Abstract
Invasive species often use habitat differently than native species and can benefit by occupying underutilized habitats during the invasion process. The Peter’s Rock Agama (Agama picticauda)—native to savannahs of sub-Saharan Africa—is successfully invading urban habitats in Florida, USA. During a field trip in urban southern Florida, we observed apparently high A. picticauda abundance around dumpsters used for human refuse, potentially because dumpsters provide refuge, thermoregulatory opportunities, abundant arthropod prey, and harbor few competitors. In this study, we surveyed abundance and built resource selection functions to better understand habitat use of A. picticauda in urban southern Florida. We tested whether hypothesized habitat features predictably influenced the abundance and occupancy of A. picticauda among sites and whether individuals used specific habitat features within sites. Across sites, we found A. picticauda abundance was positively correlated with the number of dumpsters, and, within sites, dumpsters were preferentially selected as habitat. Similarly, we also found two other anthropogenic structures, building crevices and electrical units, were positively selected habitats at population and individual scales. We hypothesize that dumpsters, crevices, and electrical units are selected resources because they are underutilized habitats by other species and they provide refuge, beneficial thermoregulatory opportunities, and in the case of dumpsters, foraging opportunities. Our study provides the first quantitative assessment of urban habitat use by non-native A. picticauda, and supports the importance of human structures as habitat. Our results suggest the intriguing possibility that the A. picticauda invasion in Florida may be exploiting a vacant niche in urban habitats during the invasion process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Main text
Invasive species often use human-modified habitats during the invasion process. The Peter’s Rock Agama (Agama picticauda) is an invasive species in southern Florida, USA (Enge et al. 2004; Nuñez et al. 2016). Native to sub-Saharan Africa, A. picticauda is a territorial, primarily insectivorous species that inhabits rocky outcroppings where it thermoregulates to achieve relatively high preferred body temperatures (35.5–37.0 °C; James and Porter 1979). In 2016, we collected invasive A. picticauda from Homestead, Florida, USA, to have stock for an incubation experiment (Steele et al. 2018). While collecting lizards, we observed individuals of all sizes and sexes frequently interacting with one another around dumpsters, using the dumpsters as refuges, and feeding on insects attracted to refuse associated with the dumpsters. Upon our approach, A picticauda typically fled beneath the dumpsters. Indeed, we collected most A. picticauda from beneath and around dumpsters due to the species’ high densities there. Our field observations led us to hypothesize that dumpsters may provide important habitat for A. picticauda with abundant thermoregulatory opportunity, food resources (both natural and anthropogenic; Ofori et al. 2018), and refuges beneath the dumpster. Interestingly, while other lizard species (e.g. anoles) occur at high densities in the area, lizards other than A. picticauda were uncommon or absent from these sites, which were mostly paved and had sparse vegetation (Online Resource 1, OR1-3). Given our hypothesis that dumpsters are high-quality sites preferentially selected by A. picticauda in south Florida, we sought to quantitatively evaluate urban habitat use by A. picticauda in an attempt to understand invasion process in the region.
To test this hypothesis, we revisited our original collection sites (N = 8; hereafter “focal sites”) in 2017, as well as nearby sites (N = 22; hereafter “random sites”). For each focal site, there were up to four random sites 115 m (m) away in each of the four cardinal directions. Several random sites were not surveyed because they were inaccessible (see below). We surveyed each site for A. picticauda and recorded the habitat that each individual was using, and then quantified the habitat available to lizards within each site. This design allowed us to assess resource selection of habitat among and within sites. Overall, we predicted that dumpsters and other anthropogenic features that provide refuges (e.g. wall crevices) were important resources selected by A. picticauda. Specifically, we made three predictions: (1) because A. picticauda is a rock-dwelling species, abundance will increase with the number of refuges, such as dumpsters and crevices, (2) occupancy probability will be greater at sites that contain dumpsters and other human-made structures compared to sites lacking such features, and (3) A. picticauda selectively use human microhabitats with narrow openings (e.g. dumpsters, crevices). Predictions 1 and 2 allowed us to test for second-order habitat selection (i.e. factors influencing the location of populations), while prediction 3 allowed us to test for third-order habitat selection (i.e. factors influencing habitat use within an individual’s home range; sensu Johnson 1980). Our study focuses on known A. picticauda sites in a small part of their non-native range and is not designed to estimate the relative importance of dumpsters and other human structures in contributing to the establishment of A. picticauda. Rather, it is designed to provide a quantitative description of post-establishment habitat use in the area.
