Abstract
Interspecific competition is assumed to have a strong influence on the population dynamics of competing species, but is not easily demonstrated for mobile species in the wild. In the Florida Keys (USA), anecdotal observations have long pointed to an inverse relationship in abundance of two large decapod crustaceans found co-occurring in hard-bottom habitat, the stone crab Menippe mercenaria and the Caribbean spiny lobster Panulirus argus. We used them to explicitly test whether competition for a renewable resource (shelter) can drive the abundance and distribution of the inferior competitor. We first explored this relationship in shelter competition mesocosm experiments to determine the competitively dominant species. Results showed that stone crabs are clearly the dominant competitors regardless of the number of lobsters present, the presence of co-sheltering species such as the spider crab, Damithrax spinosissimus, or the order of introduction of competitors into the mesocosm. We also found that lobsters use chemical cues from stone crabs to detect and avoid them. We then tested the ramifications of this competitive dominance in the field by manipulating stone crab abundance and then tracking the abundance and distribution of spiny lobsters through time. Increased stone crab abundance immediately resulted in decreased lobster abundance and increased aggregation. The opposite occurred on sites where stone crabs were removed. When we stopped removing stone crabs from these sites, they soon returned and lobster abundance decreased. This study explicitly demonstrated that interspecific competition can drive population dynamics between these species, and ultimately, community composition in these shallow water habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physiological tolerances (Spicer and Gaston 1999; Holt and Barfield 2011) and larval dispersal (Anderson et al. 2009) determine much of the potential geographic distribution of a species, but environmental factors seldom fully explain population abundance and spatial distribution (Pearson and Dawson 2003; Hampe 2004). Especially at small spatial scales, population dynamics can be driven by resource availability and selection (MacArthur and MacArthur 1961; Shulman 1984; Dunning et al. 1992), or competitive interactions (Fletcher 2007).
Competition typically results when multiple organisms simultaneously require the same limited resource and it can shape their populations (Connell 1961; Tilman 1994). It may manifest as interference to resource access (Case and Gilpin 1974; Berger and Gese 2007), exploitation that reduces resource availability (Park 1954), or as a hierarchy of resource use (Langkilde and Shine 2004). The consequence is often a change in behavior or habitat use (Kuefler et al. 2013; Liesenjohann et al. 2013), reduced fitness or recruitment (Gustafsson 1987; Martin and Martin 2001) or changes in abundance and spatial distribution (Connell 1961; Robertson 1996; Hobbs and Munday 2004).
For organisms that face intense predation, access to appropriately sized and abundant shelter is vital to survival and growth (Beck 1995). As with any resource, if shelter is limited, competition can be particularly intense. Shelter limitation can result from increases in number of shelter-dependent organisms, a decrease in shelter abundance, or barriers to the use of shelter (Beck 1997). Proximate consequences of shelter competition may include increased mortality from predation (Holbrook and Schmitt 2002; Behringer and Butler 2010), species displacement (Capelli and Munjal 1982; Usio et al. 2001), increased emigration (Butler et al. 1995), and partitioned habitat use (Langkilde and Shine 2004), especially for the inferior competitor.
Benthic macroinvertebrates are often well suited for studies of competition and behavior because they can be abundant, and densities are often easy to manipulate at small spatial scales. The Caribbean spiny lobster Panulirus argus, Latrielle 1804 is a large benthic crustacean found throughout the shallow waters of the wider Caribbean Sea and like most marine crustaceans, P. argus has a complex life history (Kanciruk and Herrnkind 1978; Mintz et al. 1994). Juvenile lobsters emerge at approximately 20 mm carapace length (CL) from the vegetation they settled in as post-larvae and seek crevice shelters occupied by conspecifics (Andree 1981; Marx and Herrnkind 1985). Juvenile spiny lobsters are particularly dependent upon shelter and exhibit behaviors that may minimize the effects of shelter limitation. They are attracted to healthy conspecifics, a behavior that may expedite the search for shelter (Childress and Herrnkind 2001) or facilitate group defense (Butler et al. 1999; Lavalli and Herrnkind 2009). Sheltering behavior is largely driven by chemoreception as lobsters are attracted to chemical cues emanating from shelters containing healthy conspecifics (Zimmer-faust et al. 1985; Horner et al. 2006), but avoid shelters with threatening chemical cues from diseased lobsters (Anderson and Behringer 2013) or octopus (Berger and Butler 2001).
In the Florida Keys (USA), much of the shelter for juvenile lobsters is provided by the abundant sponges, coral heads, and limestone solution holes found in shallow (<3 m) hard-bottom habitat. Spiny lobsters are commonly observed co-occupying shelters with spider crabs (Damithrax spinosissimus), and healthy conspecifics, but avoid Octopus spp. and diseased lobsters (Berger and Butler 2001; Behringer et al. 2006). They are also rarely found co-occupying shelter with the stone crab Menippe mercenaria, Say 1818 or the toadfish Opsanus beta, Goode and Bean 1880 (pers. obs.), suggesting competition for access to shelter.
Like lobsters, stone crabs in the post-settlement juvenile stage (≤10 mm carapace width) seek shelters similar to those used by lobsters. Their growth, recruitment, abundance, and size distribution are determined by shelter abundance and distribution (Lindberg et al. 1990; Beck 1995, 1997). Unlike lobsters, stone crabs are solitary shelter occupants, only sharing shelter while mating (Wilber 1988), and are rarely found co-occupying shelters with other crustaceans.
