Abstract
Plants are connected to habitats by functional traits which are filtered by environmental gradients. Since tree species composition in the forest canopy can influence ecosystem processes by changing resource availability, litter accumulation, and soil nutrient content, we hypothesised that non-native invasive trees can establish new environmental filters on the understorey communities. In the hardwood floodplain forests in Northern Italy, the invasive trees Robinia pseudoacacia L. and Prunus serotina Ehrh. are the dominant canopy species. We used trait data assembled from databases and iterative RLQ analysis to identify a parsimonious set of functional traits responding to environmental variables (soil, light availability, disturbance, and stand structure) and the dominant native and invasive canopy species. Then, RLQ and fourth-corner analysis was conducted to investigate the joint structure between macro-environmental variables and species traits and functional groups were identified. The trait composition of the herb-layer was significantly related to the main environmental gradients and the presence of the invaders in the canopy showed significant relationships with several traits. In particular, the presence of P. serotina may mitigate or even erase the effect of disturbances, maintaining a stable forest microclimate and thus favouring ‘true’ forest species, while R. pseudoacacia may slow down forest succession and regeneration by establishing new stable associations with a graminoid-dominated understorey. The impact of the two invasive trees on herb layer composition appears to differ, indicating that different management and control strategies may be needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous studies have shown individual invader impacts on native community-level responses, such as species richness, diversity, and composition (Hejda et al. 2009; Pyšek et al. 2012). In contrast, very few studies have investigated the functional response of plant communities to invasion (e.g., Mason et al. 2009 for graminoid and woody species; Chabrerie et al. 2010 for Prunus serotina Ehrh.; Brym et al. 2011 for Elaeagnus umbellata Thunb.; Byun et al. 2013 for Phragmites australis (Cav.) Trin. ex Steud.). Nonetheless, the degree to which non-native invaders influence native plant species composition remains poorly explored and it remains unclear for many invasive species if they act as ‘driver’ (the invader causes changes to the native community) or ‘passenger’ (the invader and the native community respond independently to environmental change) of community change (MacDougall and Turkington 2005; Chabrerie et al. 2008; Halarewicz and Zołnierz 2014). However, the impact of an invader is expected to correlate with its own population density, since any biomass (or space or energy) controlled by the invader constitutes resources no longer available to other species (Parker et al. 1999). Hence, when an invader becomes abundant in a recipient community, long-lasting shifts in species composition and ecosystem functioning are likely to occur (Chabrerie et al. 2008).
One difficulty in assessing invasion impacts is that changes can be slow and cumulative (Crooks 2005; Strayer et al. 2006), and might take many years before any effects become apparent, especially in ecosystems with low turnover rates like forests (Chabrerie et al. 2010). In contrast, functional attributes of species diversity and composition relate directly to life-history traits that are sorted by the environment. Likewise, functional traits may respond more quickly to invasion-induced environmental changes, independently from changes in species composition (Lavorel et al. 1997; Chapin et al. 2000; Diaz and Cabido 2001; Chabrerie et al. 2010). Consequently, analysing the relationship between invasion and the life-history traits of species within the recipient herb-layer community can detect early vegetation responses and/or changes of ecosystem functions which are not exposed by variations in species diversity and composition (McGill et al. 2006; Chabrerie et al. 2010).
Usually, functional traits are defined as any measurable features at the individual level that directly or indirectly affect overall fitness or performance (e.g., growth, fecundity, survival) of organisms (Violle et al. 2007; Bello et al. 2010). They are closely linked to and filtered by the given environment (Violle et al. 2007). Thus, identifying traits and their distribution is essential for understanding long-term ecosystem dynamics and how environmental change (like species invasion) drives community composition (Lacourse 2009). Recently, the concept of functional traits and trait-based approaches has been widely used in ecological research to learn more about the processes and patterns of ecosystem development and ecosystem services in response to environmental changes (Petchey and Gaston 2006; Bernhardt-Römermann et al. 2008; Naeem et al. 2009; Lavorel et al. 2013). The elegance of these approaches is that complex ecosystem dynamics can be described without following the dynamics of each species individually (Savage et al. 2007), simplifying the ecological interpretation of the complexity of a plant community (Diaz and Cabido 2001). However, the application of trait-based approaches to understanding the impact of invasive plant species is still largely limited to linking the traits of the invader with their invasiveness and to comparing the functional similarity of traits of the invader to the invaded community (e.g., Levine et al. 2003; Ordonez et al. 2010; Lamarque et al. 2011; Drenovsky et al. 2012; Jauni and Hyvönen 2012; Pyšek et al. 2012; Thuiller et al. 2012).
In this study, we analysed the relationships between different abiotic and biotic environmental variables (including the abundance of invasive tree species), understorey plant communities, and species life-history traits of a temperate European hardwood floodplain forest. In general, floodplain forests are complex and dynamic ecosystems and during succession the distribution of forest species and communities is principally filtered by strong gradients in light availability and disturbance due to the intensity and frequency of flooding (c.f. flood-shade tolerance trade-off hypothesis; Battaglia and Sharitz 2006; Mann et al. 2008). Disturbance is strongly related to forest succession as it can set plant communities back to an earlier stage of succession or may slow down the process. Additionally, variation in the temporal and spatial scales of disturbances leads to a ‘shifting mosaic’ of successional stages across the landscape (Schnitzler 1995). A number of studies have revealed that traits that shift consistently with succession are also traits that respond strongly to light availability and disturbance (Bazzaz 1979; Prach et al. 1997; Schleicher et al. 2011; Douma et al. 2012). Jointly with the abiotic environmental gradients, the composition of the dominant tree species in the canopy of these forests influences ecosystem processes by changing light and water availability, litter accumulation, and soil nutrients content, or even by phytotoxic or allelopathic effects (Gilliam 2007; Barbier et al. 2008; Blank and Carmel 2012; Catorci et al. 2013), and thus selects for specific life-history traits of the herb-layer species.