On 14 June 2017, we visited the eight focal sites and confirmed the presence of A. picticauda at each site. To demarcate the center of each focal site, we identified a point at the apparent central area of lizard habitat by observing lizards basking and fleeing toward refuges and recorded the location with GPS. We then selected additional sites by measuring 115 m in each cardinal direction from the center of each focal site (32 additional sites). While additional sites were not selected in a truly random fashion, we considered them as ‘random’ sites (hereafter, random sites) because we had no a priori knowledge of the sites and assumed they would accurately characterize nearby available habitat. We quantified habitats at all sites that were legally and safely accessible. However, we could not assess 10 of the random sites for various reasons (e.g. within inaccessible private property, center of a road), which reduced the sample to 22 random sites. Random sites were likely outside the range of the lizards in the focal sites (Harris 1964; Yeboah 1982), but near enough that they could all have been occupied if the habitat was suitable.
During 15–16 June 2017, we visited all focal and random sites, visually searched for lizards from a parked vehicle for several minutes, and finally approached lizards on foot. While observing from the vehicle, we counted the number of lizards we saw (i.e. abundance). Agama picticauda are highly conspicuous during sunny, daylight hours, as they bask, forage, or interact with conspecifics in relatively open habitats. We then approached lizards on foot. Individuals typically fled to nearby refuges such as trees, dumpsters or crevices, which we recorded as used habitat. From the center of each site, we measured a circle with 20 m radius and quantified major habitat structures within, including trees, bushes, crevices (e.g. crack in the foundation of a building), dumpsters, electrical boxes (e.g. typically green painted metal structure that house utilities), and vertical structures (e.g. wall of building). We selected a 20 m radius because that area would encompass a typical territory size (mean ± st. dev.; 286 ± 91 m2) for the species (Yeboah 1982). If the habitat structure was a potential refuge, we measured the gap of the opening (e.g. gap between asphalt and dumpster bottom, width of crevice opening). We illustrate habitat use by A. picticauda using various structures (Online Resource 1, OR1–OR3).
We assessed resource selection in three ways. First, we used linear regression to test for a relationship between species’ abundance and habitat features among sites. Second, we used second- and third-order habitat selection analyses (sensu Johnson 1980) to evaluate whether the presence of A. picticauda is influenced by habitat features both among (second-order selection) and within (third-order selection) populations. To assess second- and third-order habitat selection, we analyzed use-availability data to understand the factors influencing the relative probability of (1) the species’ occupying habitat patches among sites (second-order selection) and (2) individuals occupying habitat features within sites (third-order selection).
For each analysis, we assumed that all sites were available and that the species selects for habitat among and within sites. We developed a priori hypotheses of habitat features (i.e. covariates) that could influence the abundance or resource selection of A. picticauda. These features were both natural (e.g. number of trees) and anthropogenic (e.g. number of dumpsters). We performed an all-subsets analysis to evaluate all combinations of habitat features that might influence abundance or resource selection. After excluding covariates with ≤ 5 observations, we included the number of trees, bushes, crevices, garbage dumpsters, electrical boxes, air-conditioning units, walls, utility poles, and wooden structures as covariates in the analysis.
We used generalized linear regression models to estimate effects of habitat on lizard abundance (using a Gaussian distribution) and two scales of habitat use (using a binomial distribution). We could not use mixed-effect models to account for random, site-level effects because our sample size introduced issues of singularity and model convergence with mixed-models. For each analysis, we used package ‘MuMIn’ (Barton 2009) in the statistical Program R (R Core Team 2018) to rank models with AICc and compute model-averaged coefficients. We considered parameters useful for inference if they were included within the top-model set of ΔAICc < 2.00 and secondarily if their model-averaged 95% confidence intervals did not overlap zero (Burnham and Anderson 2010).