Here we tested for competition between juvenile Caribbean spiny lobsters and stone crabs using a renewable resource, shelter, and determined its effect on lobster abundance and distribution. We first established whether spiny lobsters and stone crabs would compete in a shelter-limited mesocosm environment, next determined if stone crab chemical cues mediated juvenile spiny lobster shelter choice, and finally, we determined the effect of stone crab abundance on juvenile spiny lobster abundance and distribution in the wild.
Methods
All animals were collected in the middle Florida Keys and maintained in isolated, aerated aquaria with ambient photoperiod (10:14 h) and water conditions (35 ± 1 ppt, 25 ± 1 °C) via a flow-through system for no more than 48 h until they were used in experiments. Aquaria were not equipped with shelter. To minimize the risk of pseudoreplication via recapture, all experimental lobsters were used only once before being released at least 2 km from any collection site.
Mesocosm shelter competition experiment
To determine if interference shelter competition occurs between juvenile spiny lobsters and stone crabs in a shelter-limited environment, we video recorded mesocosm trials in which a single shelter was offered to a lobster and stone crab in one of several treatments. Trials were performed in 68 l plastic Rubbermaid Roughneck™ bins (61 cm × 40 cm × 42 cm deep). The bottom of each container was covered in Sakrete® concrete 4 cm deep with natural hard-bottom sediment lightly coating the top to resemble natural hard-bottom substrate. Shelter was provided using half of a terracotta pot placed abutting one end of the container. Preliminary trials, in which a single lobster or single stone crab was offered shelter in the absence of the other, showed that shelter size preference varied with lobster and crab size. This is consistent with results from the field showing that lobsters (Eggleston and Lipcius 1992) and stone crabs (Beck 1995) prefer shelter scaled to their body size. Therefore, in trials with two competitors, different shelter sizes were used according to the sizes of trial animals. Terracotta pots with 11.4 cm top diameter and 10.2 cm depth were offered to lobsters <30 mm CL and stone crabs with <60 mm carapace width (CW). Pots with 15.2 cm diameter and 14.0 cm depth were offered to lobsters ≥31 mm CL and stone crabs ≥61 mm CW. Hence, test animals in each trial were roughly matched by size such that stone crabs <60 mm CW were paired with lobsters <30 mm CL (or approximately 60 mm total length), and similarly for the larger size shelter (size ranges used are reported in results below). This was done to minimize bias in shelter suitability for one of the competitors and to ensure both competitors could occupy each shelter. Trials with three or four competitors required more volume and larger shelter sizes. Thus, these trials were conducted in 380 l Rubbermaid™ stock tanks (134.6 cm long × 78.7 cm wide × 63.5 cm deep) with one shelter (30.5 cm long × 10.2 cm high × 30.5 cm wide). For each trial, the experimental mesocosm was filled with ambient seawater (35 ± 1 ppt, 25 ± 1 °C). Aerated ambient seawater in a 120 l elevated head tank drained into the experimental container at a rate of 4.0 ml s−1. In all experiments, animals were placed side-by-side in the container and confined to separate vertical 15 cm tall × 11.4 cm diameter polyvinyl chloride tubes until escape responses from handling subsided. The tubes were then removed and a video camera recorded the 2-h trial. Trials were conducted during the light photoperiod, as they would naturally be seeking shelter at this time. To remove any olfactory residue from prior trials, all equipment was rinsed with freshwater and allowed to dry for at least 10 h prior to reuse.
Prior to competition experiments, one stone crab (n = 29) or one lobster (n = 29) were tested alone to determine shelter use in the absence of competition. The shelter competition experiments consisted of four treatments. Treatment 1 tested for competition between a single lobster and a single stone crab added simultaneously (n = 33). Treatment 2 tested for competition between a single lobster and a single stone crab, but with the lobster introduced to the mesocosm 30 min prior to the stone crab (n = 29). This tested the hypothesis that initial shelter occupancy might give a lobster a competitive advantage over a stone crab. Treatment 3 included three lobsters and a single stone crab (n = 29) to assess the hypothesis that the lobsters’ gregarious nature might facilitate group shelter defense. Treatment 4 tested the potential for the addition of a spider crab to facilitate group shelter defense and included a lobster, stone crab, and spider crab (n = 29). The spider crab D. spinosissimus is a common, herbivorous crab that often co-occupies shelters with lobsters. In each competition treatment, regardless of the number of crabs or lobsters present, the dependent variables were the time spent within the shelter by a pre-determined ‘focal’ lobster and the final shelter resident.
A t test was used to compare the sheltering time of a lobster with that of a stone crab when no competitor was present. A one-way ANOVA was used to compare treatment effects (lobster only and the four lobster-stone crab competition treatments) on the time each focal lobster spent in the shelter, followed by a post hoc Bonferroni pairwise comparisons test to determine which, if any, treatments differed. A 4 × 4 contingency table analysis was used on the four competition treatments only to test if final shelter occupant was independent of the four treatments and four possible outcomes: (1) the lobster was the final occupant, (2) the stone crab was the final occupant, (3) no occupant at end of trial, or (4) both stone crab and focal lobster in shelter at end of trial. The lobster only treatment could not be included in this analysis because without a stone crab all outcomes were not possible.