Since in the study area, the Northern Italian biosphere reserve ‘Valle del Ticino’ (hereafter called Ticino Park), the two North American tree species black cherry (Prunus serotina Ehrh.) and black locust (Robinia pseudoacacia L.) are today the most frequent non-native tree species and often become the dominant species in the canopy (and/or shrub layer) of invaded forest stands (Motta et al. 2009; Chabrerie et al. 2010; Annighöfer et al. 2012; Terwei et al. 2013), the main aim was to test if these invasive trees are able to establish new environmental filters to understorey plant communities. More specifically, the following questions were addressed: (1) What are the links between the macro-environmental variables and the traits of the herb-layer species? (2) Which combinations of traits and functional groups are filtered by the environment? (3) Are there any specific traits and functional groups associated with the increase of the two non-native tree species, P. serotina and R. pseudoacacia, in the tree and shrub layer? To answer these questions we applied two increasingly used methods in plant studies (e.g., Jauni and Hyvönen 2012; Minden et al. 2012; Wesuls et al. 2012; Fischer et al. 2013; Sterk et al. 2013; Dray et al. 2014) that represent the most integrated methods to analyse trait-environment relationships (Kleyer et al. 2012; Dray et al. 2014): (1) RLQ analysis, a multivariate method for three table ordination developed by Dolédec et al. (1996); and (2) fourth-corner analysis, a method to measure and test the direct correlation between single traits and single macro-environmental variables (Legendre et al. 1997). Few studies have used the RLQ method for analysing the response of native plant communities to invasive plants (see Thuiller et al. 2006; Chabrerie et al. 2010, or Jauni and Hyvönen 2012).
Materials and methods
Study site
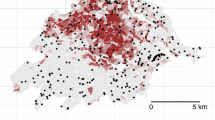
The study area was located in the North Italian UNESCO MAB Biosphere Reserve ‘Valle del Ticino’ (Ticino Park). As the largest continuous remnant of woodlands in the Po Plain and an important ecological corridor between the Alps and the Apennines, the biosphere reserve was established in 2002 in order to conserve the remaining mosaic of alluvial forests (UNESCO 2005). Situated on the border of the regions Lombardy and Piedmont, the Ticino Park is approximately 100 km long and between 10 and 20 km wide, ranging from the Lago Maggiore in the north-west (45°06′21″N, 08°34′53″E) with an elevation of about 195 m a.s.l. to the confluence of the Ticino River with the Po River in the south-east (45°46′38″N, 09°16′19″E) with an elevation of about 57 m a.s.l. (Fig. 1). The climate is sub-Mediterranean with average temperatures in January and July of 1.7 and 22.2 °C, respectively, and an average annual precipitation of 1212 mm (meteorological station Milan Malpensa Airport; Fig. 1; CNMCA 2009). The park is characterized by long drought periods at irregular intervals, especially during summer. The forest soil is a fluvic soil (FAO 1998) showing transitions from pale sandy soil to darker sand with higher amounts of loam and gravel.
Before becoming a biosphere reserve, most of the Ticino forests were managed for wood production and used as a hunting reserve (Motta et al. 2009). In general, the current forests are not older than approximately 65 years because almost all forests in the Ticino Valley were completely cut down during the harsh winters following the World War II (pers. comm. Caronni 2011). As the objective of the biosphere reserve is to develop near-natural forests in the future, consisting of native species, all silvicultural measures were stopped with the establishment of the reserve in 2002. The typical native and most abundant phytosociological forest association of the Ticino Valley is the Polygonato multiflori-Quercetum roboris (Sartori 1980), a hardwood floodplain forest on well-drained and fertile soils, mainly consisting of pedunculate oak (Quercus robur L.), European hornbeam (Carpinus betulus L.), and field elm (Ulmus minor Mill.) (Sartori 1980; UNESCO 2005) mixed with different sub-dominant tree species. In wetter parts, black poplar (Populus nigra) and hybrid poplar (P. x canadensis) are common. This association is subject to periodic flooding from the Ticino River and its tributaries; in the last 15 years, floods occurred in 2000, 2002, 2005, 2009, and 2014 (Motta 2009; pers. comm. Caronni).
