Abstract
Context
Urban landscapes are a mixture of built structures, human-altered vegetation, and remnant semi-natural areas. The spatial arrangement of abiotic and biotic conditions resulting from urbanization doubtless influences the establishment and spread of non-native species in a city.
Objectives
We investigated the effects of habitat structure, thermal microclimates, and species coexistence on the spread of a non-native lizard (Anolis cristatellus) in the Miami metropolitan area of South Florida (USA).
Methods
We used transect surveys to estimate lizard occurrence and abundance on trees and to measure vegetation characteristics, and we assessed forest cover and impervious surface using GIS. We sampled lizard body temperatures, habitat use, and relative abundance at multiple sites.
Results
At least one of five Anolis species occupied 79 % of the 1035 trees surveyed in primarily residential areas, and non-native A. cristatellus occupied 25 % of trees. Presence and abundance of A. cristatellus were strongly associated with forest patches, dense vegetation, and high canopy cover, which produced cooler microclimates suitable for this species. Presence of A. cristatellus was negatively associated with the ecologically similar non-native A. sagrei, resulting in reduced abundance and a shift in perch use of A. cristatellus.
Conclusions
The limited spread of A. cristatellus in Miami over 35 years is due to the patchy, low-density distribution of wooded habitat, which limits dispersal by diffusion. The presence of congeners may also limit spread. Open habitats—some parks, yards and roadsides—contain few if any A. cristatellus, and colonization of isolated forest habitat appears to depend on human-mediated dispersal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upon arrival in a city, non-native species encounter a spatially heterogeneous environment that varies in the types and densities of buildings, vegetation, infrastructure, and remnant natural areas (Pickett et al. 2001; Cadenasso et al. 2007; Forman 2014). This variability in habitat structure and its spatial patterning will likely influence the ability of invaders to establish and spread within an urban area. For example, exotic grey squirrels in the UK are positively associated with increased canopy cover, larger trees, and the presence of seed-bearing trees as well as supplementary feeders for birds (Bonnington et al. 2014). Thus, the arrangement of vegetation and non-vegetative features within the urban landscape influences where exotics establish and the routes by which they spread. Identifying those features associated with the occurrence of exotic species is important for understanding their current distributions and potential for future spread.
As a consequence of habitat structure modification during urbanization, city temperatures can be several degrees higher than surrounding rural areas (Akbari et al. 2001; Arnfield 2003; Chen and Jim 2008; Rizwan et al. 2008). These urban heat islands are spatially and temporally heterogeneous (Ramalho and Hobbs 2011), reflecting variation in the matrix of built structures and local vegetation and creating a thermal mosaic (Georgi and Zafiriadis 2006; Hamdi and Schayes 2008; Huang et al. 2008). This variation influences the microclimates available in a city, including air and surface temperatures, relative humidity, solar radiation, and wind speed. Thermal microclimates are critically important to ectotherms (e.g., insects, lizards, frogs), which rely on external sources of heat to regulate their body temperatures. Because temperature is fundamentally important for development, growth, survival, and reproduction in ectotherms (Angilletta 2009), organisms living in a city are likely to be sensitive to variation in vegetation and urban features that affect thermal microclimates (Ackley et al. 2015a).
In addition to the habitat structure of a city, interactions with potential competitors and predators can influence occurrence and abundance patterns (Shochat et al. 2006; Anderson and Burgin 2008). For example, abundance of a native gecko, Lepidodactylus lugubris, throughout the Pacific is strongly influenced by interactions with a competitively superior non-native gecko, Hemidactylus frenatus (Case et al. 1994), which better exploits insect resources concentrated under artificial night lighting (Petren and Case 1996). In general, more ecologically similar species are predicted to have stronger negative effects on each other at local scales through competition (Pianka 1981; Losos 1994). Thus, both biotic and abiotic factors may influence the establishment, spread, and ultimately the distribution of non-native species in a city.
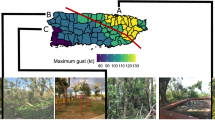
In this study, we investigate the effects of habitat structure, thermal microclimates, and species interactions on the spread of introduced Anolis lizards in the Miami metropolitan area. Our study group, Anolis lizards (or anoles), comprise a species-rich genus of small, insectivorous, diurnal lizards found in the Neotropics from the southeastern United States to South America including Caribbean islands (Losos 2009). Many Anolis species occupy both natural and human-modified areas in their native and non-native ranges ( Irschick et al. 2005; Perry et al. 2008; Marnocha et al. 2011; Kolbe et al. 2015). There are nine Anolis species established in Miami, only one of which—A. carolinensis—is native to the U.S. (Lever 2003; Kolbe et al. 2007; Kraus 2009). Four species have very restricted distributions (A. chlorocyanus, A. cybotes, A. garmani and A. porcatus), two are distributed throughout the Miami area (A. distichus and A. equestris), and one is found throughout Florida, the Gulf Coast, and southern Georgia and South Carolina (A. sagrei). In contrast to these either very restricted or widespread species, an eighth non-native species, A. cristatellus (Fig. 1, inset), is expanding its distribution in Miami, but is not yet ubiquitous. We can therefore identify factors related to its current distribution and predict whether future spread in urban areas is likely.
Anolis lizards have a number of advantages for this study. First, anoles in Miami are conspicuous, easy to detect, and sufficiently different in ecology and morphology to accurately identify to species when present. Second, the introduction history of A. cristatellus in Miami is well studied with two independent introductions from its native range in Puerto Rico (Kolbe et al. 2007). Third, the other four Anolis species that co-occur with A. cristatellus in Miami—A. carolinensis, A. distichus, A. equestris and A. sagrei—were all present prior to its introduction in the mid-1970s. These species span a range of ecological similarity; specifically, A. sagrei and A. distichus typically perch lower to the ground on tree trunks, similar to A. cristatellus, whereas A. carolinensis and A. equestris perch higher in the canopy (Losos 2009). We can therefore test the hypothesis that more ecologically similar congeners influence the presence of A. cristatellus in Miami. Lastly, the thermal biology of anoles in general, and A. cristatellus in particular, is well studied (Losos 2009). Previous studies detail the thermal preferences, thermal tolerances, and field body temperatures of A. cristatellus from numerous sites in Puerto Rico and Miami (e.g., Huey 1974; Huey and Webster 1976; Hertz 1992; Leal and Gunderson 2012; Kolbe et al. 2012; Gunderson and Leal 2012), allowing us to evaluate if the effects of urban vegetation on thermal microclimates are relevant to A. cristatellus.