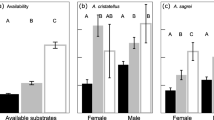
During surveys, we observed 39 individual A. picticauda at 14 of 30 total sites (observed site occupancy = 46.7%). Lizards and human structures were present at both focal sites (mean ± st. dev; A. picticauda, 3.9 ± 1.2; dumpsters, 1.4 ± 1.6; crevices, 1.3 ± 1.2; electrical units, 0.8 ± 0.5) and random sites (A. picticauda, 0.4 ± 0.7; dumpsters, 0.1 ± 0.5; crevices, 0.05 ± 0.21; electrical units, 0.3 ± 0.7). The top-model set for abundance indicated that crevices, dumpsters, and electrical units influenced A. picticauda abundance among sites (Online Resource 1, Table OR1). Model-averaged coefficients indicated that for each additional crevice, dumpster, or electrical unit, A. picticauda abundance increased by 1.01 (0.52–1.51, 95% CI), 0.73 (0.38–1.07, 95% CI) and 0.69 (0.13–1.26, 95% CI) individuals, respectively (Fig. 1). Second-order selection analyses indicated that dumpsters, electrical units, and vertical structures were important factors influencing site occupancy in the top-model set (Online Resource 1, Table OR1), but only electrical units had an effect size that did not overlap zero: for each additional electrical unit, sites were 18.6 times (1.3–300.0, 95% CI) more likely to be occupied. Analysis of third-order resource selection indicates that cement surfaces, crevices, dumpsters, and bushes influence microhabitat use (Online Resource 1, Table OR1), and the model-averaged effects of bushes (1.76; 0.49–3.02, 95% CI), crevices (2.21; 0.65–3.77, 95% CI), and dumpsters (1.93; 0.62–3.23, 95% CI) did not overlap zero. Compared to randomly available habitat, A. picticauda are 5.0 (1.6–20.7, 95% CI) times more likely to use bushes, 7.7 (1.9–43.6, 95% CI) times more likely to use crevices, and 6.1 (1.9–25.3, 95% CI) times more likely to use dumpsters.
Our analysis supports the hypothesis that dumpsters and other anthropogenic structures, like crevices and electrical units, are important habitat features for invasive A. picticauda in urban southern Florida at both the individual and population level. At the population level, abundance was positively predicted by the number of dumpsters, electrical units, and crevices present, while within sites, individuals preferred to use crevices and dumpsters. A common feature among the human-made structures is that they provide suitable refuge in the form of narrow openings within which lizards can hide. Dumpsters and electrical units are most frequently located on asphalt and concrete, which may provide favorable thermal properties for A. picticauda during thermoregulation. In particular, most dumpsters and electrical units are on paved surfaces (e.g. parking lots, alleys, concrete pads; Online Resource 1, Figure OR1), which are likely warmer and facilitate A. picticauda thermoregulation. Finally, human refuse within dumpsters may attract prey food resources for A. picticauda, or the refuse may attract the lizards directly (Powell and Henderson 2008; Uyeda et al. 2015; Ofori et al. 2018).
In this preliminary study, we have provided a quantitative description of habitat use by A. picticauda in urban southern Florida and shown that the species selectively uses dumpsters, crevices, and electrical units. Our study does not assess whether such human structures are critical to their invasion success (sensu Hulbert et al. 2020; Warner et al. 2021). We also made several observations that warrant additional study. For example, A. picticauda in our urban sites appeared to use habitat coinhabited by few (if any) potential competitors (e.g. Leiocephalus, Smith and Engeman 2004; Ameiva, Powell and Henderson 2008). Other lizard species were absent or in very low abundance during our study at the sites; we speculate whether this observation indicates that A. picticauda may be exploiting a ‘vacant niche’ in Florida (Rohde 2006). Further work is required to understand whether human structures facilitate A. picticauda invasion and how they use habitat elsewhere in their invasive range. For example, random visual transect surveys in urban and non-urban environments would provide an independent assessment of associations between A. picticauda (and other potential competitors) with potential habitat features. We also observed antagonistic interactions between males around dumpsters, and these areas included numerous females and juveniles, which indicates these sites harbor breeding populations and dumpsters may be defended as territories. Straightforward investigations could be designed to address questions such as: (1) What other habitat does A. picticauda use in urban or non-urban environments? (2) What mechanisms (e.g. thermoregulation, food availability) drive anthropogenic habitat selection in urban areas? (3) Are there fitness-associated benefits to dumpster-dwelling, such as increased reproduction or survival? (4) Does human refuse collection and deposition aid in the dispersal of A. picticauda (Kolbe et al. 2016)? Addressing such questions will provide a more mechanistic understanding how habitat use influences population demography and invasion process of A. picticauda in Florida.