Invasive tree species in the study area
Starting at the end of the nineteenth and beginning of the twentieth century, exotic, invasive species, such as black cherry (Prunus serotina Ehrh.), black locust (Robinia pseudacacia L.), tree of heaven (Ailanthus altissima (Mill.) Swingle), and red oak (Quercus rubra L.), were introduced to the area of the Ticino Park (Motta et al. 2009; Caronni 2010). Since then, the abundance of these species has greatly increased. Today, P. serotina and R. pseudoacacia, both native to North-East America (Auclair and Cottam 1971; Boring and Swank 1984), are by far the most frequently found invasive tree species, occupying 13 and 30 %, respectively, of the forested area of the park (in contrast to 23 % covered by native Polygonato multiflori-Quercetum roboris hardwood forests) (Boschetti et al. 2005). While R. pseudoacacia grows scattered all over the park area and is accepted by the park authorities as part of the forests that cannot be eradicated, P. serotina is almost absent in the southern part of the park but is expanding its range from north to south and increasing its area of distribution inside the biosphere reserve. Due to this, the goal of the park administration is to stop R. pseudoacacia from spreading further south and maintain the species at the lowest feasible level that technology, finances, and conditions will allow (pers. comm. Caronni 2011; Annighöfer et al. 2012, 2015). With regard to forest structure and composition, both species are supposed to constitute a major threat to biodiversity conservation and stability of the native ecosystem (Motta et al. 2009; Caronni 2010).
Sampling design
Vegetation sampling
Vegetation relevés were conducted within different contiguous stands belonging to the Polygonato multiflori-Quercetum roboris association (Sartori 1980), either during summer 2010 or 2011. The stands belonging to this association were identified using the forest association maps of Boschetti et al. (2005) and then 63 stands were randomly selected. The minimum size for considering a stand for this study was 30,000 m2. To avoid any edge-effects, a plot with a size of 20 m × 20 m was installed temporarily in the centre of each stand. Attention was paid to ensure that the plots represented characteristic and homogeneous stand conditions and species composition, avoiding alterations such as clearings, water edges, paths, and forest roads in the vicinity.
In each plot, all plant species in the herb-layer (height <0.7 m), shrub layer (0.7–6 m), and tree layer (>6 m) were recorded and the total cover of each species for each layer was estimated according to the method of Braun-Blanquet (1964), using the modified abundance scale from Reichelt and Wilmanns (1973) with 9 cover classes. For further analysis, the abundance values were transformed into mean %-cover of each class following Bemmerlein-Lux et al. (1994). To avoid different interpretations of the degree of vegetation cover, all observations were conducted by the same person. As there are no reliable morphological characteristics to distinguish definitively between P. nigra and P. x canadensis hybrids in the field (c.f. Aas 2006), both were pooled and regarded as native P. nigra. The nomenclature of plants follows Fischer et al. (2008), except for black locust for which we used the more common name Robinia pseudoacacia L. (Jarvis and Cafferty 2005).
Sampling and analysis of macro-environmental variables
For the herb-layer vegetation, we calculated mean indicator values for humidity and light for each plot, according to Ellenberg et al. (2001), using values adjusted for Italy by Pignatti et al. (2005). The herb-layer species were also classified according to Honnay et al. (1998) if they were ‘true’ forest species in Europe, being indicators for the ecological value of ‘old’ forests. Typical microclimates for these ‘true’ forest environments are low light availability at the forest floor, high hygrometry, and low thermic variations (c.f. also Hermy et al. 1999). The cover of the herb-layer was used to indicate possible competition between the seedlings of the tree species and other herb-layer species. In addition, the total cover of the tree layer and the cover of the native species C. betulus, P. nigra, Q. robur, and U. minor in the tree layer and of the non-native species P. serotina and R. pseudoacacia in the tree and shrub layer were used to describe the structure and composition of the different layers. For the analysis of the upper soil horizon, two soil samples of the topsoil (0–5 cm) were taken from each plot, each one in the middle of two opposite boundary lines of the plot, using a soil sample ring with a defined volume of 100 cm3. The two organic layer samples were mixed and stored as composite samples. We measured the thickness of the organic (O) layer, including litter (Ol), fragmentation (Of), and humus (Oh) layer, and the thickness of the Ah layer (mineral layer with accumulation of organic matter) in the field. In the laboratory, the samples were analysed for pH (soil solution in H2O; VDLUFA methods according to McLean 1982), plant available phosphorous (CAL extraction according to Schüller 1969), and total nitrogen and total organic carbon (quantitative elemental analysis DIN ISO 10694 according to DIN 1996) to calculate the carbon to nitrogen (C:N) ratio (Table 1). C:N ratio was used as an indicator for available nitrogen. Given that soil samples were free of CaCO3 (no reaction to 10 % HCl), organic carbon content was considered equal to total carbon content. Pearson’s correlations were performed between all macro-environmental variables. As the soil variables total organic carbon and total nitrogen were highly correlated with each other and also each of them with the variable plant available soil phosphorous (Pearson correlation coefficient >0.9), the two latter variables were excluded from further statistical analysis and only the total organic carbon was used. As a proxy for anthropogenic, management-related disturbance intensity of the forests in the past, the number of stumps (with a diameter >10 cm) per plot was counted. The quantification of disturbances by flooding would require the documentation of flooding intensity and nature (erosion or deposition processes) in the floodplain patches. As these parameters are rather difficult to document, because the stands are frequently not accessible during floods, the distance to the main river channel was used as a proxy for flooding intensity whereas the total organic carbon content of the topsoil was used as a proxy of the frequency of sediment reworking. Bornette et al. (2008) stated that carbon content has been previously demonstrated to be linked to the disturbance level of floodplain habitats (Rostan et al. 1987; Schwarz et al. 1996). Erosional processes export organic matter that accumulates between flood events whereas deposition of mineral particles leads to a decreased proportion of organic matter in the substrate (Bornette et al. 2008).