A primary goal of this study is to contrast how abiotic and biotic aspects of the urban environment influence the current distribution and abundance of a recently introduced species to better understand its potential for future spread. We survey lizards and vegetation characteristics on a tree-by-tree basis using transects across putative distribution boundaries, and test for relationships at the landscape level between the presence of A. cristatellus and GIS-based data attributes of forest cover and impervious surfaces. We predict that (1) urban vegetation structure and arrangement will influence the occurrence and abundance of A. cristatellus. In particular, we predict that A. cristatellus will be associated with denser vegetation and forested areas, which produce relatively cooler microclimates. Based on previous ecological studies (Losos 2009), we also predict (2) negative associations between A. cristatellus and its more ecologically similar congeners in Miami. Specifically, A. sagrei and A. distichus overlap most with A. cristatellus in their structural microhabitat (i.e., the height, diameter, and type of perch), which should lead to stronger interspecific competition.
Methods
Study area
We conducted this study in the Miami metropolitan area, where the initial sites of introductions for A. cristatellus are documented. Genetic analyses confirmed two independent introductions from geographically and genetically distinct native-range sources in Puerto Rico (Kolbe et al. 2007). The Key Biscayne population is from San Juan, Puerto Rico and was first detected in a residential area on the island in 1975 (Schwartz and Thomas 1975; Bartlett and Bartlett 1999). The South Miami population is from northeast Puerto Rico and was found in a different residential area in 1976 (Wilson and Porras 1983). The Key Biscayne population is ~5 km from the mainland population separated by a bridge to Virginia Key and the Rickenbacker Causeway to the mainland. The two introduction sites are ∼12 km apart across Biscayne Bay. The bulk of the study area is residential with detached single units, considerable tree cover, and low-traffic, two-lane roads. Also present are commercial districts, high-traffic roads up to six lanes, open parklands, urban forests, and waterways such as canals, lakes, and coastal areas (Fig. 1, Supplementary Fig. 1).
Study design and sampling
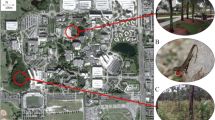
In summer 2009, we collected preliminary data on A. cristatellus presence in the Miami area by conducting block-by-block walking surveys radiating from the initial points of introduction in South Miami and Key Biscayne. Based on these data, we established five 610 × 1100 m plots in South Miami, each crossing an observed transition from presence to absence of A. cristatellus. In each plot, we established three to six roadside transects running perpendicular to the transition zone (Supplementary Fig. 2). On our initial visit to each transect in June 2010, we measured tree characteristics (Table 1) and, using binoculars when needed, observed Anolis lizards on trees with a trunk diameter >10 cm growing in the roadside public right-of-way. Although lizards use smaller trees, the availability of such trees was limited along roadsides and in yards. All species have multiple diagnostic features, which aided accurate species identification. Following this initial survey, we returned to each transect two more times to survey the same trees for the presence and total number of lizards of the five Anolis species. One to three trained observers were present for each survey, with at least two observers in most cases. Data from these transects were used to evaluate whether the presence of A. cristatellus was related to the presence of congeners and to the characteristics of the trees and surrounding vegetation (Table 1).
Given patterns of species coexistence from these transects, we conducted visual encounter surveys to determine if congener presence affects the relative abundance and habitat use of A. cristatellus (Crump and Scott 1994; Kolbe et al. 2008). Surveys consisted of walking at a constant pace for 15 min and recording the species, time, sex, and perch characteristics (i.e., height and diameter) for all undisturbed lizards observed. We compared relative abundance at sites with predominantly A. cristatellus (n = 10 surveys) to sites with A. distichus and A. sagrei in addition to A. cristatellus (n = 6 surveys). Because A. distichus and A. sagrei co-occur throughout most of Miami, we were unable to find nearby sites with only one of these species. We supplemented data on perch characteristics with opportunistic observations of all three species at the same sites.
The presence-absence data from transects, visual encounter surveys, and opportunistic surveys allowed us to map the distribution of A. cristatellus in Miami (Figs. 1, 2). In addition to the intensive sampling within the core areas of South Miami and Key Biscayne, we also investigated potential localities throughout Miami-Dade County including municipal parks and regional waste transfer stations. Preliminary surveys showed A. cristatellus was present in forest patches in some parks associated with waste transfer stations, suggesting transport of yard waste from houses to regional stations as a potential dispersal mechanism.
GIS analysis of forest cover and impervious surfaces
To complement analyses based on transect data, we conducted a geographic information system (GIS) analysis of the study area using ArcGIS version 10.2 (Environmental Systems Research Institute, Redlands, CA, USA) and publicly available GIS layers of impervious surface (MRLC 2011) and forest fragmentation (NOAA 2010). The MRLC Percent Developed Imperviousness layer, a raster dataset with 30-meter pixels, provides the average percentage of human-made impervious surface for each pixel. The NOAA forest fragmentation layer, a raster dataset with 30-meter pixels, distinguishes between four fragmentation types: (1) “core forest” refers to forested pixels that are not adjacent to any non-forested pixels, (2) “patch forest” refers to forested pixels in small patches that are not large enough to contain a 90 × 90 m block of forest, (3) “perforated forest” refers to forested pixels adjacent to small non-forested patches that are not large enough to contain a 90 × 90 m block of non-forested area, and (4) “edge forest” refers to forested pixels adjacent to larger non-forested patches that contain at least one 90 × 90 m block of non-forested area. We used GIS to generate 11 attributes describing forests and impervious surfaces (Table 2).
Thermal microclimates and lizard body temperatures
To investigate the range of possible thermal microclimates available to A. cristatellus in Miami during peak summer temperatures, we measured the temperature under two trees—one with an open canopy (Thrinax radiata, DBH = 12 cm) and another with a heavily shaded canopy (Chukrasia tabularis, DBH = 145 cm). We used painted, hollow, copper models the size of lizards with an iButton inside to estimate temperatures a lizard would experience in each location without behavioral or physiological thermoregulation (Hertz 1992; Gunderson and Leal 2012; Ackley et al. 2015a). Temperatures were recorded every 15 min on the north, south, east, and west sides of each tree at a height of 1.5 m from 1700 h on July 17 to 1000 h on July 21, 2014. To estimate the percentage of open canopy, we took hemispherical canopy photographs in each cardinal direction using a 180° fisheye lens and analyzed them using Gap Light Analyzer version 2.0 (Frazer et al. 1999).