Chemosensory driven shelter selection
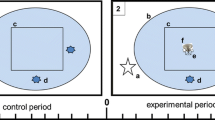
To test for the effect of stone crab chemical cues on lobster shelter selection we conducted chemosensory Y-maze experiments (Anderson and Behringer 2013). In Y-maze experiments, a lobster was placed in one end of a container and given the option of sheltering in one of two separated refuges at the other end of the container or occupying neither shelter. From each shelter a different chemical cue was emitted. A series of chemical cues known to attract (healthy conspecifics) and repel (diseased conspecifics) lobsters were compared with those of spider crabs and stone crabs, respectively. Juvenile Caribbean spiny lobsters are commonly infected with a pathogenic virus termed PaV1 (Panulirus argus Virus 1) and healthy lobsters have been shown to avoid infected lobsters using chemosensory cues (Behringer et al. 2006; Anderson and Behringer 2013). Heavily infected lobsters can be identified by gross observation of milky-white hemolymph, which is normally clear (Shields and Behringer 2004). From seasonal surveys, spider crabs were of similar size to stone crabs and reproductive adults were not uncommon where the species are sympatric. Treatments included a stone crab odor in one shelter and no odor (seawater only) in the other (n = 45), stone crab odor in one and diseased lobster odor in the other (n = 37), spider crab odor in one shelter and no odor (seawater only) in the other (n = 32), and spider crab odor in one shelter and healthy lobster odor in the other (n = 30). Four 80 l Y-mazes, with dimensions 94 cm long × 62 cm wide × 20 cm deep (Fig. 1), were constructed of epoxy-coated plywood and left with an open top for observation and placement of lobsters. A vertical partition (72 cm × 18 cm) bisected ¾ of the length of the container. This effectively separated the two shelters and their respective flows of water and chemical cues, while giving the lobster equal exposure and access to sense each cue. Unidirectional water flowed from each shelter to a drain at the opposite end of the Y-maze and was maintained at a rate of 1 cm s−1 by elevating the upstream end by 4° (Anderson and Behringer 2013). Each treatment animal was contained in one of two 120 l head tanks filled with seawater, which drained into the upstream end of one of the two sides of the Y-maze at a rate of 4.0 ml s−1. Stimulus animals in head tanks were not visible to the lobsters in the Y-maze to ensure that visual cues did not affect lobster shelter choice. Water drained from each head tank into a shelter in the Y-maze 6.35 cm from the bottom of the head tank. Crevice shelters were comprised of one concrete brick leaning against another.
Five hours prior to the start of each trial one treatment animal, or seawater only, was placed in one of the two aerated head tanks to allow accumulation of the chemical cue of interest, presumably urine. A coin toss was used to randomize placement of each treatment in the left and right side containers. As lobsters are nocturnal and seek shelter at dawn, trials started at 12:00 am and ended 1 h post-dawn. Each test lobster was placed in the open end of the Y-maze and kept in place using small hand nets as barriers until initial escape reactions from handling had ceased. Each Y-maze and head tank was rinsed with fresh water and left to dry for 10 h to avoid contamination of subsequent trials with chemical cues.
A binomial goodness of fit test was used to determine if the shelter chosen at the end of the experiment (1 h post-dawn) deviated from the predicted outcome. Lobsters do not co-occupy shelters with stone crabs in the wild, an observation that motivated this study. In contrast, lobsters shelter with spider crabs often. In treatments with a stone crab or spider crab cue compared to seawater, we expected the focal lobsters to select a shelter supplied with seawater when compared to one supplied with a stone crab cue, and select a shelter supplied with spider crab cue when compared with one supplied with only seawater (one-tailed tests). However, where we tested avoidance of stone crab and diseased lobster cues simultaneously, or attraction to spider crab and healthy lobster cues simultaneously, no particular shelter choice was expected (two-tailed tests). Significance of tests was determined at α = 0.05.
Hard-bottom shelter inhabitant surveys
Scuba surveys were conducted at nine hard-bottom sites in the Florida Keys to measure seasonal changes in shelter availability and use May–July 2012, April–March 2013, and June 2013. Sites were selected which were known from previous surveys to harbor stone crabs and lobsters and have similar abundance of crevice bearing structures.
Available shelter was defined as any sponge, coral, or solution hole ≥20 cm diameter that provided refuge in the form of holes, recesses, or crevices. Each survey consisted of two divers recording all shelter inhabitants found during a search resulting in a total survey time of 1 h. Divers haphazardly divided the search area in half to avoid multiple recordings of inhabitants. Animal processing time included recording of animal species, size [mm CL or carapace width (CW)], sex, injuries, shelter type occupied, distance to nearest neighbor, shelter size (< or ≥20 cm diameter), and whether the shelter provided refuge in the form of holes, recesses, or crevices. Processing time was not included in the 1 h survey time. Additionally, four non-overlapping 25 × 2 m belt transects were haphazardly placed in the survey area and used to determine shelter abundance. This was done to ensure consistency in shelter abundance among sites used for the stone crab density manipulations described below. A scuba diver swam along each transect with a 2 m pole held perpendicular to a 25 m measuring tape and measured each shelter encountered. For each shelter, we recorded shelter type (sponge, coral, solution hole, or gorgonian), measured the shelter diameter, and determined whether the shelter provided refuge in the form of holes, recesses, or crevices.
Linear regression analyses were used on data from each season to determine if stone crab abundance could predict lobster abundance or number of lobsters co-occupying shelters. A mixed-effects ANOVA was used to determine if shelter abundance differed significantly among sites or survey periods.