Plant trait data
Following the mechanistic and pragmatic approach of Weiher et al. (1999), we selected functional traits that were associated with the three fundamental challenges that plants face, i.e. dispersal, establishment, and persistence. A set of 16 biological traits was retained (Table 2) . The data on the traits was assembled mainly from the following four plant-trait databases: LEDA Traitbase (Kleyer et al. 2008), BIOLFLOR (Klotz et al. 2002), Electronic comparative plant ecology (Hodgson et al. 1995), and Kew Royal Botanic Gardens Seed Information Database (Royal Botanic Gardens Kew 2008). Gaps were filled by information supplemented from floras and by averaging data from congeners. If several different entries for a species were found, the numeric trait values were aggregated by taking the means of all values present in the databases. As single occurrences of species do not provide information on species distributions along environmental gradients, species that appeared in only one relevé were excluded from the analyses.
As proposed for categorical traits by Bernhardt-Römermann et al. (2008), we distinguished two cases: (1) traits where several entries of different attributes are likely (e.g., one species can be pollinated by several pollinating types), and (2) traits with only one possible attribute per species (Table 2). The second were included in the analyses without further transformation, while the first traits (woodiness, life form, leaf anatomy, pollination type, and strategy type) were dummy-transformed. For these transformations we used a simple system of linear interpolation in as many dimensions as trait stages existed. For example, the trait pollination type was transformed as follows: entomophily = [1, 0, 0]; anemophily = [0, 1, 0]; self-pollination = [0, 0, 1]; entomophily/anemophily = (entomophily + anemophily)/2 = [0.5, 0.5, 0], etc. The values in this system were weighted by the number of database entries per category. By definition, the sum per species of all values is 1 (c.f. Bernhardt-Römermann et al. 2008).
Prior to the main analysis, the trait variable seed weight (sw) was log10-transformed. To test for correlation between all traits, we used the Spearman’s rank correlation coefficient, omitting those combinations with strong correlations (ρ > 0.7) from further analyses. Due to this, the trait attributes life form phanerophyte (lif.phan) and presence of woodiness (w.wood) (highly correlated to canopy height (ch)) and annual and biennial life-history (lih.anbi) [highly correlated to perennial life-history (lih.per)] were excluded from further analysis.
Statistical analysis
Since the aim of our study was to relate species traits to environmental conditions by considering the abundances of species in the plots, we needed to analyse simultaneously three data tables, i.e. the environmental table (R), the species composition table (L), and the species-traits table (Q). An adequate and modern method for this was to conduct and to combine two complementary types of three-table analysis: RLQ analysis (Dolédec et al. 1996) to obtain a graphic display and fourth corner analysis (Dray and Legendre 2008) for statistical power (Dray et al. 2014).
First, to determine the set of traits from the total set (Table 2) which best described the variation in species composition along the given environmental gradients and to be included in the RLQ analysis (table Q), we applied an iterative RLQ analysis as proposed by Bernhardt-Römermann et al. (2008). For this, we performed RLQ analyses for each possible 2-trait combination using the sum of the correlation L metric of the first two RLQ axes as diagnostic values to evaluate the goodness-of-fit of the corresponding RLQ. Subsequently, all further traits were added step by step to produce 3-trait, 4-trait, 5-trait, etc. combinations and were evaluated by the goodness-of-fit of each of them. To minimize the need of computing power, in each step (n) only the 15 combinations performing best were chosen for the next set of combinations (n + 1). This procedure was repeated until the maximal trait number was reached. Finally, the correlation L values were plotted against the number of traits in the combination and the most parsimonious solution was defined as that point where the slope of the straight line between two trait numbers was smaller than 0.005 (see Fig. S1).
Second, with this ‘best trait set’, RLQ analysis was conducted to provide simultaneous ordinations, and to analyse the joint structure of the three datasets. It was developed to study environmental filtering in ecological communities by elucidating combinations of traits that have the highest covariance with combinations of macro-environmental variables, irrespective of whether data were quantitative or qualitative (Dolédec et al. 1996; Dray et al. 2003).
The RLQ analysis is an extension of co-inertia analysis that performs a double inertia analysis of two arrays, i.e. table R (sites × environmental variables) and table Q (species × traits) with a link expressed by a contingency table, i.e. table L (sites × species). Before the actual analysis, three separate analyses were accomplished. First, a correspondence analysis (CA) was applied on the table L. The CA gave the optimal correlations between the study sites and the species scores. Next, an ordination of table R and L was done by principal component analysis (PCA). Column weights of table L were used for ordination of table Q, by performing a Hill and Smith analysis (Hill and Smith 1976). Subsequently, the RLQ analysis combines the three separate ordinations to maximize the covariance between the study site scores constrained by the macro-environmental variables of table R and the species scores constrained by the traits of table Q. As a result, the best joint combination of the site ordination by their environmental characteristics, the ordination of species by their attributes (traits), and the simultaneous ordination of species and sites was calculated. A Monte-Carlo permutation test (n = 999) was used to test the significance of the link between the environmental table (R) and the trait table (Q). For more details on the method, including a graphical illustration, see Dolédec et al. (1996).