To estimate the range of field body temperatures (Tb) for A. cristatellus in Miami, we sampled lizards and random locations at three sites that varied in species composition and vegetative structure. For comparative purposes, we also sampled A. sagrei, which has both higher field Tb and thermal tolerances than A. cristatellus (Corn 1971; Lee 1980; Gunderson and Leal 2012; Kolbe et al. 2012, 2014). The sites included a bike path along a canal where both species were sampled, a residential area where only A. sagrei was sampled, and a forested area where only A. cristatellus was sampled. For each undisturbed lizard captured, we recorded Tb, air temperature 1 cm above the substrate where the lizard was perched, and copper lizard model temperature at the same location as the lizard using a thermocouple probe connected to a digital thermometer (Omega HH802U). We then took a hemispherical canopy photo to estimate canopy openness as described above. For comparison, we took copper lizard model temperature, air temperature, and a canopy photo at randomly selected locations within each study site. Lizard Tb and random location data were collected between 0800 and 1400 h, which is a high-activity time of day during the summer.
Data analysis
Occupancy and estimates of detection probabilities were calculated using single season occupancy models (MacKenzie et al. 2002) in the program PRESENCE (Hines 2006). Likelihood models calculated in PRESENCE all assume that (1) any site where a species is present remains occupied, (2) species may or may not be detected when present, but are not detected when absent, and (3) the detection of a species at one sampling site is independent of detection at all other sites (MacKenzie et al. 2002). A minimum of two sample occasions is required for model estimation. We conducted three repeat surveys at each sampling point. Occupancy models to calculate estimates of detection were produced with all surveyors (Rick Stanley [RS], PV, and JJK) independently as covariates as well as using full identity models including all surveyors.
We used generalized linear models (GLM; McCullagh and Nelder 1989; R Core Team 2013) with binomial (presence-absence) and continuous (abundance) response variables. This allowed us to form linear and quadratic relationships between the response and explanatory variables (Broennimann et al. 2012), which were standardized to normalize their distributions. Explanatory variables included the tree characteristics of trunk diameter, canopy diameter, distance to nearest plant, distance to nearest tree, and overstory canopy cover (Table 1). Model selection was performed using a stepwise procedure based on the Akaike information criterion (AIC; Akaike 1974). We conducted three separate analyses using presence-absence as the response variable. First, we compared transect sections with A. cristatellus present (but not necessarily occupying every tree) versus sections where A. cristatellus was absent; second, we compared the presence versus absence of A. cristatellus on all trees pooled; and third, we compared the presence versus absence of A. cristatellus on trees within only the sections of transects with A. cristatellus present. We then repeated the latter two analyses using A. cristatellus abundance as the response variable.
When analyzing the GIS-based attributes, we conducted two separate analyses. First, we divided street blocks from each transect into those with A. cristatellus present versus absent and compared attributes derived from GIS (Table 2). Second, we used presence and absence points for individual observations throughout the Miami metropolitan area to test for relationships with GIS-derived attributes, restricting the data set to no more than one observation per block.
For categorical explanatory variables (Table 1), we used likelihood ratio tests to compare tree characteristics between sections of transects with A. cristatellus present versus absent. When evaluating A. cristatellus abundance in relation to categorical tree characteristics, we used t-tests or analyses of variance (ANOVA) as appropriate. We tested for a relationship between the presence-absence of A. cristatellus and the presence-absence of the four congeners using likelihood ratio tests. We tested for effects using all trees sampled, as well as only those trees on the sections of transects with A. cristatellus present. Analyses were conducted for trees on each plot separately and with trees from all plots pooled.
Relative abundances from the visual encounter surveys were not normally distributed, so we used a Wilcoxon test to evaluate whether differences existed between sites. In particular, we predicted relative abundance of A. cristatellus would decrease when it is with other Anolis species compared to being alone. Log-transformation achieved normality for perch height and diameter, and we tested for a difference in these perch characteristics for A. cristatellus between sites with and without congeners using t-tests.
We compared lizard Tb and copper lizard model temperatures at the same locations using linear regression. Using this calibration, we adjusted model temperatures to make them directly comparable to lizard Tb for both species. We averaged model temperatures by hour and plotted them against time of day. We compared these model temperature estimates (i.e., non-thermoregulating lizards) to field Tb collected at the same time of year, and literature estimates of preferred Tb and high temperature tolerance (i.e., critical thermal maximum, or CTmax) for A. cristatellus (Huey and Webster 1976). To investigate variation in field Tb of lizards, we conducted an analysis of covariance (ANCOVA) testing for differences among groups (i.e., A. cristatellus, A. sagrei, and random locations) with air temperature, time of day, and canopy openness as covariates. We used the Johnson–Neyman procedure (White 2003) to determine the range of covariate values in which temperatures differed between groups when regression slopes were heterogeneous (i.e., a significant interaction between the main effect and covariate).
Results
Anolis cristatellus distribution in Miami
The combination of opportunistic surveys, visual encounter surveys, and transects resulted in fine-scale distribution data for A. cristatellus in key parts of the Miami metropolitan area (n = 362 presence points and n = 483 absence points; Fig. 1, Supplementary Fig. 1). This species has expanded its core range from the original point of introduction no more than 2 km to the west, south, and east in South Miami, and ~7 km to the northeast. A six-lane highway (i.e., the Dixie Highway/US 1) to the northwest of the core South Miami distribution appears to limit unaided dispersal. The introduction to Key Biscayne expanded across the majority of the island, but not across the bridge to Virginia Key or causeway to mainland Miami.
We detected seven disjunct populations ranging from <1 to 20 km from the core distribution in South Miami. These sites included several Miami-Dade County Parks (i.e., Chapman Fields, Kendall Indian Hammock, and Oak Grove) as well as the University of Miami campus and three residential areas. We found A. cristatellus at two of 13 waste transfer stations in Miami-Dade County (i.e., Chapman Fields and Sunset Kendall), but only when adjacent to forested parks. Most waste transfer stations had only a few widely spaced trees and were surrounded by residential or commercial areas. Other species were present at all waste transfer stations with A. distichus and A. sagrei being the most common.
Tree characteristics
Transect surveys yielded observations on a total of 1035 trees. At least one anole was present on 79 % of the trees, and A. cristatellus occupied 25 % of the trees (Table 3). The best models to estimate detection probabilities for each species were single season occupancy models including all three surveyors. Estimates of among-surveyor detection probability for the focal species, A. cristatellus, ranged from 0.50 to 0.96 among sites, and average detection probability for each surveyor for all sites was estimated at 0.63–0.90 (Supplementary Table 1). Total detection probability for the full model (all surveyors) was estimated at 0.88 (±0.12). These estimates for detection were high and therefore detection probabilities were not considered influential in subsequent analyses. The most likely models of tree characteristics found A. cristatellus associated with trees having larger trunks, larger canopies, greater percent of overstory canopy cover, and closer to other plants and trees (Tables 1, 4). These results suggest that A. cristatellus occupies relatively shady and densely vegetated areas.
