Abstract
Several hypotheses have been proposed to explain the defense strategies of invasive plants in new ranges. In the absence of specialist herbivores, it is believed that invasive plants may allocate fewer resources to resistance and more to growth and reproduction, thus increasing tolerance to damage in the invasive genotypes. In order to test these predictions, we compared both performance (growth and reproduction) and defense strategies (tolerance and resistance) of two populations of Taraxacum officinale, one from the native range in the French Alps, and one from the introduced range in the Chilean Andes. Individuals from the introduced population demonstrated improved reproductive traits relative to those from the native population, although there was no discernible difference in biomass accumulation. Additionally, reduced tolerance was evident in the case of the former; whereas fitness traits of native plants were unaffected by damage, invasive plants reduced growth and seed output by 25 and 30% respectively following damage treatments. Increases in levels of phenols and anthocyanins, produced as a defense response to herbivory, were observed in introduced plants. Our results suggest that reallocation of resources to reproduction may be an important factor favouring invasive success of T. officinale in Chile, and that a higher investment in chemical resistance traits in this population may also be a factor in this regard.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive plant species often show different strategies that allow them to thrive and spread in the area where they are introduced. Several hypotheses have been put forward to explain the success of exotic plant invasions. One of the most widely accepted mechanisms is the escape from natural enemies (“Enemy release hypothesis”, Keane and Crawley 2002), which states that the success of an introduced plant is attributable to the absence of natural enemies in the new range. Additionally, the “Evolution and increased competitive ability” (EICA hypothesis, Blossey and Nötzold 1995) predicts that the absence of natural enemies allows invasive plants to reallocate resources from defense to growth and reproduction. Thus, invasive plants would evolve a reduced resistance to herbivory accompanied by improved growth and reproduction, leading to genotypes with a superior competitive ability. A large body of evidence has emerged to support these hypotheses (Hierro et al. 2005; Qin et al. 2013; Vila and Weiner 2004; Willis and Blossey 1999). Nevertheless, recent meta-analyses have revealed inconsistencies in the evidence for superior performance in invading populations (Chun et al. 2010; Felker-Quinn et al. 2013), suggesting that there may in fact be different non-mutually exclusive mechanisms operating to explain plant invasions (Qin et al. 2013). The latter emphasizes that no single hypothesis can explain plant invasion as a whole and that proposed invasion mechanisms may act synergistically to promote plant invasion (Lau and Schultheis 2015).
Although invasive plants colonizing new ranges may escape specialist herbivores, they are often exposed to a suite of new generalist herbivores (Keane and Crawley 2002; Müller-Schärer et al. 2004). Introduced plants must therefore rely on any defensive strategy against predation by herbivores they may encounter in the new range. These strategies are divided between (i) resistance, i.e. plant traits that reduce the preference or performance of herbivores, such as deterrent compounds including phenols, anthocyanins, alkaloids, terpenoids (Lambers et al. 1998; Lev-Yadun and Gould 2009; Fürstenberg-Hägg et al. 2013), and (ii) tolerance, i.e., the ability of plants to maintain fitness after damage (Strauss and Agrawal 1999). A trade-off between both strategies predicts that reduced levels of plant resistance may translate into greater tolerance to herbivory, and vice versa (Fineblum and Rausher 1995; Nuñez-Farfán et al. 2007). Thus, according to the EICA hypothesis that fast-growing traits are favored in exotic plants, it is expected that invasive populations are likely to demonstrate higher levels of tolerance, as opposed to resistance (Rogers and Siemann 2005; Zou et al. 2008). Previous studies, however, have shown inconsistent results. Whereas some studies support greater tolerance and/or lower resistance in invasive populations (Huang et al. 2010; Yang et al. 2014), others conversely demonstrate higher resistance and lower tolerance relative to native populations (Oduor et al. 2011).