Third, to quantify the explicit trait-environment relationship and to test for its significance, we used the multivariate version of the fourth-corner statistic (Dray and Legendre 2008). This method measures the link between species traits and macro-environmental variables by a Pearson correlation coefficient (for one quantitative variable), and by a Pseudo-F (for six qualitative variables). A permutation model (with 999 permutations) was applied using a combination of two different permutation models (for details see Dray and Legendre 2008). The null hypothesis H0 was that species traits are unrelated to the environmental characteristics of the sites. The first model tested the null hypothesis H1 that the species assemblages and the environmental characteristics of the locations where they were found are unrelated (permutation of site vectors in the table L, model 2 in Legendre et al. 1997). In the second model, the permutation procedure tested the null hypothesis H2 that the distribution of the species and the traits they possess are unrelated (permutation of the species vectors of the table L, model 4 in Legendre et al. 1997). As proposed by Dray and Legendre (2008), the two models were combined in order to attain the correct level of Type I error. If both permutation tests were significant, H0 can be rejected and thus the environmental characteristics, species distributions, and traits were considered to be effectively linked. In this sense, if α1 is the significant level at which H1 is rejected, and α2 is the significant level at which H2 is rejected, then α0 = α1 × α2 is the significant level at which H0 is rejected, thereby α1 = α2 = √α0. Considering a trait to be significantly correlated if both p-values associated to models 2 and 4 were lower than α = 0.05, the significance level will be α1 = α2 = √0.05 = 0.22. In our study, a Bonferroni correction for the eighteen macro-environmental variables was used to finally obtain the significance level (α0 = 0.05/20 = 0.0025). As a consequence, the adjusted significance levels to reject H1 and H2 (in permutation models 2 and 4, respectively) was considered to be α1 = α2 = √0.0025 = 0.05 (c.f. Dray and Legendre 2008; Gallardo et al. 2009). In combination, RLQ analysis and the fourth-corner statistic represent straightforward methods for exploring trait-environment relationships, and complement each other by mixing graphical exploratory analysis with formal statistical tests to unravel processes that remain hidden when analysing different datasets separately (Wesuls et al. 2012). The fourth-corner method was adapted by Dray and Legendre (2008) to deal with species abundances instead of presence-absence data.
Finally, subsequent to RLQ analysis, functional groups were generated by conducting hierarchical clustering of species scores and were projected on the first two RLQ axes (following the script of Kleyer et al. 2012) using Ward’s method (Everitt et al. 2011). The optimal number of groups was determined by means of Caliński and Harabasz’s index (Caliński and Harabasz 1974). These clusters showed the distribution of functional groups in the trait-environment space (c.f. Kleyer et al. 2012; Minden et al. 2012). All statistics were conducted using R 3.0.1 (R Core Team 2013) and the package ade4 (Dray and Dufour 2007).
Results
RLQ analysis
The iterative RLQ analysis of the macro-environmental variables (Fig. S1) identified seven traits (that split up into 21 trait attributes; c.f. Table 2) as having a high, but parsimonious, explanatory power for the distribution of species across sites: seed weight (sw), seed-bank type (sb), dispersal type (dt), start of flowering period (fls), leaf phenology (lp), lateral spread (las), and absence of woodiness restricted to graminoids (w.grass). These traits might be roughly grouped into traits that describe the competitive ability and persistence of the herb-layer species (w.grass, lp, las), the dispersal ability and regeneration (dt, sw, sb, las), and processes of crucial life-history stages (fls, sb). With this optimised trait-set, the RLQ ordination was calculated.
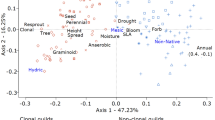
The first two axes of the RLQ ordination contributed 79 % of the total inertia (57 and 22 % respectively). The Monte-Carlo test indicated that the co-structure between R and Q was significant (p < 0.001, with 999 random permutations). The first two RLQ axes accounted for most of the variance of the corresponding axes in the separate analyses of environmental descriptors (77 % for PCA of the R-table) and species traits (80 % for Hill and Smith analysis of the Q-table). This demonstrates the strength of the link between environmental filters and species traits, indicating how macro-environmental variables structure vegetation and the trait composition of occurring species. The results were best summarized by projecting macro-environmental variables (Fig. 2), traits (Fig. 2), plots (Fig. 3), and species (ESM Appendix, Fig. S2) in the RLQ diagram. Correlations between environmental variables and the first two RLQ axes are shown in Table 1.
Ordination diagram of the first two RLQ axes displaying the plots in the field; simple circles indicate plots with R. pseudoacacia in the tree layer and circles with a cross indicate plots with P. serotina in the tree layer; the size of the circles corresponds to the cover value of the two species; a black dot indicates a plot where one or both of the two species is/are absent
The first RLQ axis mainly corresponded to a positive ‘acidity-nutrient-humidity’ gradient of the top soil in combination with a ‘light’ gradient spanned by the variables pH (S_pH), nitrogen availability (expressed by a decreasing C:N ratio, S_C_N), humidity (expressed by Ellenberg soil humidity value, H_mean, and cover of Populus sp. in the tree layer, Popu), and light availability at the ground floor (expressed by the Ellenberg light value, L_mean) (Fig. 2). To a smaller degree, it also corresponded to a negative gradient of the distance to the main river channel (Dist), the number of stumps per plot (Stumps), and total cover tree layer (Cov_T) and cover of P. serotina in the shrub layer (Prun_S). The second axis correlated best with an increase of herb-layer cover (Cov_H) and decrease of shrub layer cover (Cov_S), as well as decrease of Q. robur cover in the tree layer (Quer), discriminating almost between tree and shrub, herb, and graminoid species in the understorey. The other variables showed only small correlations with the first two axes (Table 1).