Sections of transects with A. cristatellus present had a greater proportion of native trees (χ2 = 12.3, df = 1, P < 0.001, n = 937) and trees with smooth bark (χ2 = 14.4, df = 4, P < 0.01, n = 1035) as compared to transect sections with A. cristatellus absent. In contrast, transect sections with and without A. cristatellus did not differ in the proportion of palm trees (χ2 = 0.5, df = 1, P = 0.46, n = 1028) or the number of trunks on trees (χ2 = 4.3, df = 2, P = 0.12, n = 1035).
Models for the abundance of A. cristatellus showed similar results with increased abundance associated with trees having larger trunks and canopies, greater percent of canopy cover, and closer to other plants (Table 4). Anolis cristatellus abundance was twice as high on non-palm compared to palm trees (t = 2.7, df = 1026, P < 0.01) and highest on trees with multiple trunks (F2,1032 = 14.9, P < 0.0001), which were often large Ficus trees. Abundance did not differ between native and non-native trees (t = −1.08, df = 935, P = 0.28) or among bark textures (F4,1030 = 1.57, P = 0.18).
Analyses of A. cristatellus presence using GIS-based attributes were consistent with transect surveys. Blocks with A. cristatellus present had more trees per km, greater canopy cover, denser vegetation, and less impervious surface area (Table 5a). Similarly, when analyzing the presence-absence points across Miami, A. cristatellus was present at locations with less impervious surface and closer to larger blocks of forest but not smaller forest patches (Fig. 2; Supplementary Fig. 3; Table 5a). The percentage of forested area was three times greater in the core area of A. cristatellus’ distribution compared to the study area as a whole (Supplementary Table 2). Moreover, the percentage of the core area with high impervious surface (>40 %) was about half as much as the study area as a whole (Supplementary Table 2).
Congener presence
Pooling all trees sampled, A. distichus and A. sagrei were both significantly more likely to be absent when A. cristatellus was present than expected by chance, with effects involving A. sagrei being much stronger (Table 6a). The presence of A. carolinensis or A. equestris had no effect. When evaluating each plot separately, a negative effect was observed with A. sagrei for most plots, and with A. carolinensis and A. distichus in a few plots (Table 6a). This suggests congeneric interactions may differ among plots. All comparisons for individual transects were non-significant (results not shown).
Dividing each transect into sections based on A. cristatellus presence or absence, only A. sagrei was more likely to be absent where A. cristatellus was present (Table 6b). There was no interaction with the less abundant species A. carolinensis and A. equestris. In contrast to the analyses of all trees pooled, this analysis revealed no relationship between occurrence of A. distichus and A. cristatellus (Table 6b). Potential interactions for A. cristatellus appear to be strongest with A. sagrei, followed by A. distichus, but little evidence existed for interactions with A. carolinensis or A. equestris.
Relative abundance and habitat use
Relative abundance estimates from visual encounter surveys were consistent with the negative relationship between the presence of A. cristatellus and two of its congeners in Miami. Anolis cristatellus was four times more abundant at sites with no congeners than in sites occupied by A. distichus and A. sagrei (mean ± SE: 45.3 ± 2.5 vs. 11.0 ± 3.2 per survey; Wilcoxon: Z = 3.21, P < 0.01). Furthermore, at sites with congeners, A. cristatellus perched nearly twice as high (mean ± SE: 79.0 ± 4.2 vs. 47.2 ± 1.8; t = 6.38, df = 608, P < 0.0001) and on trunk substrates twice as wide (mean ± SE: 18.6 ± 1.5 vs. 9.5 ± 0.67; t = 6.1 df = 604, P < 0.0001), suggesting a possible shift in habitat use in the presence of congeners.
Thermal microclimates and lizard body temperatures
We investigated the thermal consequences of canopy cover by comparing copper lizard model temperatures under trees with open versus shaded canopies. The percentage of overstory canopy cover ranged from 31–46 % for the open canopy tree versus 89–91 % for the shaded canopy tree (Supplementary Fig. 4). Model temperatures for the two trees were similar through the night from ~1900 to ~0800 h (Fig. 3). After 0800 h, model temperatures on the open canopy tree increased quickly, exceeding both shaded tree temperatures and preferred temperatures of A. cristatellus from 1000 to 1800 h. While there was little variation in model temperatures among the sides of the shaded tree, temperatures on the sides of the open tree differed substantially from one another, with a maximum difference of 5.7 °C at 1000 h.
Mean temperatures for copper lizard models placed on the trunks of two trees, one with an open canopy and the other with a shaded canopy, in each cardinal direction. Points are hourly means collected over a 3.5-day period in July 2014 (error bars are omitted for clarity). Patterned shading (gray) shows the range of field Tb for A. cristatellus in Miami during each hour from 0800 to 1400 from this study as well as the preferred Tb (light gray) and the critical thermal maximum (dotted line) of A. cristatellus measured for populations from Puerto Rico (Huey and Webster 1976)
Lizard Tb and model temperatures showed a strong positive correlation (r = 0.91; P < 0.0001, n = 52), suggesting that models accurately reflected lizard body temperatures. ANCOVA results showed all three covariates had significant positive effects on Tb/model temperatures (canopy openness: F1,83 = 46.42, P < 0.0001; air temperature: F1,83 = 7.97, P = 0.006; time of day: F1,83 = 23.51, P < 0.0001; whole model R2 = 0.67). Anolis sagrei field body temperatures (mean ± SE: 31.2 °C ± 0.4) were significantly higher than A. cristatellus temperatures (mean ± SE: 28.8 °C ± 0.4; F2,83 = 3.79, P = 0.03; Tukey’s HSD post hoc test P < 0.05; Fig. 4). However, because the species by canopy openness interaction was significant this main effect should not be interpreted directly but only in conjunction with the covariate. The relationship between temperature and canopy openness had a much steeper slope for A. cristatellus compared to A. sagrei and random points (P < 0.05 for comparison of slopes; Fig. 4). The Johnson–Neyman procedure supported Tb differences between A. cristatellus and both A. sagrei and random points for relatively closed canopies (i.e., <15 % openness). In summary, all covariates had significant effects on lizard Tb, but A. cristatellus had lower Tb compared to A. sagrei and the two species appeared to thermoregulate differently in closed canopy areas.
