Abstract
Post-introduction evolution of increased growth or reproduction has been observed in many species of invasive plants; however, it is not consistently associated with a loss of defense, as predicted by the influential evolution of increased competitive ability (EICA) hypothesis. Inconsistent support for the EICA hypothesis likely reflects the fact that, although invasive plants are released from attack by some enemies, typically specialists, they often do not escape attack from generalists. Thus, different types of defense (e.g., structural versus chemical) may evolve in different directions following introduction. We used a common garden experiment to test whether a shift in allocation among defenses (as opposed to a simple increase or decrease in a single defense) is associated with increased growth in introduced Verbascum thapsus populations. Introduced populations had significantly greater shoot biomass than natives. However, root biomass was similar between ranges, and highly variable, resulting in only marginal differences in total biomass. Mean investment in all three defenses was remarkably similar between the native and introduced populations, providing no evidence for range-level, post-introduction evolution of defense. This finding was consistent with the fact that, despite significant population-level variability for all defenses, there was little evidence of trade-offs between growth and defense or among different types of defense. These results suggest that evolution of increased growth in V. thapsus is not fueled by decreased allocation to defense, and that selection on defense may vary more at the population scale than the continental scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species commonly escape many of their natural enemies, leading to a release from top-down population regulation (e.g., Elton 1958; Memmott et al. 2000; Keane and Crawley 2002; DeWalt et al. 2004; but see Colautti et al. 2004; Chun et al. 2010). For invasive plants, release from enemies may alter the selection regime such that particular defenses are no longer under positive selection, and in fact may be under negative selection if their production incurs a fitness cost (Strauss et al. 2002; Müller-Schärer et al. 2004). This can then result in an evolutionary loss of defenses, which is predicted to favor growth and reproduction (evolution of increased competitive ability or EICA hypothesis; Blossey and Nötzold 1995).
Post-introduction evolution of increased growth or reproduction has been observed in many species, as predicted by the EICA hypothesis (e.g., Leger and Rice 2003; Wolfe et al. 2004; Blumenthal and Hufbauer 2007). However, it is not consistently associated with a loss of defense (reviewed in Hinz and Schwarzlaender 2004; Bossdorf et al. 2005), perhaps because introduced plants are often attacked by generalist herbivores in the new range, and thus do not completely escape herbivory (Memmott et al. 2000; Müller-Schärer et al. 2004; Parker et al. 2006). If introduced plants are predominantly attacked by generalists (here we focus on insects), then qualitative defenses (i.e. toxins), which deter generalists and are relatively inexpensive for a plant to produce, should be maintained in the new range. In contrast, quantitative defenses (i.e., structural defenses and/or digestibility-reducing chemicals), which are effective against specialists and generalists, but are typically costly to produce, should decrease (Müller-Schärer et al. 2004; Joshi and Vrieling 2005). Resulting increases in growth are then thought to stem from a shift in allocation from relatively costly quantitative defenses to less costly qualitative defenses (Müller-Schärer et al. 2004).
There are few studies that have tested the expanded EICA hypothesis (sensu Müller-Schärer et al. 2004) by measuring chemical and structural defense in addition to some aspect of growth or reproduction (but see Joshi and Vrieling 2005; Franks et al. 2008; Ridenour et al. 2008). Most tests of the EICA hypothesis directly quantify only one type of defense (e.g., Joshi and Vrieling 2005; Lewis et al. 2006; Cano et al. 2009; but see Franks et al. 2008; Ridenour et al. 2008) or use more general feeding assays that cannot pinpoint which specific defensive traits might differ between ranges (e.g., Blossey and Nötzold 1995; Siemann and Rogers 2003; Leger and Forister 2005). Studies that do provide a detailed test of the expanded EICA hypothesis (sensu Müller-Schärer et al. 2004), are, taken together, inconclusive (Joshi and Vrieling 2005; Franks et al. 2008; Ridenour et al. 2008). For example, in clear support of the hypothesis, Joshi and Vrieling (2005) found that introduced populations of Senecio jacobaea (Asteraceae) grew larger than their native counterparts, and in addition were better protected against generalist herbivores (Mamestra brassicae and Spodoptera exigua) while less protected against a specialist (Tyria jacobaeae). In contrast, Ridenour et al. (2008) reported that introduced populations of Centaurea maculosa (Asteraceae) are not only larger, but also better defended, against both specialists and generalists than their native counterparts. They interpreted these findings to be evidence for a lack of trade-offs between growth and defense.