Associated trait attributes of the ‘acidity-nutrient-humidity-light’ gradient of the first axis were short-term persistent seed-bank type (sb.shor), dispersal type hydrochory (dt.hyd), late start of flowering period (fls.m6, fls.m7_12), hivernal leaf phenology (lp.hiv), no lateral spread (las.s1, las.s3) (all positively), and seed weight (sw), dispersal type anemochory (dt.ane), evergreen leaf phenology (lp.eve), and small lateral spread (las.s2) (all negatively). The second axis was positively related to an unspecific dispersal type (dt.unsp), evergreen and hivernal leaf phenology (lp.eve, lp.hiv), small and medium lateral spread (las.s2, las.s3, las.s4), and absence of woodiness in combination with graminoids (w.grass) (Fig. 2). The second axis was negatively associated with seed weight (sw) and extensive lateral spread (las.s5).
Whereas the first and second RLQ axes were mainly stretched by the abiotic macro-environmental variables, the presence of the two invasive species P. serotina and R. pseudoacacia in the tree and shrub layer only showed low correlations with the axes in the two-dimensional projection. Both axes were not able to discriminate between invaded and uninvaded plots (Fig. 3).
Fourth-corner analysis
The results of the permutation tests of the fourth-corner analysis revealed numerous significant correlations between macro-environmental variables and plant traits and were consistent with the RLQ ordination (Table 3). All traits attributes showed a significant relationship (p < 0.05) with at least two variables. Moreover, the traits seed weight (sw), short-term persistent seed-bank (sb.shor), vernal and evergreen leaf phenology (lp.ver, lp.eve), late start of flowering period (fls.m7_12), and small lateral spread (las.s2) possessed the highest number of significant relationships and a strong relationship with at least two of the environmental variables (except for sb.shor). In addition, eleven trait attributes had significant correlations with at least one of the two non-native species in the shrub or tree layer. Strongest relationships (p < 0.001) were found between seed weight (sw) and cover of herb-layer (Cov_H) and between evergreen leaf phenology (lp.eve) and Ellenberg light value (L_mean). It should be pointed out that the macro-environmental variables that show most significant relationships with the trait attributes were cover of herb-layer (Cov_H), soil pH value (S_pH), Ellenberg light value (L_mean), Ellenberg humidity value (H_mean), and cover of shrub layer (Cov_S), but also cover of R. pseudoacacia (Robi_T) in the tree layer. A detailed summary of the results is shown in Table 3.
Species functional clustering
The cluster analysis of the herb-layer species yielded five stable clusters that were clearly separated in the trait-environment space (Fig. 4; clusters A to E), showing how multiple trait expressions combine into functional groups (FG). This partition can be interpreted in terms of traits as shown in Fig. 5. For an overview of the species that were pooled in the five cluster groups, see Table S3 (ESM appendix). The first FG, FG-A, contained mainly non-woody but also some woody species, with vernal and partly also evergreen leaves, a high proportion of zoochorous species, start of flowering in early summer, and mainly transient seed-banks. The second cluster, FG-B, was characterised by typical species of softwood floodplain forests (composed of Salix sp. and Populus sp.), many of them short living annual and biennial species with small seed weights, vernal leaf phenology, and flowering start in late summer or autumn. FG-C was the biggest and the most homogeneous cluster group. It was mostly composed of woody shrub and tree species, with a high proportion of ‘true’ forest species. In addition, it contained only a handful of small herbaceous plants but all of these species are as well typical for ‘true’ forest environments. It was dominated by heavy and animal dispersed seeds, a transient seed-bank, an only vernal leaf phenology, and the absence of graminoids. Almost all species showed a very high lateral spread and an early start of flowering before June. The smallest cluster, FG-D, contained mostly non-woody species, mostly herbs that are also related typically to the softwoods and wetter parts of the floodplain. Thus, hydrochorous seed dispersal dominated in this group. In addition, it showed a high proportion of short living therophytes which produce very light seeds, build often persistent seed-banks, and start flowering late in the year. Finally, the species of FG-E were mainly grass, sedge, and rush species or ferns. These species showed small seed weights, mainly a persistent seed-bank and an evergreen leaf phenology, only small and medium lateral spread, and a rather late flowering start.
Ordination diagram of the first two RLQ axes displaying the five species cluster groups A to E; point positions correspond to the species positions in Fig. S2 (ESM Appendix); for abbreviations see Table 2
Trait attributes of each cluster for best trait set; the letters A to E refer to the functional groups in Fig. 4; boxplots show the quantitative variable with its ranges in each cluster; vertical bar graphs show the qualitative variables with scaling in % of the different categories in each cluster (full bar corresponds to 100 %); dashed lines indicate absence of individuals for a particular category; for abbreviations see Table 2
In general, including all FG, the ‘true’ forest species were clearly concentrated towards the left side of the ordination graph (Figs. 4, 5). Concerning the environmental gradients, FG-B and especially FG-D showed a clear relationship with the wetter sites with more available soil nitrogen, increasing pH value, and increasing light availability. In contrast, FG-A, FG-C, and FG-E were correlated with an increasing tree layer and P. serotina cover, and management-related disturbance. Also drier and poorer sites in a bigger distance to the main river channel, as well as decreasing light availability were associated with these groups.