Stone crab density manipulations
To test for the effect of stone crab presence on lobster abundance and distribution, we manipulated the densities of stone crabs on nine hard-bottom sites throughout the Florida Keys (Fig. 2). Seven of the nine sites from the seasonal surveys described above were used for stone crab density manipulation. A paucity of stone crabs required the establishment of two new sites previously un-surveyed. These additional sites were surveyed using the methods above and met our selection criteria. All had similar shelter abundance, past recordings of both lobsters and stone crabs, and were at least 2 km apart. The experimental study began immediately following the shelter inhabitant surveys.
A 25 × 25 m quadrat was placed haphazardly in each of the selected hard-bottom areas. Stone crabs added to sites were tethered to concrete bricks and placed within sheltering distance of haphazardly chosen but appropriately sized shelters. To tether the crabs, monofilament line was wrapped around the carapace to form a bridle and reinforced with cyanoacrylate glue. This allowed the crab uninhibited movement and use of limbs. A snap swivel was attached to the monofilament line on the dorsal carapace as a connection point for a 13.6 kg wire leader attached to the block. The tethers allowed stone crabs to use shelters within a 0.3 m radius of the brick. Each brick was 10.2 cm × 5.7 cm × 20. 3 cm and did not provide refuge for tethered crabs.
Surveys consisted of a single scuba diver recording pertinent data on each inhabitant within the quadrat. Three treatments were used in this experiment, including: (1) the removal of all stone crabs from three sites, (2) The doubling of the number of stone crabs at three sites, and (3) leaving three sites unmanipulated to serve as controls. Only stone crabs with 60–95 mm CW were used on addition sites as this was the size range available from collection areas. The stone crab addition treatment included doubling the densities of stone crabs within quadrants, but was never at an unrealistically high density not found in a past survey. Stone crabs removed from sites were transplanted to areas at least 5 km away. A census of all inhabitants at each site was conducted at t = 0, t = 24 h, t = 48 h, t = 1 week, and t = 2 week. Treatments at each site were maintained by replacing missing stone crabs on addition sites and removing stone crabs from removal sites during each survey. Due to logistical constraints, crab removals stopped after t = 48 h, which allowed the system to return to pre-manipulation conditions.
A 2 × 2 repeated-measures MANOVA was used to determine if stone crab abundance (Factor 1, 3 levels) and time (Factor 2, 5 levels) affected lobster abundance and aggregation. Dependent variables were the density of lobsters within a site, percentage of lobsters found co-occupying shelter with conspecifics, and mean lobster aggregation size. Post hoc multiple comparison tests were used to determine which treatments differed. SPSS version 22 (SPSS IBM, New York, U.S.A.) was used to perform these analyses.
Results
Mesocosm shelter competition experiments
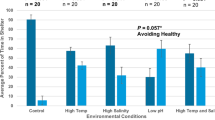
Mean lobster size was 30.6 ± 11.8 s.d. mm CL, mean stone crab size was 54.9 ± 14.1 s.d. mm CW and mean spider crab size was 60.1 ± 14.1 s.d. mm CW. A t test showed lobsters (n = 29) and stone crabs (n = 29) occupied shelter for similar amounts of time in the absence of a competitor (t = 1.88, df = 27, P = 0.331). Sheltering time and final shelter occupant did not differ between small and large animals (t = 5.49, df = 27, P = 0.742). Therefore, these groups were combined for further analyses. The amount of time lobsters occupied shelter decreased significantly in the presence of a stone crab. A one-way ANOVA showed that the time a lobster spent sheltering was dependent on treatment (F = 10.32, df = 30, P = 0.012). ANOVA assumptions of normality, equal variances, and lack of outliers were met with raw data. Post hoc Bonferroni multiple comparisons analysis revealed the only significantly different treatment was the control in which a lobster had no competitor present (Table 1). When only a stone crab was present (n = 33), the focal lobster spent 141.8% less time in the shelter than when no stone crab competitor was present (n = 29). The outcome was similar with multiple lobsters in addition to the stone crab (n = 30, 125.6% less time in shelter), the presence of a spider crab (n = 30, 127.4% less time in the shelter), and initial occupancy of the shelter by the lobster (n = 31, 124.2% less time in the shelter). The presence of multiple lobsters, presence of a spider crab, and initial shelter occupancy by the lobster did not significantly differ in their effect on the time focal lobsters occupied the shelter (Fig. 3). Contingency table analysis of only the competition treatments (Table 2) showed that the final shelter occupant at the end of each trial was the stone crab and this did not differ significantly among treatments (χ 2 = 16.92, df = 9, P = 0.4197). The final shelter occupant was a stone crab in 67.2% of trials across all treatments, a lobster in 12.5% of trials, neither in 19.5%, of trials, and both in 0.8% of trials. The lobster only treatment could not be included in the contingency table analysis because the possible outcomes of this treatment were not the same as the competition treatments.
Influence of chemical cues on shelter choice
Average size of the focal lobsters was 35.4 ± 7.7 s.d. mm CL, average stone crab size was 59.3 ± 7.7 s.d. mm CW, average spider crab size was 74.1 ± 11.3 s.d. mm CW, average healthy stimulus lobster size was 41.1 ± 9.4 s.d. mm CL, and average diseased stimulus lobster size was 28.5 ± 5.9 s.d. mm CL. As expected, a one-tailed binomial test showed focal lobsters chose seawater-only shelters significantly more (66 versus 34%) in stone crab versus seawater-only treatments (n = 41, P = 0.040) suggesting an avoidance of stone crab chemical cues (Table 3; Fig. 4). Also as expected, focal lobsters selected shelters supplied with a spider crab chemical cue significantly more (68 versus 32%) than seawater only (n = 32, P = 0.041). Shelter choice in the stone crab versus diseased lobster treatments did not differ from random (n = 32, P = 0.117).