The Ridenour et al. (2008) findings contribute to a long-standing debate regarding the existence of costs associated with trade-offs between plant growth and defense (e.g., Mole 1994; Bergelson and Purrington 1996; Herms and Mattson 1992; Koricheva 2002; Strauss et al. 2002). This debate is directly relevant to the predictions of the EICA hypothesis: if trade-offs are weak or imperceptible in a system, there is little reason to expect an increase in growth or reproduction to come at the expense of investing in defense. The first study to provide a detailed analysis of fitness costs associated with defense (Bergelson and Purrington 1996) reported that plants exhibit a trade-off in only 33% of cases. However, a follow-up review that included more recent work reached a quite different conclusion, showing that costs are detectable in 76% of cases (Strauss et al. 2002). A recent meta-analysis (Koricheva 2002) highlighted that several factors determine the shape of the function describing costs, including environmental factors and the type of defense compounds explored. Because investigations of the EICA hypothesis lend themselves to correlation analysis, they provide a tool to directly test for inverse relationships between growth and defense or between different types of defense.
Here we quantify variation among populations and between ranges in three types of defense (two structural and one chemical) in the introduced weed Verbascum thapsus L. (Scrophulariaceae; common mullein). We used a common garden approach to test whether a shift in allocation among defenses (as opposed to a simple increase or decrease in a single defense) is associated with increased growth in introduced populations. We predicted that introduced mullein would invest more in biomass, more or similarly in chemical defense (iridoid glycosides) against generalists, and less in structural defense (trichomes and leaf toughness) against specialists and generalists, than do native populations. By simultaneously measuring growth and several defense traits, we were also able to explore whether there is a negative relationship between growth and defense or between different types of defense. A negative relationship provides evidence for an underlying assumption of the EICA hypothesis: that defenses are costly and impose a trade-off between the ability to grow and defend (sensu Herms and Mattson 1992).
Methods
Study system
Common mullein is a monocarpic perennial (typically biennial) forb that was repeatedly introduced to the eastern United States, first by Puritan settlers in the 1600s for its medicinal properties and later by English and German settlers for use as a piscicide (Wilhelm 1974; Gross and Werner 1978, Mitich 1989). It was also directly imported to the US from Germany in the early 1900s (Henkel 1917). It now occurs in all 50 of the United States, having spread rapidly from its points of introduction in the east to Michigan by 1839 and the Pacific Coast by 1876 (Brewer et al. 1876; Gross and Werner 1978). It is designated as noxious in Colorado and Hawaii. Mullein has a large native range, occurring throughout Europe and Asia. Although there are currently no molecular reconstructions of its introduction history, the timing of its introduction and its well-documented ethnobotanical history support the contention that Europe was the source of the introduction, especially since there were few trade or travel connections between Asia and the US in the 17th century.
Mullein has several characteristics typical of weedy invaders. It produces up to 175,000 seeds per plant and forms long-lived seed banks (Gross and Werner 1978). Mullein flourishes in response to disturbance, and therefore may depress recruitment by co-occurring natives in early-seral communities (Pitcairn 2000). Although this species tends to be fugitive, infestations can persist for many years in the introduced range, especially following fire (Fornwalt et al. 2011) or in areas subject to chronic disturbance (e.g., black-tailed prairie dog [Cynomys ludovicianus] colonies; Alba, pers. obs.). A recent biogeographic comparison of native (n = 21) and introduced (n = 32) populations showed that introduced populations are significantly larger and more dense than native populations, with larger individual plants (Alba and Hufbauer, in review). Additionally, introduced plants are less severely damaged by insect herbivores than their native counterparts (Alba and Hufbauer, in review), and they have been released from attack by several specialist insects, including Cucullia verbasci L. (Noctuidae) (Maw 1980) and several species of weevil (Gross and Werner 1978; Alba and Hufbauer, in review).