Relationships between lizard field body temperature or copper lizard model temperature and significant covariates from the ANCOVA: a canopy openness, b air temperature, and c time of day for A. cristatellus (black circles), A. sagrei (white circles), and copper lizard models at random locations (gray circles) in South Miami. Separate slopes are shown for the significant temperature by canopy openness interaction
Discussion
Since its introduction to South Miami over 40 years ago, A. cristatellus has spread only modestly by diffusion (~0.2–0.25 km/yr), much slower than some of the other introduced Anolis species in Miami and invasive species in general (Lockwood et al. 2007; Davis 2009). A recent analysis shows an order of magnitude faster spread rates on average for exotic lizards and snakes (~3 km/yr) and invaders to the Nearctic (~5 km/yr; Liu et al. 2014). Results from our study suggest that both abiotic and biotic factors contribute to the limited spread of A. cristatellus in urban Miami. The fragmentation of suitable habitat is an abiotic constraint. The presence of A. cristatellus is strongly associated with forest habitats, which result in cooler and more humid microclimates (e.g., Wong and Yu 2005; Georgi and Zafiriadis 2006; Millward et al. 2014; Ackley et al. 2015a; Fig. 3). Because forests are patchily distributed in Miami (Fig. 2a), dispersal by diffusion will be limited by fragmentation caused by canals, non-forest habitats, and areas of impervious surface, such as buildings, roads, and parking lots (Fig. 2). Therefore, human-mediated dispersal may be an important mechanism for moving A. cristatellus to isolated patches of suitable habitat, which lizards are unable to reach via natural diffusion.
Interactions with ecologically similar congeners may be a biotic constraint. Anolis cristatellus is spreading into areas occupied by one or more additional Anolis species. As expected, we found negative associations between A. cristatellus and ecologically similar A. sagrei and A. distichus, but weak or no relationship between the occurrence of A. cristatellus and A. carolinensis or A. equestris, which typically perch higher in the canopy (Losos 2009). Ultimately, the relative abundance of each species and the extent to which they overlap on niche axes, such as structural habitat and thermal microclimate, will determine whether and how quickly A. cristatellus spreads to new areas.
Effect of urban vegetation on the spread of A. cristatellus
The presence and abundance of introduced A. cristatellus in Miami are positively associated with larger trees, denser vegetation, greater canopy cover, proximity to forest, and less impervious surface. These features are indicative of forest patches in the urban environment including parks and certain residential areas. Previous studies show patterns of urban vegetation can be related to numerous factors including socio–economics, remnant natural habitats, and neighborhood age and history (e.g., Nowak et al. 1996; Martin et al. 2004; Grove et al. 2006; Jenerette et al. 2007; Luck et al. 2009; Boone et al. 2010; Forman 2014). Anolis cristatellus was present in several tropical hardwood hammocks, including parks outside of its core distribution in South Miami. These disjunct populations suggest dispersal limitation, not lack of suitable habitat, slows the spread of A. cristatellus outside its core area in South Miami. The patchwork of scarce suitable forested habitat in Miami will continue to limit the spread of A. cristatellus by diffusion, making human transport an important dispersal mechanism. The presence of A. cristatellus at forested parks located adjacent to spatially isolated regional waste transfer stations suggests yard waste collection and transport may be one such method of dispersal.
Vegetation in some residential areas within the core distribution of A. cristatellus can change rapidly over short distances, likely affecting the ability of A. cristatellus to spread to new areas. The transition from presence to absence of A. cristatellus coincides with an abrupt increase in impervious surface and a loss of forest habitat in some areas (see Fig. 2). The current distribution of A. cristatellus includes mostly higher-income neighborhoods including parts of Coconut Grove, Coral Gables, Pinecrest, and Key Biscayne (American Community Survey 2013; see also Ackley et al. 2015b). Socio-economic factors influence surface temperatures primarily through their impact on vegetation cover (Grove et al. 2006; Jenerette et al. 2007; Boone et al. 2010); such that areas with dense, mature tree canopies will produce relatively cooler microclimates suitable for A. cristatellus. These underlying effects of urban vegetation on available microclimates provide a mechanistic understanding of the current distribution of A. cristatellus in Miami. Other studies of urban and fragmented landscapes show species presence connected with other key resources, such as prey availability (e.g., Sullivan et al. 2014), shelter availability (e.g., Fischer et al. 2005), and structural habitat (e.g., Sarre et al. 1995; Garden et al. 2007; Santos et al. 2008) as well as urban development (e.g., Germaine and Wakeling 2001). Future studies should test whether socio-economic factors are correlated with vegetation and microclimates, and thus potentially useful for predicting the spread of A. cristatellus in Miami.
Thermal microclimates
We found substantial temperature differences between copper lizard models on open versus shaded trees (Fig. 3). Non-thermoregulating lizards would experience a temperature difference of up to a 7.6 °C in the morning (1000 h) and a 5.7 °C in the afternoon (1600 h). Open trees, but not shaded ones, experienced temperatures exceeding observed field Tb for A. cristatellus in the summer in Miami (Fig. 3). Denser overstory vegetation will produce relatively cooler microclimates favorable for A. cristatellus in the city. Shade from vegetation cooled buildings up to 11.7 °C during summer conditions in Toronto, Canada (Millward et al. 2014), and shade from individual trees in city parks decreased average air temperatures by 10 % and increased relative humidity by 18 % in Thessaloniki, Greece (Georgi and Zafiriadis 2006). Ackley et al. (2015a), using copper lizard models, found that microclimates in areas with mesic landscaping were 5–10 °C cooler than those in native xeric landscapes, even though the mean surface temperature of Phoenix, Arizona, USA was 3 °C warmer than the surrounding desert. Interestingly, surface temperatures in Phoenix were related to vegetation during the daytime and the proportion of paved area during the night (Buyantuyev and Wu 2010). Daytime temperatures may limit activity or approach maximum thermal limits, whereas nighttime temperatures likely influence metabolic costs during times of inactivity. Whether the distribution of A. cristatellus in Miami is limited by daytime temperatures driven by vegetation, nighttime temperatures related to impervious surfaces, or both is a key question for future study.