Hard-bottom shelter inhabitant surveys
Linear regressions for each season showed a significant positive relationship between stone crab abundance and number of small lobsters co-occupying shelters with conspecifics in Winter and Summer 2013, and number of large lobsters co-occupying shelters with conspecifics in Summer 2013 (Table 4). Small (<30 mm CL) and large (≥30 mm CL) lobsters occur in hard-bottom, but exhibit ontogenetic divergence in shelter use (Butler and Herrnkind 2000) and were therefore analyzed separately. Stone crab abundance and lobster abundance were only significantly related for large lobsters in Summer 2013. Mean lobster size was 37.1 ± 8.9 s.d. mm CL, and mean stone crab size was 57.3 ± 8.9 s.d. mm CW.
Juvenile lobsters were the most abundant shelter inhabitants in all surveys, followed by stone crabs, spider crabs, and other rare inhabitants (Fig. 5). Those other rare inhabitants included toad fish, swimming crabs (family Portunidae), and other small spider crabs (family Majidae).
A mixed-effects ANOVA showed that shelter abundance did not vary among sites (F (8,27) = 1.991, P = 0.87) or sampling period (F (2,54) = 1.166, P = 0.32) and there was no significant interaction between site and sampling period (F (16,54) = 0.701, P = 0.78). All assumptions of mixed-effects ANOVA were met with the raw data. Gorgonians were the most abundant structures on nearly all sites, but provide little refuge. No lobsters or crabs were recorded sheltering under gorgonians, so this group was omitted from analysis.
Stone crab density manipulations
Tethered stone crabs added to the three addition sites ranged from 53.1 to 99.4 mm CW with a mean of 71.8 ± 9.2 s.d. mm CW while those removed from sites ranged from 48.3 to 81.6 mm CW with a mean of 60 ± 12.7 s.d. mm CW. Lobster size across all surveys ranged from 18.4 to 67.8 mm CL with a mean of 35.6 ± 12.5 s.d. mm CL. All assumptions for a 2 × 2 repeated-measures MANOVA were met using the raw data. The interaction term, Time × Treatment, was not statistically significant and so the assumption of homogeneity among regression slopes was met. Assumption of equal variance of residuals was confirmed with Levene’s test for lobster abundance. The multivariate tests showed a statistically significant between-subjects effect of treatment (Wilk’s Lambda = 0.129, F (12,76) = 1.945, P = 0.024) and a borderline significant within-subjects effect of time (Wilk’s Lambda = 0.783, F (26,44) = 7.622, P = 0.048). The between-subjects univariate tests showed a significant effect of treatment on lobster abundance (F (2,6) = 17.7, P = 0.033), and the number of lobsters co-occupying shelters (F (2,6) = 21.2, P = 0.019) (Table 5). Neither Time nor Time × Treatment interaction were significant. Post hoc pairwise multiple comparisons revealed that the addition of stone crabs to sites caused a decrease in lobster abundance relative to removal sites and control sites (Table 6). Conversely, the removal of stone crabs caused an increase in lobster abundance relative to the addition sites and control sites. However, the initial increase was followed by a decrease in lobster abundance after crab removals stopped at t = 48 h (Table 6; Fig. 6). The addition of stone crabs caused an increase in lobster co-occupancy relative to removal and control sites, removal of stone crabs caused a decrease in co-occupancy relative to addition sites, but not control sites (Table 6; Fig. 7). Mean lobster aggregation size (i.e., number of lobsters per shelter) did not change with Time or Treatment (Table 6; Fig. 8).
Discussion
This study presents empirical evidence for resource (shelter) competition between juvenile Caribbean spiny lobsters and stone crabs that results in significant shifts in population abundance and distribution. In mesocosm experiments, stone crabs excluded lobsters from shelter regardless of the number of lobsters present, the presence of another cohabitant (spider crab), or order of introduction to the mesocosm. Lobsters appear to avoid physical interactions with stone crabs by detecting and avoiding stone crab chemical cues. These laboratory results were mirrored on natural hard-bottom sites where stone crab abundance was positively associated with increased aggregation of among lobsters, and in field experiments where increased stone crab density resulted in decreased lobster abundance and increased aggregation. The opposite occurred on stone crab removal sites. Exclusion of lobsters from shelters by stone crabs effectively reduces the shelter available to lobsters. Exclusion from shelter could in turn result in decreased survival, increased emigration, and altered spatial distribution that changes the composition of the benthic community.