Mullein invests heavily in both structural and chemical defense against herbivores. Mullein leaves are covered with dense trichomes, structures that reduce feeding by many insects including caterpillars (e.g., Khan et al. 1986, Agren and Schemske 1993), leafhoppers (reviewed in Levin 1973), beetles (e.g., Dimock and Tingey 1988) and grasshoppers (Woodman and Fernandes 1991). Another potentially important structural defense is leaf toughness, which has been shown to deter insect feeding (Coley 1983; Choong 1996) and to reduce insect performance (Feeny 1970; Clissold et al. 2009) on multiple plant species. Mullein also produces toxic secondary metabolites including the iridoid glycosides aucubin and catalpol (Khuroo et al. 1988; Pardo et al. 1998). These chemicals deter generalists (e.g., Bowers and Puttick 1988) and can attract specialists that use them as oviposition and feeding cues and are able to detoxify or sequester them (e.g., Bowers 1984; Bowers and Puttick 1988; Pereyra and Bowers 1988; Nieminen et al. 2003). Catalpol is the final product of the biosynthetic pathway (Damtoft 1994), suggesting that higher proportions of catalpol reflect greater energetic investment by the plant. Additionally, catalpol is more strongly deterrent to generalists than aucubin (Bowers and Puttick 1988). As such, the ratio of aucubin to catalpol may be an important driver of herbivore feeding preferences in addition to their total amount.
Experimental design
We used a common garden approach to explore whether mullein populations exhibit variation in biomass, trichome length, leaf toughness, and iridoid glycoside content, with the specific aim to test whether introduced and native mullein populations differ for these traits. Plants from 10 introduced and 4 native sites were grown in a greenhouse from field-collected seed (see Table 1 for locations of sample sites). Although limited samples were available from the native range, the sites are within the geographic range reported to be the source of mullein introductions into North America (see Study System). Despite this, the relatively low replication requires some caution in interpreting the experimental results. We grew three replicates of each of 10 maternal lines per site (with the exception of the Romania and Ithaca, NY sites, which had 5 and 6 maternal lines, respectively) for a total of 393 plants. We measured above-ground biomass, trichome length, and leaf toughness on all three replicates of each maternal plant, while root biomass and iridoid glycosides were measured on one replicate of each maternal plant.
In June 2008, seeds were sown into germination trays containing Sunshine #3 germination mix (DWF Grower Supply, Denver CO) and placed on a mist bench (average daytime temp., 24.8°C; average daytime relative humidity, 59.5%; average nighttime temp., 19.9°C; average nighttime relative humidity, 77.4%). Excess seed was sown and seedlings were thinned as necessary to avoid competition. The length of one cotyledon per seedling was measured with calipers to provide an estimate of maternal provisioning. We took this measurement to help us determine whether observed differences in biomass between native and introduced plants might be a result of maternal effects. Germination trays were re-randomized at regular intervals to avoid micro-climatic effects. At four weeks, seedlings were transplanted into 1-gallon pots containing a mixture of 75% Sunshine #2 potting soil (DWF Grower Supply, Denver, CO), 15% turface (L.L. Johnson Distributing Co., Fort Collins, CO), and 10% sand (Bath Garden Center, Fort Collins, CO) and moved to greenhouse benches (average daytime temp., 21.9°C; average daytime relative humidity, 64.5%; average nighttime temp., 18.4°C; average nighttime relative humidity, 72.6%) for the remainder of the experiment, where they were re-randomized once every 2 weeks. Plants were watered as needed and fertilized once with Osmocote (a slow-release NPK fertilizer) per the manufacturer’s directions. To control an outbreak of thrips and fungus gnats, all plants were treated a single time with a Permethrin-based (2.5%) multipurpose insecticide and Gnatrol (a biocontrol insecticide using Bacillus thuringiensis), respectively.
Plants were harvested for growth and defense measurements at 8 weeks of age. Mullein rosettes must reach a threshold size in order to successfully overwinter (Gross 1980); thus, the rate at which biomass is accumulated early in life has a critical influence on final fitness. Indeed, individuals that germinate early in the season (and thus can achieve greater rosette size before overwintering) produce larger inflorescences and more seed than those germinating later in the season (Gross 1980). We also note that, although we conducted our common garden experiment in the introduced range, it is unlikely that the greenhouse conditions or potting soil favored introduced populations.
Biomass Measurements
All rosettes were oven dried at 50°C to a constant mass and then weighed. We measured root biomass on a subset of individuals (1 individual of each maternal line in each population, n = 131 individuals). Roots were gently washed free of their potting soil prior to drying and weighing.
