing in a Virginia salt marsh. Ecology 82:2830–2845. https://doi.org/10.2307/2679964
Steneck RS, Watling L (1982) Feeding capabilities and limitation of herbivorous molluscs: a functional group approach. Mar Biol 68:299–319. https://doi.org/10.1007/bf00409596
Taylor CR, Maloiy GMO, Weibel ER, Langman VA, Kamau JMZ, Seeherman HJ, Heglund NC (1981) Design of the mammalian respiratory system. III. Scaling maximum aerobic capacity to body mass: wild and domestic mammals. Respir Physiol 44:25–37. https://doi.org/10.1016/0034-5687(81)90075-x
Tegner MJ, Dayton PK, Edwards PB, Riser KL (1995) Sea urchin cavitation of giant kelp (Macrocystis pyrifera C. Agardh) holdfasts and its effects on kelp mortality across a large California forest. J Exp Mar Biol Ecol 191:83–99. https://doi.org/10.1016/0022-0981(95)00053-t
Tracey SR, Baulch T, Hartmann K, Ling SD, Lucieer V, Marzloff MP, Mundy C (2015) Systematic culling controls a climate driven, habitat modifying invader. Biol Invasions 17:1885–1896. https://doi.org/10.1007/s10530-015-0845-z
Valentine JF, Heck KL (1999) Seagrass herbivory: evidence for the continued grazing of marine grasses. Mar Ecol Prog Ser 176:291–302. https://doi.org/10.3354/meps176291
Van Alstyne KL, Whitman SL, Ehlig JM (2001) Differences in herbivore preferences, phlorotannin production, and nutritional quality between juvenile and adult tissues from marine brown algae. Mar Biol 139:201–210. https://doi.org/10.1007/s002270000507
Viejo RM, Åberg P, Cervin G, Lindegarth M (1999) The interactive effects of adult canopy, germling density and grazing on germling survival of the rockweed Ascophyllum nodosum. Mar Ecol Prog Ser 187:113–120. https://doi.org/10.3354/meps187113
Watanabe JM (1984) The influence of recruitment, competition, and benthic predation on spatial distributions of three species of kelp forest gastropods (Trochidae: Tegula). Ecology 65:920–936. https://doi.org/10.2307/1938065
Wilkinson DM, Sherratt TN (2016) Why is the world green? The interactions of top–down and bottom–up processes in terrestrial vegetation ecology. Plant Ecol Divers 9:127–140. https://doi.org/10.1080/17550874.2016.1178353
Wittstock U, Agerbirk N, Stauber EJ, Olsen CE, Hippler M, Mitchell-Olds T, Gershenzon J, Vogel H (2004) Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc Natl Acad Sci 101:4859–4864. https://doi.org/10.1073/pnas.0308007101
Wootton JT (1997) Estimates and tests of per capita interaction strength: diet, abundance, and impact of intertidally foraging birds. Ecol Monogr 67:45–64. https://doi.org/10.2307/2963504
Acknowledgements
We thank A. Muth and M. Graham for support in developing the kelp culturing methods; N. Low, P. Leary, and J. Lee for help with the aquarium system; K. Chang-Haines, A. Wachtell, M. Pobis, J. Salazar, T. Leggett, L. Anderson, A. Charlesworth, C. Aragon, and A. Meislin for help in the field and laboratory; J. Cohen for the illustrations; and J. Barry, L. Crowder, and R. Dirzo for advice on the experimental design and data analyses.
Funding
This work was supported by the National Science Foundation (DEB-1212124, DGE-114747, BioOce 1736830 and 1722513), The Women Divers Hall of Fame, and the Myers Oceanographic and Marine Biology Trust.
Author information
Authors and Affiliations
Contributions
CAN and FM conceived and designed the experiments. CAN performed the experiments, collected and analyzed the data, and wrote the first draft. Both authors contributed equally to subsequent revisions of the analyses and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Communicated by Pablo Munguia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ng, C.A., Micheli, F. Size-dependent vulnerability to herbivory in a coastal foundation species. Oecologia 193, 199–209 (2020). https://doi.org/10.1007/s00442-020-04655-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04655-3