Copper lizard model temperatures do not account for the ability of lizards to thermoregulate. If suitably cool microhabitats were nearby, lizards in open areas could behaviorally thermoregulate to preferred temperatures by shuttling between warm and cool spots, at the cost of increased movement rates. The cost of thermoregulation is predicted to be lower in more open sites because of the shorter distance to sunny patches, which lowers the energetic cost of shuttling between sun and shade (Huey 1974; Huey and Slatkin 1976; Huey and Webster 1976; Angilletta 2009). Accordingly, previous studies of A. cristatellus in Puerto Rico found that lizards actively thermoregulate in open habitats, but thermoconform in less variable, closed canopy habitats (Huey and Webster 1976). This versatility in thermoregulatory behavior suggests that A. cristatellus might occupy both open and closed canopy sites in Miami; however, A. cristatellus is generally restricted to relatively closed canopy locations (<22 % canopy openness; Fig. 4).
Our results suggest at least two possible explanations for this pattern. The first is that A. cristatellus uses relatively cooler microclimates strictly due to its thermal requirements: open canopy areas in Miami may be too warm relative to the preferred temperature and upper thermal limit of A. cristatellus, and thus not suitable for this species (Fig. 3). A second possibility is that A. cristatellus is excluded from warmer areas by the presence of A. distichus and A. sagrei, which both occupy warmer thermal niches than A. cristatellus (Huey and Webster 1976; Lee 1980; Gunderson and Leal 2012; Leal and Gunderson 2012; Kolbe et al. 2012, 2014; this study). The relative importance of these two factors on limiting the spread of A. cristatellus in the Miami area is an open question. These hypotheses need to be comprehensively evaluated by including sites where each species is present in the absence of the other as well as locations where they coexist. The importance of microclimates to competitive interactions between the species, allowing coexistence or contributing to competitive exclusion, warrants further investigation.
During the summer in Miami, shade from urban vegetation is expected to produce microclimates closer to the preferred body temperature of A. cristatellus as compared to more open areas (Fig. 3). Higher activity rates are predicted when lizards are closer to their preferred temperature (Gunderson and Leal 2015), allowing lizards to better forage, mate, defend their territories, and escape from predators. Mean body temperatures for A. cristatellus in Miami (28.8 °C) and at low-elevation, mesic sites in Puerto Rico (~29 °C from numerous sites; Huey and Webster 1976; Hertz 1992; Gunderson and Leal 2012) were similar to preferred temperatures for lizards from three locations in Puerto Rico (range = 29.0–29.6 °C; Huey and Webster 1976; Fig. 3). This suggests that some aspects of the thermal niche of A. cristatellus are conserved between introduced populations in South Miami and their low-elevation source population in northeast Puerto Rico (Kolbe et al. 2007). This similarity in field body temperatures occurs despite shifts in other aspects of their thermal niche, specifically the introduced population in South Miami rapidly acquired the ability to tolerate lower temperatures relative to its source population in Puerto Rico (see Kolbe et al. 2012; Leal and Gunderson 2012).
Effect of species interactions on the spread of A. cristatellus
Interspecific interactions, primarily competition, are thought to be important factors structuring both native and introduced Anolis lizard communities (Losos et al. 1993; Losos 2009). Previous experimental studies of anoles have found effects on abundance and structural habitat use consistent with interspecific competition when species coexist (e.g., Pacala and Roughgarden 1982; Rummel and Roughgarden 1985; Leal et al. 1998; Stuart et al. 2014). In accordance with predictions based on ecological similarity (primarily perch height), A. cristatellus presence showed the strongest negative association with A. sagrei, followed by A. distichus, and in a few cases with A. carolinensis and A. equestris. Thus, ecological similarity of interacting species may provide important information for predicting patterns of establishment and range expansion dynamics for introduced species.
The negative relationship between A. cristatellus and A. sagrei in Miami may be explained by resource competition and agonistic interference (Salzburg 1984; Losin 2012). When A. cristatellus was experimentally removed from plots 5 years after its initial introduction in 1981, A. sagrei rapidly shifted back to the structural habitat previously occupied by A. cristatellus—off the ground, on to trunks, and to shadier sites (Salzburg 1984). We found consistent patterns, with A. cristatellus occupying higher and broader perches as well as shadier microhabitats when sympatric with A. sagrei. Additionally, A. cristatellus was far less abundant when coexisting with congeners compared to when alone. However, competitive interactions between A. cristatellus and A. sagrei may have changed over time with their coexistence. Thirty years later, at the same site as Salzburg’s experiment, another removal experiment did not influence habitat use or body condition of these two species (Losin 2012). Furthermore, A. sagrei lizards found sympatric with A. cristatellus were less aggressive toward this species compared to A. sagrei from allopartric populations (Losin 2012). Aggressive individuals facilitated the rapid range expansion of western bluebirds in the northwestern U.S., but following displacement of mountain bluebirds, their aggressive behavior decreased rapidly (Duckworth and Badyaev 2007). Given that A. sagrei is ubiquitous in Miami and A. cristatellus is still spreading, the opportunity exists to study resource use and aggression of A. sagrei before and after the arrival of A. cristatellus.
Summary
The occurrence of introduced A. cristatellus in Miami is strongly associated with forest habitat—dense vegetation, high canopy cover and low impervious surface—and the lack of congeners, particularly A. sagrei. Given the correlative nature of our analyses, it is difficult to tease apart the causal effects of urban vegetation and species interactions for limiting the spread of A. cristatellus. However, because A. sagrei already occupies nearly all habitats in Miami and forest habitat is highly fragmented across the city, we predict that dispersal to forest habitat will be the primary factor limiting future spread of A. cristatellus. Human-mediated, sometimes long-distance, dispersal is likely to contribute to spread as well as movement by diffusion through corridors of suitable habitats.