Defense measurements
Measurements of trichome length and leaf toughness were made on freshly harvested leaves. We controlled for differences in defense due to leaf age and size by harvesting leaves of similar rank, randomly choosing from the two leaves within a rank, and measuring the length of each leaf to include as a covariate in statistical analyses. Leaves were cut in half and each half randomly assigned to trichome or leaf toughness measures. Trichome length was measured under an ocular micrometer at 40× magnification (Woodman and Fernandes 1991). We removed a 0.6-cm-diameter circle of tissue from between the second and third secondary veins (moving away from the leaf tip), near the midrib. The circle was gently held on end with tweezers, and the length of trichomes was measured from the epidermal layer out. The length of the trichomes did not include the occasional longer hairs, but was taken to be the dominant layer or mat of hairs (sensu Woodman and Fernandes 1991).
Leaf toughness measurements were made at the same location on the other half of each leaf using a Lloyd LF-Plus universal testing machine customized to work as a leaf penetrometer. The penetrometer forces a blunt circular probe (7.0686 mm2) through the leaf at a constant speed, and measures force applied to the probe continuously with a 20 Newton load cell, accurate to within 1% of the force measurement. We recorded both the total work required to puncture a leaf and the maximum force required to puncture a leaf, but report only the latter (load at maximum load in kN), as it was less sensitive to measurement error. For simplicity, we use the term “leaf toughness” throughout.
Iridoid glycosides were quantified in a subset of individuals (1 individual from each maternal line in each population, n = 131) using gas chromatography (detailed in Gardner and Stermitz 1988; Bowers and Stamp 1993). Briefly, we ground dried rosettes to a fine powder and extracted 50 mg subsamples in methanol. The extract was then partitioned between water and ether to remove chlorophyll and hydrophobic compounds. We added an internal standard (phenyl-β-d-glucose) to the remaining sample, which was then derivatized with Tri-Sil-Z (Pierce Chemical, Rockford Illinois, USA) prior to injection on a gas chromatograph (Hewlett Packard 5890 equipped with an autoinjector).
Statistical analyses
All statistical analyses were conducted in SAS, v. 9.1 (SAS, Cary Institute, NC 2002). We first tested for differences in cotyledon size due to invasive status (i.e., continent of origin) using mixed model ANOVA with continent of origin as a fixed effect and site with continent as a random effect. After ruling out continent-level differences in maternal provisioning based on cotyledon size, we continued with the remaining analyses.
We tested for differences in biomass and levels of defense due to continent of origin using mixed model ANOVA. We evaluated the use of latitude as a covariate in the model for shoot biomass and altitude as a covariate in the models for trichome length and leaf toughness. As we found no effect of latitude and altitude on the response variables (latitude effect on shoot biomass, P = 0.39; altitude effect on trichome length, P = 0.68; altitude effect on leaf toughness, P = 0.27), we analyzed shoot biomass, trichome length, and leaf toughness with continent of origin as a fixed effect and population within continent and maternal line within population as random effects. The models for root biomass, total biomass, shoot:root ratio, and iridoid glycoside content did not include the random effect of maternal line within site because we did not have replication at that level. Models testing for differences in trichome length and leaf toughness included leaf length as a covariate to control for differences in leaf age. We used the least square means statement to test for differences based on continent of origin. To test the significance of the random effects of site and maternal line, we generated likelihood-ratio statistics and compared them against a chi-square distribution with one degree of freedom (Littell et al. 1996). When necessary, data were transformed (square root: shoot biomass, root biomass, and leaf toughness; arcsine square root: aucubin and catalpol proportions) to improve normality and homogeneity of variance.
To test for trade-offs between biomass and the three defenses, and between the three defenses themselves, we generated correlation coefficients using the PROC CORR procedure (Table 2). We used family means when possible (for shoot biomass, trichome length, and leaf toughness). We did not have replication within families for total biomass, iridoid glycoside content, and the proportion of iridoids made up of catalpol. “Global” trade-offs were evaluated by generating correlations that included data points from all populations in the two ranges (Table 2). We additionally evaluated trade-offs separately for each population to ensure that the global correlation coefficients did not obscure any trade-offs present at the population scale. To test whether native and introduced populations had significantly different global correlation coefficients, we used a mixed model ANOVA (fixed effect = continent; random effect = population with continent).
Results
Maternal effects
Cotyledon size did not differ between introduced and native populations (introduced, 3.25 mm2 ± SE 0.03; native, 3.45 mm2 ± SE 0.04; P = 0.27), providing no evidence that maternal provisioning differed between continents.