T. officinale Weber (dandelion) is considered one of the most aggressive invasive plants globally (Holm et al. 1997), and, since first introduced in the late 19th century, has become an important invader in Latin America in general and in high-elevation ecosystems of the Andes in particular (McDougall et al. 2011). Using a common garden approach, we compared the performance and defense strategies of plants grown from seeds collected in alpine environments in its native range in the French Alps, and in an introduced range in the Chilean Andes. Specifically, we addressed the following questions: (1) Are differences in growth and reproduction between the two populations evident? (2) Is tolerance to damage higher in the invasive population than in the native one? (3) Does the colonizing population demonstrate reduced resistance (phenol and anthocyanin content) relative to the native population?
Materials and methods
Taraxacum officinale (Asteraceae) (dandelion) originates in central Europe, but has colonized most countries around the world (Holm et al. 1997). It is a stemless, deeply rooted perennial herb, having a thick taproot and leaves in rosettes at the soil level. Each plant has one or more 2–5 cm diameter capitula or flower heads terminally positioned on 5–45 cm long, hollow, cylindrical peduncles. Each capitulum has a composite of 50–250 small bright yellow ligulate or ray florets (Holm et al. 1997). Propagules are mainly dispersed by wind. T. officinale is generally apomictic, although sexually reproducing biotypes have been described. It was introduced to Chile from Europe ca. 150 years ago, and first recorded in the city of Santiago in 1870. Multiple introductions have probably occurred since then, and it can now be found growing in sites with diverse climatic characteristics, disturbance regimes, and along a wide altitudinal range (Matthei 1995; Fuentes et al. 2014).
Bulk seed collections of more than fifty T. officinale individuals were made in the Queyras Mountains at 2.000 m elevation in the South Western French Alps (native range), and in the Molina River valley at >2.500 m elevation in the central Chilean Andes (introduced range). A small number of seeds per individual plant was collected from a large number of sampled plants at different places close to each other in both ranges. Within each range (population), seeds were pooled before sorting them into experimental treatments in order to minimize possible maternal effects. Thus, it seems unlikely that the experimental seeds come from a single mother plant. Seeds were transported to the laboratory in Chile and germinated in Petri dishes at 20 °C, with a photoperiod of 12 h light. No differences were observed in the germination responses between both populations. When seedlings attained the third true-leaf stage, they were transplanted into 1 L plastic plots filled with potting soil.
The common garden experiment was carried out (January–March 2006) in a greenhouse at the Universidad de Concepción, Chile (36°S, 73°W), where the mean maximum and minimum temperatures during the experiments were 24° and 12 °C respectively. Plants of each population were randomly assigned to two different damage treatments (damage and control). Thus, the following four treatments were obtained: (1) Native population-damaged, (2) Native population-undamaged, (3) Introduced population-damaged and (4) Introduced population-undamaged (N = 20–30 plants per treatment). Damage treatment comprised 50% defoliation with scissors (50% of leaf area removed in all the leaves of a target individual), whereas control plants remained unclipped. We selected the mentioned kind of damage because it is a common pattern observed in the field (see online supplementary material). Plants of T. officinale are usually attacked by caterpillars and different mammalian herbivores under natural conditions, including chamois in the native range, and rabbits and horses in the introduced range. In the Andes (the introduced range) for instance, a survey conducted at two different sites at 3400 m a.s.l. indicated that more than 60% of the T. officinale individuals showed signs of herbivory (see online supplementary material). In these sites plants were exposed to caterpillars (Ormiscodes sp.) and mammal herbivory (rabbits or horses) that can destroy from the great majority to a few portion of all leaves of a single T. officinale individual (see online supplementary material). As the mentioned herbivores consumed T. officinale at different proportions, we estimated an average of 50% per plant as a fair damage level. All plants were watered to field capacity every two days. At the end of the experiment (after three months), the following traits were recorded: aboveground biomass (g), number of leaves, number of flowers and number of seeds produced.