Stone crabs dominate shelter competition, emit chemical cues aversive to lobsters, and affect lobster abundance and distribution when shelter is limited. Nonetheless, stone crabs have not excluded lobsters from all hard-bottom habitats and lobsters can be the most abundant macroinvertebrates in some areas. Several processes may allow lobsters and stone crabs to coexist. For example, common predators or parasites may keep stone crab abundance below the level necessary to saturate shelter availability. Octopuses are common predators in hard-bottom and readily prey upon crustaceans such as stone crabs and lobsters (Berger and Butler 2001). Additionally, stone crabs are susceptible to the blood parasite, Hematodinium sp., which can reach a high prevalence in many crustacean populations (Stentiford and Shields 2005), and could keep stone crabs and lobsters from saturating shelter availability. Similarly, P. argus juveniles suffer high mortality from the pathogenic virus PaV1 that could keep their populations depressed (Shields and Behringer 2004; Moss et al. 2013). Co-existence could also be facilitated by inconsistent and unequal larval recruitment. The 5- to 7-month planktonic larval duration of the Caribbean spiny lobster allows for transport of larvae throughout the Caribbean (Butler et al. 2011). Larvae that recruit to the Florida Keys are spawned throughout the Caribbean. The diversity of sources for spiny lobster larvae (Kough et al. 2013) may buffer poor recruitment from one area. In contrast, stone crab larvae have a planktonic larval duration of only 27–30 days (Porter 1960) which may preclude them from dispersing as far as spiny lobster larvae. Therefore, localized, unfavorable conditions for stone crab spawning or larval survival may significantly impact recruitment to juvenile stages.
Ontogenetic changes in resource requirements may also limit interspecific competition to only a part of species’ life history. Wang (1975) found that shell competition between the hermit crabs Pagurus pollicaris and Pagurus longicarpus was limited to juvenile size ranges because as adult P. pollicaris grew larger than adult P. longicarpus they were eventually released from competition. In the Florida Keys, spatial overlap between spiny lobsters and stone crabs primarily occurs during their juvenile stage, but as they reach adulthood lobsters migrate to coral reefs where stone crabs are rarely found. Although this habitat shift may afford lobsters an eventual ontogenetic release from competition, shelter competition may contribute to a bottleneck in recruitment to the adult population.
Our results showed that the addition of two lobsters or a spider crab did not improve the likelihood that a lobster would be successful in competing for a shelter, and thus did not support the group defense hypothesis (Codella and Raffa 1995; Lavalli and Herrnkind 2001), at least in the sense of defending a shelter against an aggressive competitor. However, we did not test the effect of multiple lobsters with prior residency of the shelter, and we recognize that even in trials where the introduction of a stone crab was delayed, this may not have been enough time to affect the outcome of a trial. Blank and Figler (1996) found that prior occupancy of 24 h gave the crayfish Procambarus clarkii an advantage over Procambarus zonangulus in shelter competition. In contrast, O'Neill and Cobb (1979) found that 24 h prior residency did not significantly affect the outcome of shelter competition between conspecific Homarus americanus.
Lobsters were consistently the most common shelter inhabitants in hard-bottom areas followed by stone crabs and spider crabs. Stone crabs and spider crabs comprised 24 and 18% of total shelter inhabitants, respectively. However, addition of stone crabs to hard-bottom sites resulted in significantly lower lobster abundance and increased shelter co-occupancy among those lobsters remaining within 24 h. In contrast, the removal of stone crabs from sites resulted in greater lobster abundance and decreased aggregation as lobsters dispersed to shelters previously occupied by stone crabs. Stone crab removal was only repeated during the initial daily surveys. Consequently, the extended periods between t = 48 h, t = 1 week, and t = 2 week allowed stone crabs to repopulate the sites. Stone crab densities on sites increased during intervals between these removals and this coincided with decreases in lobster abundance. This further supports our findings that increased stone crab abundance is related to decreased lobster abundance and suggests that if spiny lobsters and stone crabs co-occur in shelter-limited areas, lobsters will be excluded from those shelters.
Spiny lobsters are not notoriously aggressive animals. Hence, it is not surprising that they are ultimately excluded from shelters, and furthermore, that they have adapted a mechanism to avoid direct interactions with stone crabs. Our y-maze shelter selection experiments showed that lobsters avoid shelters occupied by stone crabs using only chemical cues, but can also decipher these from spider crab chemical cues to which they are attracted. In fact, lobsters were attracted to shelters emitting spider crab and healthy lobster chemical cues to a similar degree, which suggests their attraction to spider crab cues may facilitate a guide effect akin to that described for conspecific lobsters (Childress and Herrnkind 1997, 2001). Juvenile lobsters live in shallow, often well-lit environments in which vision can be useful, but sheltering behavior has been shown time and again to be strongly driven by chemoreception. Juvenile P. argus use chemoreception to avoid shelters containing diseased conspecifics (Anderson and Behringer 2013) or octopuses (Berger and Butler 2001), but use the chemical cues of healthy conspecifics to find shelter (Ratchford and Eggleston 1998). Interestingly, shelter choice between stone crab and diseased lobster odors was similar, suggesting that these chemical cues may represent a similar degree of threat. Furthermore, when given the option of sheltering with one of these two aversive chemical odors or going without shelter, many lobsters remained out of the shelters. Where stone crabs and diseased lobsters are abundant, lobsters may be left with few options other than emigration.
Conclusions
Early evidence of competitive exclusion was based on the assumption that communities existed in competitive equilibrium (Alley 1982). However, the dynamic nature of populations and environmental variability often preclude competitive exclusion. Our study shows that when the abundance of a competitor abruptly and continually reduces resource availability, an inferior competitor may eventually be excluded. However, low intensity competition is more likely to occur, resulting in changes in competitor behavior and redistribution with regard to resource use.