Biomass
Introduced plants had significantly greater shoot biomass than native plants (F 1,12 = 10.43; P = 0.007; Fig. 1a), but root biomass was similar between populations from the two ranges (F 1,12 = 0.21; P = 0.66; Fig. 1b). As a result of this, the difference in total biomass was only marginally significant (F 1,12 = 2.02; P = 0.09; Fig. 1c). The shoot:root ratios did not significantly differ (F 1,12 = 0.63; P = 0.43; Fig. 1d). There was no significant population-level variation in biomass (shoot, P = 0.32; root, P = 0.5) or shoot:root ratios (P = 0.22), nor was there significant within-population (maternal plant) variation in shoot biomass (P = 0.38). As such, we present only the continental means for the biomass data.
Defense
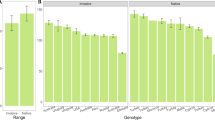
Defenses were remarkably similar between the native and introduced ranges. There were no significant differences between introduced and native populations for trichome length (F 1,12 = 0.12; P = 0.74; Fig. 2a), leaf toughness (F 1,12 = 0.05; P = 0.83; Fig. 2b), percent dry weight of iridoid glycosides (F 1,12 = 0; P = 0.99; Fig. 2c), or the proportion of total iridoids made up of catalpol (F 1,12 = 0.77; P = 0.40; Fig. 2d). In contrast to the striking similarity of defense investment at the continent scale, there was highly significant among-population variation for all defenses (trichome length, P = 0.005; leaf toughness, P < 0.0001; percent dry weight of iridoids, P < 0.0001; proportion catalpol, P = 0.005; Fig. 2a–d). There was no significant within-population (maternal plant) variation in the defenses with replication at that level (trichome length: P = 0.5; leaf toughness: P = 0.5).
Continent and population means (±SE) of a trichome length, b leaf toughness, c iridoid glycoside content (with separate standard error bars presented for aucubin and catalpol), and d the proportion of iridoids composed of catalpol. Continent means are not significantly different (P < 0.05) in any case. In contrast, there is significant population-level variability for all defenses (see “Results”). Populations ordered as in Table 1
Cost of defense
Correlation coefficients expressing the relationship between all pairwise comparisons of total biomass, shoot biomass, trichome length, leaf toughness, and iridoid glycoside content revealed no compelling evidence of trade-offs.. The only significant global correlation coefficients (generated using all data points from all populations in both ranges) were positive (Table 2). When evaluating populations separately (14 populations × 13 pairs of traits = 182 comparisons), we detected only 8 significantly negative correlations (cf. Table 2 for populations exhibiting negative trade-offs). A mixed model ANOVA showed that correlation coefficients did not significantly differ between native and introduced populations for any pair of traits (data not shown).
Discussion
Our goal was to provide a detailed test of the expanded EICA hypothesis (Müller-Schärer et al. 2004) by quantifying growth plus several types of defense that are predicted to deter mainly specialist (trichomes, leaf toughness) or generalist (iridoid glycosides) insects. We found partial support for the EICA hypothesis in that shoot biomass of introduced plants was significantly greater than that of natives (Fig. 1a). However, root biomass was similar between ranges, and highly variable, resulting in only marginal differences in total biomass (Fig. 1c). The different conclusions reached based on the results for shoot biomass (clear support for EICA) versus total biomass (weak support for EICA) highlights the importance of estimating whole-plant growth rather than only aboveground growth, which is sometimes done, likely because of logistical constraints (e.g., Blumenthal and Hufbauer 2007; Cano et al. 2009). Our results also suggest that aboveground biomass was more strongly selected to increase in the introduced range than was belowground biomass; this indicates that in some invasive populations, potentially adaptive changes in plant architecture (e.g., a shift in the relative investment in above- versus belowground parts) may be present even if total investment in growth is similar between ranges.