Concentrations of phenols and anthocyanins, compounds linked to plant defense response to herbivory (Lambers et al. 1998; Lev-Yadun and Gould 2009), were measured in control plants from both populations. Total phenolic content in fresh leaves (µg/g) was measured using the Folin-Dennis assay (AOAC 1997). Phenol extraction was performed according to Giner-Chávez (1996). A calibration curve for tannic acid was used for determining the total of phenolic compounds in the sample. For the measurement of anthocyanins, leaves were extracted with acidified methanol (1% v/v HCl) for 24 h in darkness at 4 °C with occasional shaking. Distilled water and chloroform were mixed and added to the extract. The mixture was then centrifuged for 15 min at 5000 rpm. The absorbance of the upper phase was determined at 530 and 657 nm. The concentration of anthocyanins as mg g−1 dry weight was determined using the following equation: Anthocyanins = [OD530−0.25 OD657] × V/[dwt × 1000]. OD = optical density, V = total volume of the extract (mL); dwt = weight of the dry leaf tissue (g). Chemical traits were only measured in control plants due the goal of this study was to assess the prediction derived from the EICA hypothesis that introduced plants, because of the loss of natural enemies, should constitutively present less chemical defenses than native plants.
A two-way ANOVA was applied to evaluate the effects of Origin and Damage on growth and reproduction traits, and a one-way ANOVA was used to test the effect of the Origin on the phenol and anthocyanin content. Post-hoc comparisons were made by Tukey HSD. All statistical analyses were performed with Statistica 6.0.
Results
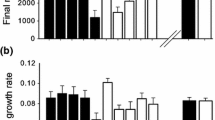
Native and introduced plants of T. officinale differed significantly in terms of the number of leaves, number of inflorescences and number of seeds inflorescence−1 (Table 1). For aboveground biomass there were no significant differences between origins (Fig. 1a). Plants from the native range produced more leaves than plants from the introduced range (Fig. 1b); introduced plants produced more inflorescences however, as well as more seeds per inflorescence, than plants from the native range (Fig. 1c, d).
Growth traits (a aboveground biomass and b number of leaves) and reproductive traits (c inflorescences and d seed output) of French (native) and Chilean (introduced) populations of Taraxacum officinale under undamaged and damaged (50% of mechanical defoliation) conditions. Asterisks indicate the significant level (Tukey HSD): *P < 0.05, **P < 0.01, ***P < 0.001, ns indicates no significant differences
The damage treatment had significant effects on aboveground biomass and fitness traits in both populations (Table 1); nevertheless, neither the introduced nor the native population was able to tolerate the damage. Whereas aboveground biomass, number of inflorescences and number of seeds per inflorescence decreased significantly for introduced plants after damage, no differences between damaged and undamaged plants were observed for these traits in native individuals (Fig. 1b, c, d). The content of phenols (mg/g) and anthocyanins (mg/g) was significantly different between origins (Phenols: native population = 2.49 ± SE 0.32, introduced population = 5.76 ± SE 0.85, F1.8 = 12.88, P = 0.007; Anthocyanins: native population = 2.66 ± SE 0.19, introduced population = 3.88 ± SE 0.38, F1.8 = 7.85, P = 0.023), in both cases markedly higher in plants of the introduced range relative to those of the native area.
Discussion
In the present study, we assessed the prediction that the invasive success of T. officinale in Chile can be attributed to superior performance and higher tolerance to herbivory relative to a population from the native range. As expected, in the absence of herbivory, the introduced population invested more resources in reproduction than the native population. These findings concur with previous studies (Blair and Wolfe 2004; Brown and Eckert 2005; Hodgins and Rieseberg 2011; Stastny et al. 2005) and partially support EICA predictions, showing superior competitive ability for the introduced population of T. officinale, at least in terms of reproduction. With respect to aboveground biomass, the introduced population showed no increase in growth, producing even fewer leaves than the native population. Although most studies support increased growth in invasive plants (Graebner et al. 2012; Huang et al. 2012; Kumschick et al. 2013; Leger and Rice 2003; Siemann and Rogers 2001; Stastny et al. 2005), others reported no differences in biomass between introduced and native genotypes (Alba et al. 2011; Li et al. 2012).