Discussion
Species traits associated with the environmental gradients
RLQ and fourth-corner analyses—conducted with the traits of the best trait set—reflected well, how macro-environmental variables and dominant tree cover type of both native and non-native species had a joint effect influencing the functional trait composition of the understorey vegetation. We found that decreasing light, soil humidity, and nutrient (N) availability, along with increasing soil acidity were the most important macro-environmental factors, explaining the occurrence of the herb-layer vegetation. Accordingly, the distribution of the understorey species along the first RLQ axis followed principally these environmental gradients that are characteristic for floodplain forests (Battaglia and Sharitz 2006), driving forest regeneration, and succession from ‘new’ or more disturbed and open forests to ‘old’ or more closed and stable forests. As described by Sartori and Bracco (1996) for the alluvial hardwood forests of the Ticino Valley, these gradients correspond to a combination of different zonation and succession stages from (1) light mixed forests of Q. robur and U. minor, showing a high presence or dominance of Populus sp. and Alnus glutinosa with increased natural disturbance due to more frequent and more intense flooding, to (2) mixed forests of Q. robur and U. minor (with the latter having a more dominant role), (3) mixed forests of dominant Q. robur, and (4) mixed forests of Q. robur and C. betulus (with the latter having a more dominant role) (c.f. also Sartori 1980; Siebel and Bouwma 1998), forming more and more a stable or ‘true’ forest environment along the gradient (Decocq and Hermy 2003). Along the second RLQ axis, variables connected to aspects of the forest structure, like absence or presence of herb or shrub layer, become more important, arranging the understorey species according to different ecological guilds. This axis almost completely differentiated between shrub and tree species on one side (negative part) and graminoids on the other side (positive part). Thus, the alignment along this axis could also be interpreted as different intermediate succession stages of mixed Q. robur forests ranging from stands with dense graminoid communities in the herb-layer with an almost absent shrub layer and a scattered canopy with more abundant R. pseudoacacia in the upper part, to a shrub layer-dominated situation with a high amount of woody species and decreasing herb-layer but increasing canopy layer cover with more abundant U. minor in the lower part of the ordination graph.
With regard to the selected traits seed weight, seed-bank type, dispersal type, start of flowering period, lateral spread, leaf phenology, and absence of woodiness (restricted to graminoids), we assume that the differentiation of their abundance between the functional groups was mainly driven by the change in environmental conditions (i.e. the main environmental gradients). More in detail, the characteristics and distribution of these traits as observed in our study can be interpreted as expressions of different strategies linked to different succession stages.
For example, in the Ticino Park, flowering but also leaf phenology was strongly related to light availability due to canopy and shrub layer cover. Usually, the mean beginning of the flowering period is connected to species that exploit the early spring peak of photosynthetically active radiation before leaves of trees emerge, thereby reducing interspecific competition, and probably, avoiding summer drought stress by growing in the off season (Catorci et al. 2012). Furthermore, as long-lived leaves may store lipids and proteins that are translocated to the new leaves at the beginning of the growing season, also persistent evergreen green leaves were positively related to spring and early summer flowering strategies (Catorci et al. 2012, 2013). Thus, in different studies it was found that the proportion of species with later and longer flowering periods are higher in young forests than in the old ones (e.g., Graae and Sunde 2000; Catorci et al. 2012), supporting our results.
Second, the role of vegetative lateral spread was observed to increase with succession from lighter to denser forests. Consistent with Prach et al. (1997), an extensive lateral spread above and below ground is a characteristic of competitive plants whereas limited lateral spread is a characteristic of ruderals (Grime 1977). Thus, it can be suggested that sexual reproduction is reduced under closed canopies of older succession stages (Prach and Pyšek 1994; Eriksson 1997) while clonal growth is generally less abundant in disturbed habitats and more abundant in shaded ones (Catorci et al. 2012). High lateral spread as a ‘guerrilla tactic’ could also be advantageous in densely rooted grass communities (Saar et al. 2012). However, the last point was not confirmed in our study.
Third, the regeneration strategy of species changed with increasing competition for light. In accordance to our results, a number of studies have shown an increase in seed mass during succession (Bazzaz 1979) while the role of a persistent seed-bank decreases (Prach et al. 1997). Dispersal ability and regeneration of forest plants is strongly connected to seed weight and seed-bank type. Seed size and weight are generally linked to seed-bank type, so that species with larger seeds do not tend to form persistent seed-banks (Thompson 1987; Verheyen et al. 2003). In addition, the large seeds of many herbs, shrubs, and trees of woodlands are suspected to be of crucial importance in seedling emergence through tree litter (Thompson 1987). However, the results of Endels et al. (2007) showed, especially for alluvial forests, that species with unassisted dispersal and large seeds are severely limited in their potential to colonize recent forests.