We detected no difference in trichome length, leaf toughness, or iridoid glycoside content when comparing plants from mullein’s native and introduced ranges. That none of the traits showed evidence of post-introduction evolution provides a compellingly consistent pattern—one that stands in contrast to the equally clear pattern of significant population-level variation present for each defense. It is possible that our low population replication for the native range failed to capture existing differences in defense phenotypes at the continent scale, and this interpretation cannot be ruled out given the variation that exists among populations. However, two lines of evidence suggest that our findings are accurate. First, there were no non-significant trends toward differences in defense. In fact, the means for all three types of defense were virtually identical between ranges (Fig. 2). Second, correlation analysis suggests that our findings of no effect reflect a biological reality of the system: there is only very weak evidence of trade-offs between either biomass and defense, or between the three types of defense (Table 2). Overall, populations with large plants also tended to have plants with relatively tough leaves, and high concentrations of chemical defenses. Adler et al. (1995) found results similar to ours, in that they detected no trade-off between allocation to biomass and iridoid glycoside content in Plantago lanceolata. This finding may well reflect a true lack of a physiological trade-off; alternatively, it could reflect a greater degree of variation among genotypes in the ability to assimilate carbon than variation in the allocation of carbon to growth versus defense (Adler et al. 1995). Although the conditions under which trade-offs manifest are complicated (Koricheva 2002), and their existence can be difficult to detect (Bergelson and Purrington 1996; Strauss et al. 2002), it is nonetheless striking that we found little evidence for trade-offs between any of the several traits measured (Table 2).
Although the EICA hypothesis explicitly predicts differences at the continent scale, considering all levels of genetic structuring (including among- and within-population variation) can help researchers interpret either the presence or absence of differences between ranges. For example, here we found that none of the biomass traits (shoot, root, and total biomass) exhibit significant population variation, with a mean difference in shoot biomass instead manifesting at the continent scale (Fig. 1a). Conversely, all of the defenses showed significant population-level variation, with no indication of mean differences between ranges. If our sample populations accurately capture mean investment in defense, it suggests that selection operating at local or regional scales may be stronger than the directional selection predicted to operate at the continent scale.
There are many examples of geographic variability in selection (“selection mosaics”) on plant-insect interactions (e.g., Berenbaum and Zangerl 1998; Gomulkiewicz et al. 2000; Thompson and Cunningham 2002). Such geographic structuring leads to population differentiation for traits associated with the interactions, thereby precluding a “globally favored” phenotype spanning all populations of a species (or in the context of invasions, all populations in a species’ native or introduced ranges; Thompson 1997). We also found no maternal variation for any of the traits that had replication of maternal lines (shoot biomass, trichome length, leaf toughness). A lack of within-population variation suggests that, even if selection were acting on these traits, populations may not possess the requisite genetic variability to respond rapidly. In the case of shoot biomass, the combination of continent-scale genetic differentiation and minimal within or between population variation may reflect the introduction of pre-adapted genotypes rather than a rapid response to selection following introduction.
Although several studies do provide support for the EICA hypothesis (e.g., Siemann and Rogers 2003; Blair and Wolfe 2004; Wolfe et al. 2004), the balance of studies, including ours, provide partial or no support (reviewed in Hinz and Schwarzlaender 2004, Bossdorf et al. 2005). The next step is to determine why the hypothesis appears to explain invasion dynamics in some systems but not others. While common garden experiments effectively measure the results of evolutionary processes, they cannot directly quantify the source, direction, or strength of selection on traits that are relevant to invasion (Endler 1986; Kalisz 1986), nor discriminate between rapid adaptation and other modes of genetic divergence such as the differential introduction of pre-adapted genotypes. Here we have suggested that it can be useful to directly test the conditions required for EICA, such as variation in and tradeoffs between growth and defense, both within and among populations. We would also suggest that the next generation of studies in this area should incorporate direct measurements of selection gradients on traits associated with competitive ability and defense (cf. Franks et al. 2008; Murren et al. 2009) so that the identity and role of putative selection pressures (e.g., specialist and generalist enemies) acting in each range can be confirmed.