Results contradict our prediction that the introduced population should exhibit greater tolerance, while the native population should be more resistant and less able to compensate for damage. Whereas growth and reproduction in the native population were not negatively affected by damage, the introduced population demonstrated significant reductions in both growth and seed output (25 and 30% respectively) in damage treatments, suggesting that the invasive population is unable to compensate for tissue loss. Even though the invasive population seems to tolerate worse the defoliation than the native population, it still produced considerably more seeds and flowers after damage, demonstrating that the invasive population displays a better reproductive ability than the native one.
Chemical resistance traits (phenols and anthocyanins) in T. officinale were higher in the introduced population relative to the native one, providing convincing evidence to suggest that the invasive population allocates more resources to resistance than to tolerance. Contrasting results have been found regarding defense strategies in invasive plant species. Similar to our results, Oduor et al. (2011) observed lower tolerance but higher resistance in the exotic plant Brassica nigra; lower resistances, however, were detected for the Chinese invasive tree Sabium sebiferum (Zou et al. 2008) and for the exotic plant Peuraria montana (Yang et al. 2014). Significantly higher concentrations of phenols were detected in introduced T. officinale populations in Korea relative to the native congener T. mongolicum (Kim and Lee 2011). Furthermore, Kim and Lee (2011) reported high levels of phenolic acids in several species of Asteraceae, of which T. officinale is a member, raising the possibility that plants of the Asteraceae family may be inherently better suited for invasion due to their particular chemical nature. Consistent with these studies, our results support the idea that invasive plant species may gain a competitive advantage through the effects of secondary metabolites to which herbivore species in the new range have not had the opportunity to adapt (Callaway and Ridenour 2004). On the other hand, phenols and anthocyanins that deter feeding by herbivores may also serve other functions in plants, such as photoprotection (Steyn et al. 2002; Agati et al. 2013). Considering that both the native and the introduced population of T. officinale belong to an Alpine environment, a potential role of secondary metabolites in photoprotection of this plant species cannot be discarded.
Overall, our results partially validate EICA. On one hand, the invasive population of T. officinale in Chile produced a remarkably higher seed output than the native one. On the other hand however, and in contrast to the EICA, the introduced population allocated more resources to the production of toxic compounds as a resistance strategy than the native population. The lack of tolerance to herbivory by introduced plants is likely a factor of higher investment in chemical traits; however, further evidence would be required to confirm this. A previous report on the invasive mechanisms of T. officinale in Chile (Molina-Montenegro et al. 2012) demonstrated that T. officinale responds plastically in several ecophysiological traits, displaying greater competitive ability than a co-occurring native. Moreover, Quiroz et al. (2009), who also compared native and invasive populations of T. officinale from France and Chile respectively, reported better performance in plants from the introduced range than the native one. Our findings complement previous research on the mechanisms of the invasive success of T. officinale in Chile, suggesting that significant reallocation of plant resources to reproduction may likely be an important contributing factor in this regard. In addition, greater investment in chemical defences by the introduced population is undoubtedly a novel advantage in the competitive ability of this invasive species in Chile. Further studies involving larger numbers of populations are needed to elucidate the evolution of defensive strategies of the dandelion in order to explain the singular success of this ubiquitous plant in South America.