References
Alley TR (1982) Competition theory, evolution, and the concept of an ecological niche. Acta Biotheor 31:165–179
Anderson JR, Behringer DC (2013) Spatial dynamics in the social lobster Panulirus argus in response to diseased conspecifics. Mar Ecol-Prog Ser 474:191–200
Anderson BJ, Akcakaya HR, Araujo MB, Fordam DA, Martinez-Meyer E, Thuiller W, Brook BW (2009) Dynamics of range margins for metapopulations under climate change. Proc R Soc B 276:1415–1420
Andree SW (1981) Locomotory activity patterns and food items of benthic postlarval spiny lobsters, Panulirus argus. M.S. Thesis, Florida State University, Tallahassee, Florida
Beck MW (1995) Size-specific shelter limitation in stone crabs: a test of the demographic bottleneck hypothesis. Ecology 76(3):968–980
Beck MW (1997) A test of the generality of the effects of shelter bottlenecks in four stone crab populations. Ecology 78(8):2487–2503
Behringer DB, Butler MJ IV (2010) Disease avoidance influences shelter use and predation in Caribbean spiny lobster. Behav Ecol Sociobiol 64:747–755
Behringer DC, Butler MJ IV, Shields JD (2006) Ecology: avoidance of disease in social lobsters. Nature 441:421
Berger DK, Butler MJ IV (2001) Octopuses influence den selection by juvenile Caribbean spiny lobster. Mar Freshw Res 52:1049–1053
Berger KM, Gese EM (2007) Does interference competition with wolves limit the distribution and abundance of coyotes? J Anim Ecol 76:1075–1085
Blank GS, Figler MH (1996) Interspecific shelter competition between the sympatric crayfish species Procambarus clarkia (Girard) and Procambarus zonangulus (Hobbs and Hobbs). J Crust Biol 16(2):300–309
Butler MJ IV, Hunt JH, Herrnkind WF, Childress MJ, Bertelsen R, Sharp W, Matthews T, Field JM, Marshall HG (1995) Cascading disturbances in Florida Bay, USA: cyanobacteria blooms, sponge mortality, and implications for juvenile spiny lobsters Panulirus argus. Mar Ecol Prog Ser 129:119–125
Butler IV MJ, Herrnkind WF (2000) Puerulus and juvenile ecology. In: Phillips BF, Cobb JS, Kittaka J (eds) Spiny Lobster Management, 2nd edn. Blackwell Scientific Press, Oxford, pp 276–301
Butler MJ IV, MacDiarmid AB, Booth JD (1999) The cause and consequence on ontogenetic changes in social aggregation in New Zealand spiny lobsters. Mar Ecol Prog Ser 188:179–191
Butler MJ IV, Paris CB, Goldstein JS, Matsuda H, Cowen RK (2011) Behavior constrains the dispersal of long-lived spiny lobster larvae. Mar Ecol Prog Ser 422:223–237
Capelli GM, Munjal BJ (1982) Aggressive interactions and resource competition in relation to species displacement among crayfish of the genus Orconectes. J Crust Biol 2(4):486–492
Case TJ, Gilpin ME (1974) Interference competition and niche theory. Proc Natl Acad Sci USA 71(8):3073–3077
Childress MJ, Herrnkind WF (1997) Den sharing by juvenile Caribbean spiny lobsters (Panulirus argus) in nursery habitat: cooperation or coincidence? Mar Freshw Res 48:751–758
Childress MJ, Herrnkind WF (2001) The guide effect influence on the gregariousness of juvenile Caribbean spiny lobsters. Anim Behav 62:465–472
Codella SG Jr, Raffa KF (1995) Contributions of female oviposition patterns and larval behavior to group defense in conifer sawflies (Hymenptera: Diprionidae). Oecologia 103:24–33
Connell JH (1961) The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42:710–723
Dunning JB, Danielson BJ, Pulliam HR (1992) Ecological processes that affect populations in complex landscapes. Oikos 65:169–175
Eggleston DB, Lipcius RN (1992) Shelter selection by spiny lobster under variable predation risk, social conditions, and shelter size. Ecology 73(3):992–1011
Fletcher RJ (2007) Species interactions and population density mediate the use of social cues for habitat selection. J Anim Ecol 76:598–606
Gustafsson L (1987) Interspecific competition lowers fitness in collard flycatchers Ficedula albicollis: an experimental demonstration. Ecology 68(2):291–296
Hampe A (2004) Bioclimate envelope models? What they detect and what they hide. Global Ecol Biogeogr 13:469–476
Hobbs JPA, Munday PL (2004) Intraspecific competition controls spatial distribution and social organization of the coral dwelling goby Gobiodon histro. Mar Ecol Prog Ser 278:253–259
Holbrook SJ, Schmitt RJ (2002) Competition for shelter space causes density-dependent predation mortality in damselfishes. Ecology 83:2855–2868
Holt RD, Barfield M (2011) Theoretical perspectives on the statics and dynamics of species borders in patchy environments. Am Nat 178(4):6–25
Horner AJ, Nickles SP, Weissburg MJ, Derby CD (2006) Source and specificity of chemical cues mediating shelter preference of Caribbean spiny lobsters (Panulirus argus). Biol Bull 211:128–139
Kanciruk P, Herrnkind W (1978) Mass migration of spiny lobster, Panulirus argus (Crustacean: Palinuridae): behavior and environmental correlates. Bull Mar Sci 28(4):601–623
Kough AS, Paris CB, Butler MJ IV (2013) Larval connectivity and the international management of fisheries. PLoS ONE 8(6):e64970