References
Adler LS, Schmitt J, Bowers MD (1995) Genetic variation in defensive chemistry in Plantago lanceolata (Plantaginaceae) and its effect on the specialist herbivore Junonia coenia (Nymphalidae). Oecologia 101:75–85
Agren J, Schemske DW (1993) The cost of defense against herbivores: an experimental-study of trichome production in Brassica rapa. Am Nat 141:338–350
Alba C, Hufbauer R (in review) A biogeographic comparison of Verbascum thapsus ecology reveals differences in performance, herbivory, and surrounding plant community
Berenbaum MR, Zangerl AR (1998) Chemical phenotype matching between a plant and its insect herbivore. Proc Natl Acad Sci USA 95:13743–13748
Bergelson J, Purrington CB (1996) Surveying patterns in the cost of resistance in plants. Am Nat 148:536–558
Blair AC, Wolfe LM (2004) The evolution of an invasive plant: an experimental study with Silene latifolia. Ecology 85:3035–3042
Blossey B, Nötzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J Ecol 83:887–889
Blumenthal DM, Hufbauer RA (2007) Increased plant size in exotic populations: a common-garden test with 14 invasive species. Ecology 88:2758–2765
Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11
Bowers MD (1984) Iridoid glycosides and host-plant specificity in larvae of the buckeye butterfly, Junonia coenia (Nymphalidae). J Chem Ecol 10:1567–1577
Bowers MD, Puttick GM (1988) Response of generalist and specialist insects to qualitative allelochemical variation. J Chem Ecol 14:319–334
Bowers MD, Stamp NE (1993) Effects of plant age, genotype, and herbivory on Plantago performance and chemistry. Ecology 74:1778–1791
Brewer WH, Watson S, Gray A (1876) Botany of California. Welch Bigelow and Co, University Press, Cambridge
Cano L, Escarre J, Vrieling K, Sans FX (2009) Palatability to a generalist herbivore, defence and growth of invasive and native Senecio species: testing the evolution of increased competitive ability hypothesis. Oecologia 159:95–106
Choong MF (1996) What makes a leaf tough and how this affects the pattern of Castanopsis fissa leaf consumption by caterpillars. Funct Ecol 10:668–674
Chun YJ, van Kleunen M, Dawson W (2010) The role of enemy release, tolerance and resistance in plant invasions: linking damage to performance. Ecol Lett 13:937–946
Clissold FJ, Sanson GD, Read J, Simpson SJ (2009) Gross versus net income: how plant toughness affects performance of an insect herbivore. Ecology 90:3393–3405
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Coley PD (1983) Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol Monogr 53:209–233
Damtoft S (1994) Biosynthesis of catalpol. Phytochemistry 35:1187–1189
DeWalt SJ, Denslow JS, Ickes K (2004) Natural-enemy release facilitates habitat expansion of the invasive tropical shrub Clidemia hirta. Ecology 85:471–483
Dimock MB, Tingey WM (1988) Host acceptance behavior of Colorado potato beetle larvae influenced by potato glandular trichomes. Physiol Entomol 13:399–406
Elton CS (1958) The ecology of invasions by animals and plants. University of Chicago Press, 196 p
Endler JA (1986) Natural selection in the wild. Princeton University Press, Princeton
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Fornwalt PJ, Kaufmann MR, Stohlgren TJ (2011) Impacts of mixed-severity wildfire on exotic plants in a Colorado ponderosa-pine Douglas-fir forest. Biol Invasions 12:2683–2695
Franks SJ, Pratt PD, Dray FA, Simms EL (2008) No evolution of increased competitive ability or decreased allocation to defense in Melaleuca quinquenervia since release from natural enemies. Biol Invasions 10:455–466
Gardner DR, Stermitz FR (1988) Hostplant utilization and iridoid glycoside sequestration by Euphydryas anicia individuals and populations. J Chem Ecol 14:2147–2168
Gomulkiewicz R, Thompson JN, Holt RD, Nuismer SL, Hochberg ME (2000) Hot spots, cold spots, and the geographic mosaic theory of coevolution. Am Nat 156:156–174
Gross KL (1980) Colonization by Verbascum thapsus (mullein) of an old-field in Michigan: experiments on the effects of vegetation. J Ecol 68:919–927
Gross KL, Werner PA (1978) The biology of Canadian weeds 28. Verbascum thapsus and Verbascum blattaria. Can J Plant Sci 58:401–413
Henkel A (1917) Weeds used in medicine. US Department of Agriculture Farmers’ Bulletin no. 188
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Hinz HL, Schwarzlaender M (2004) Comparing invasive plants from their native and exotic range: what can we learn for biological control. Weed Technol 18:1533–1541
Joshi J, Vrieling K (2005) The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett 8:704–714