References
Agati G, Brunetti C, Di Ferdinando M, Ferrini F, Pollastri S, Tattini M (2013) Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiol Biochem 72:35–45
Alba C, Bowers MD, Blumenthal D, Hufbauer R (2011) Evolution of growth but not structural or chemical defense in Verbascum thapsus (common mullein) following introduction to North America. Biol Invasions 13:2379–2389
AOAC (1997) Official methods of analysis, 20th edn. Association of Official Analytical Chemists, Washington
Blair A, Wolfe L (2004) The evolution of an invasive plant: an experimental study with Silene latifolia. Ecology 85:3035–3042
Blossey B, Nötzold R (1995) Evolution of increased competitive ability in invasive non-indigenous plants: a hypothesis. J Ecol 83:887–889
Brown JS, Eckert CG (2005) Evolutionary increase in sexual and clonal reproductive capacity during biological invasion in an aquatic plant Butomus umbellatus (Butomaceae). Am J Bot 92:495–502
Callaway R, Ridenour W (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Chun YJ, van Kleunen M, Dawson W (2010) The role of enemy release, tolerance and resistance in plant invasions: linking damage to performance. Ecol Lett 13:937–946
Felker-Quinn E, Schweitzer JA, Bailey JK (2013) Meta-analysis reveals evolution in invasive plant species but little support for Evolution of Increased Competitive Ability (EICA). Ecol Evol 3:739–751
Fineblum WL, Rausher MD (1995) Tradeoff between resistance and tolerance to herbivore damage in a morning glory. Nature 377:517–520
Fuentes N, Sánchez P, Pauchard A, Urrutia J, Cavieres L, Marticorena A (2014) Plantas Invasoras del Centro-Sur de Chile: Una guia de campo. Ediciones del IEB
Fürstenberg-Hägg J, Zagrobelny M, Bak S (2013) Plant defense against insect herbivores. Int J Mol Sci 14:10242–10297
Giner-Chávez B (1996) Condensed tannins in tropical forages. Doctoral Thesis. Cornell University, Ithaca, New York, US
Graebner RC, Callaway RM, Montesinos D (2012) Invasive species grows faster, competes better, and shows greater evolution toward increased seed size and growth than exotic non-invasive congeners. Plant Ecol 213:545–553
Hierro JL, Maron JL, Callaway RM (2005) A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. J Ecol 93:5–15
Hodgins K, Rieseberg L (2011) Genetic differentiation in life-history traits of introduced and native common ragweed (Ambrosia artemisiifolia) populations. J Evol Biol 24:2731–2749
Holm L, Doll L, Holm E, Pacheco J, Herberger J (1997) World weeds: natural histories and distributions. John Wiley, New York
Huang W, Siemann E, Wheeler GS, Zou JW, Carrillo J, Ding JQ (2010) Resource allocation to defence and growth are driven by different responses to generalist and specialist herbivory in an invasive plant. J Ecol 98:1157–1167
Huang W, Carrillo J, Ding JQ, Siemann E (2012) Interactive effects of herbivory and competition intensity determine invasive plant performance. Oecologia 170:373–382
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Kim YO, Lee EJ (2011) Comparison of phenolic compounds and the effects of invasive and native species in East Asia: support for the novel weapons hypothesis. Ecol Res 26:87–94
Kumschick S, Hufbauer RA, Alba C, Blumenthal DM (2013) Evolution of fast-growing and more resistant phenotypes in introduced common mullein (Verbascum thapsus). J Ecol 101:378–387
Lambers H, Chapin F, Pons T (1998) Plant physiological ecology. Springer, New York
Lau JA, Schultheis EH (2015) When two invasion hypotheses are better than one. New Phytol 205:958–960
Leger EA, Rice KJ (2003) Invasive California poppies (Eschscholzia californica Cham.) grow larger than native individuals under reduced competition. Ecol Lett 6:257–264
Lev-Yadun S, Gould K (2009) Role of anthocyanins in plant defence. In: Gould K, Davies K, Winefield C (eds) Anthocyanins: biosynthesis, functions, and applications. Springer, New York, pp 21–48