References
Ackley JW, Angilletta MJ Jr, DeNardo D, Sullivan B, Wu J (2015a) Urban heat island mitigation strategies and lizard thermal ecology: landscaping can quadruple potential activity time in an arid city. Urban Ecosyst. doi:10.1007/s11252-015-0460-x
Ackley JW, Wu J, Angilletta MJ Jr, Myint SW, Sullivan B (2015b) Rich lizards: how affluence and land cover influence the diversity and abundance of desert reptiles persisting in an urban landscape. Biol Conserv 182:87–92
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Akbari H, Pomerantz M, Taha H (2001) Cool surfaces and shade trees to reduce energy use and improve air quality in urban areas. Sol Energy 70:295–310
American Community Survey (2013) United States Census Bureau. http://www.census.gov. Accessed Aug 2015
Anderson L, Burgin S (2008) Patterns of bird predation on reptiles in small woodland remnant edges in peri-urban north-western Sydney, Australia. Landscape Ecol 23:1039–1047
Angilletta MJ Jr (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Arnfield AJ (2003) Two decades of urban climate research: a review of turbulence, exchanges of energy and water, and the urban heat island. Int J Climatol 23:1–26
Bartlett RD, Bartlett PP (1999) A field guide to Florida reptiles and amphibians. Gulf Publishing, Houston
Bonnington C, Gaston KJ, Evans KL (2014) Squirrels in suburbia: influence of urbanisation on the occurrence and distribution of a common exotic mammal. Urban Ecosyst 17:533–546
Boone CG, Cadenasso ML, Grove JM, Schwarz K, Buckley GL (2010) Landscape, vegetation characteristics, and group identity in an urban and suburban watershed: why the 60 s matter. Urban Ecosyst 13:255–271
Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L, Yoccoz NG, Thuiller W, Fortin M-J, Randin C, Zimmermann NE, Graham CH, Guisan A (2012) Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecol Biogeogr 21:481–497
Buyantuyev A, Wu J (2010) Urban heat islands and landscape heterogeneity: linking spatiotemporal variations in surface temperatures to land-cover and socioeconomic patterns. Landscape Ecol 25:17–33
Cadenasso ML, Pickett STA, Schwarz K (2007) Spatial heterogeneity in urban ecosystems: reconceptualizing land cover and a framework for classification. Front Ecol Environ 5:80–88
Case TJ, Bolger DT, Petren K (1994) Invasions and competitive displacement among house geckos in the tropical Pacific. Ecology 75:464–477
Chen WY, Jim CY (2008) Assessment and valuation of the ecosystem services provided by urban forests. Ecology, planning, and management of urban forests international perspective. Springer, New York, pp 53–83
Corn MJ (1971) Upper thermal limits and thermal preferenda for three sympatric species of Anolis. J Herpetol 5:17–21
Crump ML, Scott NJ Jr (1994) Visual encounter surveys. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LAC, Foster MS (eds) Measuring and monitoring biological diversity: standard methods for amphibians. Smithsonian Institution Press, Washington D.C., pp 84–92
Davis MA (2009) Invasion biology. Oxford University Press, Oxford
Duckworth RA, Badyaev AV (2007) Coupling of dispersal and aggression facilities the rapid range expansion of a passerine bird. PNAS 104:15017–15022
Fischer J, Lindenmayer DB, Barry S, Flowers E (2005) Lizard distribution patterns in the Tumut fragmentation “Natural Experiment” in south-eastern Australia. Biol Conserv 123:301–315
Forman RTT (2014) Urban ecology. Cambridge University Press, Cambridge
Frazer GW, Canham CD, Lertzman KP (1999) Gap light analyzer (GLA), version 2.0: imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. Copyright © 1999. Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies, New York
Garden JG, McAlpine CA, Possingham HP, Jones DN (2007) Habitat structure is more important than vegetation composition for local-level management of native terrestrial reptile and small mammal species living in urban remnants: a case study from Brisbane, Australia. Austral Ecol 32:669–685
Georgi NJ, Zafiriadis K (2006) The impact of park trees on microclimate in urban areas. Urban Ecosyst 9:195–209
Germaine SS, Wakeling BF (2001) Lizard species distributions and habitat occupation along an urban gradient in Tucson, Arizona, USA. Biol Conserv 97:229–237
Grove JM, Troy AR, O’Neil-Dunne JPM, Burch WR, Cadenasso ML, Pickett STA (2006) Characterization of households and its implications for the vegetation of urban ecosystems. Ecosystems 9:578–597
Gunderson AR, Leal M (2012) Geographic variation in vulnerability to climate warming in a tropical Caribbean lizard. Funct Ecol 26:783–793
Gunderson AR, Leal M (2015) Patterns of thermal constraint on ectotherm activity. Am Nat 185:653–664
Hamdi R, Schayes G (2008) Sensitivity study of the urban heat island intensity to urban characteristics. Int J Climatol 28:973–982
Hertz PE (1992) Temperature regulation in Puerto Rican Anolis lizards: a field test using null hypotheses. Ecology 73:1405–1417
Hines JE (2006) PRESENCE—software to estimate patch occupancy and related parameters. USGS-PWRC. http://www.mbr-pwrc.usgs.gov/software/presence.html
Huang L, Li J, Zhao D, Ahu J (2008) A fieldwork study on the diurnal changes of urban microclimate in four types of ground cover and urban heat island of Nanjing, China. Build Environ 43:7–17
Huey RB (1974) Behavioral thermoregulation in lizards: importance of associated costs. Science 184:1001–1003
Huey RB, Slatkin M (1976) Cost and benefits of lizard thermoregulation. Q Rev Biol 51:363–384
Huey RB, Webster TP (1976) Thermal biology of Anolis lizards in a complex fauna: the cristatellus group on Puerto Rico. Ecology 57:985–994
Irschick DJ, Carlisle E, Elstrott J, Ramos M, Buckley C, Vanhooydonck B, Meyers J, Herrell A (2005) A comparison of habitat use, morphology, clinging performance and escape behaviour among two divergent green anole lizard (Anolis carolinensis) populations. Biol J Linn Soc 85:223–234
Jenerette GD, Harlan SL, Brazel A, Jones N, Larsen L, Stefanov WL (2007) Regional relationships between surface temperature, vegetation, and human settlement in a rapidly urbanizing ecosystem. Landscape Ecol 22:353–365
Kolbe JJ, Battles AC, Avilés-Rodríguez KJ (2015) City slickers: poor performance does not deter Anolis lizards from using artificial substrates in human-modified habitats. Funct Ecol. doi:10.1111/1365-2435.12607
Kolbe JJ, Colbert PL, Smith BE (2008) Niche relationships and interspecific interactions in Antiguan lizard communities. Copeia 2008:261–272
Kolbe JJ, Ehrenberger JC, Moniz HA, Angilletta MJ (2014) Physiological variation among invasive populations of the Brown Anole (Anolis sagrei). Physiol Biochem Zool 87:92–104
Kolbe JJ, Glor RE, Schettino LR, Lara AC, Larson A, Losos JB (2007) Multiple sources, admixture, and genetic variation in introduced Anolis lizard populations. Conserv Biol 21:1612–1625