Kalisz S (1986) Variable selection on the timing of germination in Collinsia verna (Scrophulariaceae). Evolution 40:479–491
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Khan ZR, Ward JT, Norris DM (1986) Role of trichomes in soybean resistance to cabbage looper, Trichoplusia ni. Entomologia Experimentalis Et Applicata 42:109–117
Khuroo MA, Qureshi MA, Razdan TK, Nichols P (1988) Sterones, iridoids and a sesquiterpene from Verbascum thapsus. Phytochemistry 27:3541–3544
Koricheva J (2002) Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83:176–190
Leger EA, Forister ML (2005) Increased resistance to generalist herbivores in invasive populations of the California poppy (Eschscholzia californica). Divers Distrib 11:311–317
Leger EA, Rice KJ (2003) Invasive California poppies (Eschscholzia californica Cham.) grow larger than native individuals under reduced competition. Ecol Lett 6:257–264
Levin DA (1973) Role of trichomes in plant defense. Q Rev Biol 48:3–15
Lewis KC, Bazzaz FA, Liao Q, Orians CM (2006) Geographic patterns of herbivory and resource allocation to defense, growth, and reproduction in an invasive biennial, Alliaria petiolata. Oecologia 148:384–395
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models. SAS Institute, Cary
Maw MG (1980) Cucullia verbasci an agent for the biological control of common mullein (Verbascum thapsus). Weed Sci 28:27–30
Memmott J, Fowler SV, Paynter Q, Sheppard AW, Syrett P (2000) The invertebrate fauna on broom, Cytisus scoparius, in two native and two exotic habitats. Acta Oecol Int J Ecol 21:213–222
Mitich LW (1989) Common mullein: the roadside torch parade. Weed Technol 3:704–705
Mole S (1994) Trade-offs and constraints in plant-herbivore defense theory: a life-history perspective. Oikos 71:3–12
Müller-Schärer H, Schaffner U, Steinger T (2004) Evolution in invasive plants: implications for biological control. Trends Ecol Evol 19:417–422
Murren CJ, Chang CC, Dudash MR (2009) Patterns of selection of two North American native and nonnative populations of monkeyflower (Phrymaceae). New Phytol 183:691–701
Nieminen M, Suomi J, Van Nouhuys S, Sauri P, Riekkola ML (2003) Effect of iridoid glycoside content on oviposition host plant choice and parasitism in a specialist herbivore. J Chem Ecol 29:823–844
Pardo F, Perich F, Torres R, Delle Monache F (1998) Phytotoxic iridoid glucosides from the roots of Verbascum thapsus. J Chem Ecol 24:645–653
Parker JD, Burkepile DE, Hay ME (2006) Opposing effects of native and exotic herbivores on plant invasions. Science 311:1459–1461
Pereyra PC, Bowers MD (1988) Iridoid glycosides as oviposition stimulants for the buckeye butterfly, Junonia coenia (Nymphalidae). J Chem Ecol 14:917–928
Pitcairn MF (2000) Verbascum thapsus L. In: Bossard CC, Randall JM (eds) Invasive plants of California’s wild lands. University of California Press, Hoshovsky
Ridenour WM, Vivanco JM, Feng YL, Horiuchi J, Callaway RM (2008) No evidence for trade-offs: Centaurea plants from America are better competitors and defenders. Ecol Monogr 78:369–386
SAS System for Windows, v. 9.1 (2002) SAS Institute Inc. Cary
Siemann E, Rogers WE (2003) Reduced resistance of invasive varieties of the alien tree Sapium sebiferum to a generalist herbivore. Oecologia 135:451–457
Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol 17:278–285
Thompson JN (1997) Evaluating the dynamics of coevolution among geographically structured populations. Ecology 78:1619–1623
Thompson JN, Cunningham BM (2002) Geographic structure and dynamics of coevolutionary selection. Nature 417:735–738
Wilhelm G (1974) Mullein: plant piscicide of mountain folk culture. Geogr Rev 64:235–252
Wolfe LM, Elzinga JA, Biere A (2004) Increased susceptibility to enemies following introduction in the invasive plant Silene latifolia. Ecol Lett 7:813–820
Woodman RL, Fernandes GW (1991) Differential mechanical defense: herbivory, evapotranspiration, and leaf hairs. Oikos 60:11–19
Acknowledgments
We thank Alecu Diaconu, Brad Harmon, Hariet Hinz, John Parker, René Sforza, and Jennifer Williams for collecting seeds; Erik Hardy for measuring leaf toughness; and Christa Fettig and two anonymous reviewers for providing helpful feedback on an earlier version of the manuscript. We also thank the Colorado Native Plant Society for partial funding of the chemical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alba, C., Deane Bowers, M., Blumenthal, D. et al. Evolution of growth but not structural or chemical defense in Verbascum thapsus (common mullein) following introduction to North America. Biol Invasions 13, 2379–2389 (2011). https://doi.org/10.1007/s10530-011-0050-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0050-7