Li YP, Feng YL, Barclay G (2012) No evidence for evolutionarily decreased tolerance and increased fitness in invasive Chromolaena odorata: implications for invasiveness and biological control. Plant Ecol 213:1157–1166
Matthei OJ (1995) Manual de las malezas que crecen en Chile. Alfabeta, Santiago
McDougall KL, Alexander JM, Haider S, Pauchard A, Walsh NG, Kueffer C (2011) Alien flora of mountains: global comparisons for the development of local preventive measures against plant invasions. Divers Distrib 17:103–111
Molina-Montenegro M, Penuelas J, Munne-Bosch S, Sardans J (2012) Higher plasticity in ecophysiological traits enhances the performance and invasion success of Taraxacum officinale (dandelion) in alpine environments. Biol Invasions 14:21–33
Müller-Schärer H, Schaffner U, Steinger T (2004) Evolution in invasive plants: implications for biological control. Trends Ecol Evol 19:417–422
Nuñez-Farfán J, Fornoni J, Valverde L (2007) The evolution of resistance and tolerance to herbivores. Annu Rev Ecol Evol Syst 38:541–566
Oduor AMO, Lankau RA, Strauss SY, Gomez JM (2011) Introduced Brassica nigra populations exhibit greater growth and herbivore resistance but less tolerance than native populations in the native range. New Phytol 191:536–544
Qin R-M, Zheng Y-L, Valiente-Banuet A, Callaway RM, Barclay GF, Silva Pereyra C, Feng YL (2013) The evolution of increased competitive ability, innate competitive advantages, and novel biochemical weapons act in concert for a tropical invader. New Phytol 197:979–988
Quiroz CL, Choler P, Baptist F, González-Teuber M, Molina-Montenegro M, Cavieres L (2009) Alpine dandelions originated in the native and introduced range differ in their responses to environmental constraints. Ecol Res 24:175–183
Rogers WE, Siemann E (2005) Herbivory tolerance and compensatory differences in native and invasive ecotypes of Chinese tallow tree (Sapium sebiferum). Plant Ecol 181:57–68
Siemann E, Rogers WE (2001) Genetic differences in growth of an invasive tree species. Ecol Lett 4:514–518
Stastny M, Schaffner U, Elle E (2005) Do vigour of introduced populations and escape from specialist herbivores contribute to invasiveness? J Ecol 93:27–37
Steyn WJ, Wand SJE, Holcroft DM, Jacobs G (2002) Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol 155:349–361
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Vila M, Weiner J (2004) Are invasive plant species better competitors than native plant species? evidence from pair-wise experiments. Oikos 105:229–238
Willis AJ, Blossey B (1999) Benign environments do not explain the increased vigour of non-indigenous plants: a cross-continental transplant experiment. Biocontrol Sci Technol 9:567–577
Yang X, Huang W, Tian B, Ding J (2014) Differences in growth and herbivory damage of native and invasive kudzu (Peuraria montana var. lobata) populations grown in the native range. Plant Ecol 215:339–346
Zou JW, Siemann E, Rogers WE, DeWalt SJ (2008) Decreased resistance and increased tolerance to native herbivores of the invasive plant Sapium sebiferum. Ecography 31:663–671
Acknowledgements
We thank the Department of Botany at Universidad de Concepción, Chile for the greenhouse facility in their dependencies. We are also grateful to José Becerra for his kind support with the analyses of the chemical compounds. This research was funded by CONICYT doctoral Fellowship to C.L. Quiroz and F ICM P05-02 and CONICYT PFB-023 supporting the Institute of Ecology and Biodiversity (IEB).
Funding
This research was funded by CONICYT doctoral Fellowship to C.L. Quiroz, FONDECYT 1130952, and F ICM P05-02 and CONICYT PFB-023 supporting the Institute of Ecology and Biodiversity (IEB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
González-Teuber, M., Quiroz, C.L., Concha-Bloomfield, I. et al. Enhanced fitness and greater herbivore resistance: implications for dandelion invasion in an alpine habitat. Biol Invasions 19, 647–653 (2017). https://doi.org/10.1007/s10530-016-1309-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1309-9