Kuefler D, Avger T, Fryxell JM (2013) Density- and resource-dependent movement characteristics in a rotifer. Funct Ecol 27:323–328
Langkilde T, Shine R (2004) Competing for crevices: interspecific conflict influences retreat-site selection in montane lizards. Oecologia 140(4):684–691
Lavalli KL, Herrnkind WF (2001) Collective defense by spiny lobster (Panulirus argus) against triggerfish (Balistes capriscus): effects of number of attackers and defenders. New Zeal J Mar Freshw Res 43(1):15–28
Lavalli KL, Herrnkind WF (2009) Defensive strategies of Caribbean spiny lobsters: effects of group size and predator group size. New Zeal J Mar Freshw Res 43(1):15–28
Liesenjohann T, Palme R, Eccard JA (2013) Differential behavioral and endocrine responses of common voles (Microtus arvalis) to nest predators and resource competitors. BMC Ecol 13:33
Lindberg WJ, Frazer TK, Stanton GR (1990) Population effects of refuge dispersion for adult stone crabs (Xanthidae, Menippe). Mar Ecol Prog Ser 66:239–249
MacArthur R, MacArthur J (1961) On bird species diversity. Ecology 42:594–598
Martin PR, Martin TE (2001) Ecological and fitness consequences of species coexistence: a removal experiment with wood warblers. Ecology 82(1):189–206
Marx JM, Herrnkind WF (1985) Macroalgae (Rhodophyta: Laurencia spp.) as habitat for juvenile spiny lobster, Panulirus argus. Bull Mar Sci 36:423–431
Mintz JD, Lipcius RN, Eggleston DB, Seebo MS (1994) Survival of juvenile Caribbean spiny lobster: effects of shelter size, geographic location and conspecific abundance. Mar Ecol Prog Ser 112:255–266
Moss J, Behringer DC, Shields JD, Baeza A, Aguilar-Perera A, Bush PG, Dromer C, Herrera- Moreno A, Gittens L, Matthews TR, McCord MR, Scharer MT, Reynal L, Truelove N, Butler MJ (2013) Distribution, prevalence, and genetic analysis of Panulirus argus virus 1 (PaV1) from the Caribbean Sea. Dis Aquat Org 104:129–140
O'Neill DJ, Cobb JS (1979) Some factors influencing the outcome of shelter competition in lobsters (Homarus americanus). Mar Behav Phy 6(1):33–45
Park T (1954) Experimental studies of interspecies competition. II. Temperature, humidity, and competition in two species of Tribolium. Physiol Zool 27:177–238
Pearson RG, Dawson TP (2003) Predicting the impacts of climate change on the distribution of species: are bioclimatic envelope models useful? Global Ecol Biogeogr 12:361–371
Porter H (1960) Zoeal stages of the stone crab Menippe mercenaria (Say). Chesap Sci 1(3–4):168–171
Ratchford SG, Eggleston DB (1998) Size- and scale-dependent chemical attraction contribute to an ontogenetic shift in sociality. Anim Behav 56:1027–1034
Robertson DR (1996) Interspecific competition controls abundance and habitat use of territorial Caribbean damselfishes. Ecology 77(3):885–899
Shields JD, Behringer DC (2004) A new pathogenic virus in the Caribbean spiny lobster Panulirus argus from the Florida Keys. Dis Aquat Org 59:109–118
Shulman MJ (1984) Resource limitation and recruitment patterns in a coral reef fish assemblage. J Exp Mar Biol Ecol 74:85–109
Spicer JI, Gaston KJ (1999) Physiological diversity and its ecological implications. Blackwell Science, Oxford
Stentiford GD, Shields JD (2005) A review of the parasitic dinoflagellates Hematodinium species and Hematodinium-like infections in marine crustaceans. Dis Aquat Organ 66:47–70
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75(1):2–16
Usio N, Konishi M, Nakano S (2001) Species displacement between an introduced and a ‘vulnerable’ crayfish: the role of aggressive interactions and shelter competition. Biol Invasions 3:179–185
Wang D (1975) Agonistic and shell fighting behaviors of two sympatric species of hermit crabs. M.S. thesis, University of Delaware, Lewes
Wilber DH (1988) The influence of sexual selection and predation on the mating and postcopulatory guarding. Behav Ecol Sociobiol 24(6):445–451
Zimmer-Faust RK, Tyre JE, Case JF (1985) Chemical attraction causing aggregation in the spiny lobster, Panulirus interruptus (Randall), and its probable ecological significance. Biol Bull 169:106–118
Acknowledgements
We thank D. Cleveland, J. Anderson, R. Squibb, B. Gutzler, C. Butler, J. Butler, and J. Spadaro for field and laboratory assistance. J. Hart was partially supported by a graduate research assistantship from the University of Florida School of Natural Resources and the Environment. This research was supported by NOAA Florida Sea Grant College Program grant R/LR-B-65 and National Science Foundation grant OCE-0928398 to DCB.
Author contribution statement
DCB and JEH conceived and designed the study, JEH conducted the experiments, and DCB and JEH wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Pete Peterson.
Rights and permissions
About this article
Cite this article
Behringer, D.C., Hart, J.E. Competition with stone crabs drives juvenile spiny lobster abundance and distribution. Oecologia 184, 205–218 (2017). https://doi.org/10.1007/s00442-017-3844-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3844-1