Kolbe JJ, VanMiddlesworth PS, Losin N, Dappen N, Losos JB (2012) Climatic niche shift predicts thermal trait response in one but not both introductions of the Puerto Rican lizard Anolis cristatellus to Miami, Florida, USA. Ecol Evol 2:1503–1516
Kraus F (2009) Alien reptiles and amphibians: a scientific compendium and analysis. Springer, Dordrecht
Leal M, Gunderson AR (2012) Rapid change in the thermal tolerance of a tropical lizard. Am Nat 180:815–822
Leal M, Rodríguez-Robles JA, Losos JB (1998) An experimental study of interspecific interactions between two Puerto Rican Anolis lizards. Oecologia 117:273–278
Lee JC (1980) Comparative thermal ecology of two lizards. Oecologia 44:171–176
Lever C (2003) Naturalized reptiles and amphibians of the world. Oxford University Press, Oxford
Liu X, Li X, Liu Z, Tingley R, Kraus F, Guo Z, Li Y (2014) Congener diversity, topographic heterogeneity and human-assisted dispersal predict spread rates of alien herpetofauna at a global scale. Ecol Lett 17:821–829
Lockwood JL, Hoopes MF, Marchetti MP (2007) Invasion ecology. Blackwell Publishing, Malden
Losin NJE (2012) The evolution and ecology of interspecific territoriality: studies of Anolis lizards and North American wood-warblers. Dissertation, University of California Los Angeles
Losos JB (1994) Integrative approaches to evolutionary ecology: Anolis lizards as model systems. Ann Rev Ecol Syst 25:467–493
Losos JB (2009) Lizards in an evolutionary tree: ecology and adaptive radiation of Anoles. University of California Press, Berkeley
Losos JB, Marks JC, Schoener TW (1993) Habitat use and ecological interactions of an introduced and a native species of Anolis lizard on Grand Cayman. Oecologia 95:525–532
Luck GW, Smallbone LT, O’Brien R (2009) Socio-economics and vegetation change in urban ecosystems: patterns in space and time. Ecosystems 12:604–620
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
Marnocha E, Pollinger J, Smith TB (2011) Human-induced morphological shifts in an island lizard. Evol Appl 4:388–396
Martin CA, Warren PS, Kinzig AP (2004) Neighborhood socioeconomic status is a useful predictor of perennial landscape vegetation in residential neighborhoods and embedded small parks of Phoenix, AZ. Landsc Urban Plan 69:355–368
McCullagh P, Nelder J (1989) Generalized linear models, 2nd edn. Chapman and Hall/CRC, Boca Raton
Millward AA, Torchia M, Laursen AE, Rothman LD (2014) Vegetation placement for summer built surface temperature moderation in an urban microclimate. Environ Manag 53:1043–1057
Multi-Resolution Land Characteristics (MRLC) Consortium (2011) The national land cover database: percent developed imperviousness. Available from http://www.mrlc.gov
National Oceanic and Atmospheric Administration (NOAA) Office for coastal management (2010) Coastal change analysis program (C-CAP) regional land cover database. Available from http://www.coast.noaa.gov/dataregistry/search/dataset/info/forestfrag
Nowak DJ, Rowntree RA, McPherson EG, Sisinni SM, Kerkmann ER, Stevens JC (1996) Measuring and analyzing urban tree cover. Landsc Urban Plan 36:49–57
Pacala S, Roughgarden J (1982) Resource partitioning and interspecific competition in two two-species insular Anolis lizard communities. Science 217:444–446
Perry G, Buchanan BW, Fisher RN, Salmon M, Wise SE (2008) Effects of artificial night lighting on amphibians and reptiles in urban environments. In: Mitchell JC, Jung Brown RE, Bartholomew B (eds) Urban herpetology. Society for the Study of Amphibians and Reptiles, pp 239–256
Petren K, Case TJ (1996) An experimental demonstration of exploitation competition in an ongoing invasion. Ecology 77:118–132
Pianka ER (1981) Competition and niche theory. In: May RM (ed) Theoretical ecology. Blackwell, oxford, pp 167–196
Pickett STA, Cadenasso ML, Grove JM, Nilon CH, Pouyat RV, Zipperer WC, Costanza R (2001) Urban ecological systems: linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annu Rev Ecol Syst 32:127–157
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available from http://www.R-project.org/
Ramalho CE, Hobbs RJ (2011) Time for a change: dynamic urban ecology. Trends Ecol Evol 27:179–188
Rizwan AM, Dennis YCL, Liu C (2008) A review of the generation, determination and mitigation of urban heat island. J Environ Sci 20:120–128
Rummel J, Roughgarden J (1985) Effects of reduced perch-height separation on competition between two Anolis lizards. Ecology 66:430–444
Salzburg MA (1984) Anolis sagrei and Anolis cristatellus in Southern Florida: a case study in interspecific competition. Ecology 65:14–19
Santos T, Diaz JA, Perez-Tris J, Carbonell R, Tellera JL (2008) Habitat quality predicts the distribution of a lizard in fragmented woodlands better than habitat fragmentation. Animal Conserv 11:46–56
Sarre S, Smith GT, Meyers JA (1995) Persistence of two species of gecko (Oedura reticulata and Gehyra variegata) in remnant habitat. Biol Conserv 71:25–33
Schwartz A, Thomas R (1975) A checklist of West Indian amphibians and reptiles. Special Publication. Carnegie Museum of Natural History 1:1–216
Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191
Stuart YE, Campbell TS, Hohenlohe PA, Reynolds RG, Revell LJ, Losos JB (2014) Rapid evolution of a native species following invasion by a congener. Science 346:463–466
Sullivan BK, Sullivan KO, Vardukyan D, Suminski T (2014) Persistence of horned lizards (Phrynosoma spp.) in urban preserves of Central Arizona. Urban Ecosyst 17:707–717
White CR (2003) Allometric analysis beyond heterogeneous regression slopes: use of the Johnson–Neyman technique in comparative biology. Physiol Biochem Zool 76:135–140
Wilson LD, Porras L (1983) The ecological impact of man on the south Florida herpetofauna. University of Kansas Museum of Natural History Special Publication No. 9:1–89
Wong NH, Yu C (2005) Study of green areas and urban heat island in a tropical city. Habitat Int 29:547–558
Acknowledgments
This research was supported by grants from the Harvard University Center for the Environment to RTTF and JBL, and the National Geographic Society, National Science Foundation (DEB-1354897) and University of Rhode Island Council for Research to JJK. We thank Rick Stanley for help in the field, Matthew Girard for producing maps of our study area, David Lee for assistance with tree identification, and Neil Losin and Nathan Dappen for logistical support in Miami. Miami-Dade Parks Natural Areas Management granted permission for us to work in county parks, and we thank Patrick Griffith and staff allowing us to work at the Montgomery Botanical Center.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kolbe, J.J., VanMiddlesworth, P., Battles, A.C. et al. Determinants of spread in an urban landscape by an introduced lizard. Landscape Ecol 31, 1795–1813 (2016). https://doi.org/10.1007/s10980-016-0362-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-016-0362-1