Availability of data and code
All relevant data and code is archived here: http://dx.doi.org/10.35099/aurora-49.
References
Barton K (2009) MuMIn: multi-model inference. R Package version 0120
Burnham KP, Anderson DR (2010) Model selection and multimodel inference: a practical information-theoretic approach, second. New York City, NY USA
Enge KM, Krysko KL, Talley BL (2004) Distribution and ecology of the introduced African rainbow lizard, Agama agama africana (Sauria: Agamidae), in Florida. Florida Sci 67:303–310
Harris V (1964) The life of the rainbow lizard. Hutchinson & Co, Bengaluru
Hulbert AC, Hall JM, Mitchell TS, Warner DA (2020) Use of human-made structures facilitates persistence of a non-native ectotherm. Biol Invasions 22:2017–2031
James FC, Porter WP (1979) Behavior-microclimate relationships in the African rainbow lizard, Agama agama. Copeia 1979:585–593
Johnson DH (1980) The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65–71
Kolbe JJ, VanMiddlesworth P, Battles AC et al (2016) Determinants of spread in an urban landscape by an introduced lizard. Landsc Ecol 31:1795–1813
Nuñez LP, Krysko KL, Avery ML (2016) Confirmation of introduced Agama picticauda in Florida based on molecular analyses. Bull Fla Mus Nat Hist 54:138–146
Ofori BY, Martey P, Attuquayefio DK (2018) Observations of the African rainbow lizard (Agama picticauda Peters 1877) from Ghana feeding on bread. Herpetol Notes 11:955–957
Powell R, Henderson RW (2008) Urban herpetology in the West Indies. Urban Herpetol 3:389–404
Rohde K (2006) Nonequilibrium ecology. Cambridge University Press, Cambridge
R Core Team (2018) R: a language and environment for statsitcal computing
Smith H, Engeman R (2004) A review of the colonization dynamics of the northern curly-tailed lizard (Leiocephalus carinatus arimouri) in Florida. Fla F Nat 32:107–113
Steele AL, Wibbels T, Warner DA (2018) Revisiting the first report of temperature-dependent sex determination in a vertebrate, the African redhead agama. J Zool 306:16–22
Uyeda LT, Iskandar E, Kyes RC, Wirsing AJ (2015) Encounter rates, agonistic interactions, and social hierarchy among garbage-feeding water monitor lizards (Varanus salvator bivittatus) on Tinjil Island, Indonesia. Herpetol Conserv Biol 10:753–764
Warner DA, Hall JM, Fargevieille A et al (2021) Dependence on a human structure influences the extinction of a non-native lizard population after a major environmental change. Biol Invasions 23:825–842
Yeboah S (1982) Observations on territory of the rainbow lizard, Agama agama. Afr J Ecol 20:187–192
Acknowledgements
We thank Sarin Tiatragul for providing housing and Jonathan Bjork for insights on waste management. Daniel Warner provided useful discussions, resources, and comments. Natalie Claunch, Christina Romagosa, Brad Udell, and Emilie Snell-Rood and her lab provided helpful comments on the manuscript.
Funding
NSF DBI-1402202 to Timothy Mitchell. Joshua M. Hall acknowledges financial support from the Alabama Graduate Research Scholars Program (GRSP) funded through the Alabama Commission for Higher Education and administered by the Alabama EPSCoR.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by Timothy Mitchell and Brian Folt. All authors contributed to field observations. Brian Folt performed data analysis. The first draft of the manuscript was written by Timothy Mitchell and all authors critically revised prior drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Research was approved by the Auburn University Institutional Animal Care and Use Committee (2016–2881).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mitchell, T.S., Folt, B. & Hall, J.M. Dumpsters and other anthropogenic structures as habitat for invasive African rock agama lizards in Florida. Biol Invasions 23, 2689–2693 (2021). https://doi.org/10.1007/s10530-021-02537-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02537-0