In general, stable forest ecosystems are characterized by species with slow growth, an early start of flowering, high vegetative spread, large seeds, and a transient seed-bank (Graae and Sunde 2000). Moreover, high lateral spread, and long seed longevity, as well as high seed mass are considered important for rapid recovery. Recovery and resistance of the vegetation are properties of resilience that ensure the capacity of the ecosystem to maintain functioning (Sterk et al. 2013). Hence, as stated by Hermy et al. (1999), the stress-tolerant strategy type is significantly more common among ‘true’ forest species than expected when compared with other forest plant species. ‘True’ forest species are generally slow colonizers and are indicative for high forest quality or old forests (Hermy et al. 1999; Brown and Boutin 2009). In contrast, in new forests or early succession stages, high light availability determines the establishment of short life-cycle species (i.e. ruderal species) which are adapted to efficient resource acquisition and are more abundant than in old forests (Graae and Sunde 2000; Catorci et al. 2012). Such species may be considered as being ‘transient’ sensu Grime (1977); they spread rapidly in the forest undergrowth by means of the local seed-bank or the seed-rain from the surrounding landscape (Catorci et al. 2012) and have long lasting effects on species composition (Gilliam 2007; Brown and Boutin 2009).
In summary, the revealed trait shifts in the Ticino Park floodplain forests, related to different succession stages, were consistent with those of other studies conducted in temperate forests (e.g., Bazzaz 1996; Garnier et al. 2004; Aubin et al. 2009; Catorci et al. 2012, 2013). Catorci et al. (2012) stated that changes in plant traits during forest succession reflect the development from fast growing species which acquire external resources rapidly and hence dominate the early stages, towards slower growing species which tend to conserve internal resources more efficiently. As indicated by Grime et al. (1997), this pattern appears to reflect a trade-off between attributes conferring an ability for high rates of resource acquisition in productive habitats, such as in high light conditions of young stands, and those responsible for retention of resource capital in unproductive conditions, like in the shaded understorey of mature stands (Catorci et al. 2012).
Relationships between invasive P. serotina and R. pseudoacacia and understorey plants
In general, P. serotina is thought to not be tolerant to flooding (Bourtsoukidis et al. 2014) and is often missing in intact floodplains with their natural fluvial dynamics (Roloff et al. 1994). In the floodplain of the Ticino Park, P. serotina was absent in the wetter parts of the forests and associated principally with drier soils. This was consistent with the findings of Verheyen et al. (2007), referring to other studies conducted in the Netherlands, Belgium, Germany, and Poland that confirmed the higher abundance of P. serotina on drier soils. In contrast to other areas in Central Europe where P. serotina was intentionally introduced to dry and poor soils, its dispersal into the Ticino Park occurred by natural means. The less frequent presence on wetter sites could be explained by the presence of soil-borne diseases (Verheyen et al. 2007). A possible cause for such diseases could be Pythium spp. which is a so called water mould, known to be responsible for low densities of seedlings near conspecifics of P. serotina inside its native range (e.g., Packer and Clay 2003). Pythium spp. tend to have the highest pathogenicity on moist soils (Martin and Loper 1999). In addition, P. serotina was also restricted to more acidic soils with lower nitrogen content. These findings corroborate those of Chabrerie et al. (2007, 2008) for P. serotina in France and Godefroid et al. (2005) in Belgium and suggest that it occupies a similar niche between regions with different climates within Europe. In contrast, P. serotina dominated in forests of Poland with increasing soil moisture, soil reaction, and nitrogen capacity (Halarewicz and Zołnierz 2014). However, for the Ticino Park, this may also be associated with the relatively low overall variability of the values across the studied plots.
Thus, in our study area, the stands invaded by P. serotina were orientated towards the drier parts of the forests, in a large distance to the main river, with dominance of C. betulus, a denser canopy, and with lower levels of soil nitrogen and light availability. These stands were rather characterized by ‘true’ forest species (e.g., Convallaria majalis, Maianthemum bifolium, Paris quadrifolia, Polygonatum multiflorum, Pulmonaria officinalis, Viola reichenbachiana, and Vinca minor) (c.f. Honnay et al. 1998; Hermy et al. 1999), being associated with early spring flowering and transient seeds, which are related to a stress-tolerant strategy type (Grime 1977). These traits are typical for stable forest ecosystems (Catorci et al. 2012). In contrast, uninvaded stands were characterized by hygrophilous species and also ruderal species with rather large ecological amplitudes, i.e. generalists (Decocq et al. 2004). While the proportion of ‘true’ forest species increased with increasing canopy cover of P. serotina, we propose that after disturbance events P. serotina rapidly fills and closes the gaps (c.f. Closset-Kopp et al. 2007). Thus, P. serotina may provide or maintain in invaded stands a microclimate that is close to a ‘true’ forest microclimate of the native Q. robur-C. betulus association (including low light availability at the forest floor, high hygrometry, and low thermic variations). Our findings were consistent with those of Closset-Kopp et al. (2007), Chabrerie et al. (2007), and Chabrerie et al. (2010) suggesting that these characteristics of P. serotina erase the effects of large- and mid-scale disturbances of the forest matrix. Thus, they lead to a homogenisation of the forest environment, favouring ‘true’ forest species.
In the shrub layer, P. serotina invasion was less related to the main environmental gradients than in the tree layer, being mostly associated with dense oak forests, increasing management-related disturbance and organic layer thickness, and decreasing herb-layer cover. Here, P. serotina was significantly filtering for higher seed weight which was in agreement with the results of Mason et al. (2009), arguing that large-seeded species may use metabolic reserves in the seed to tolerate shading. In addition, species with a late flowering were inhibited. A delayed start of flowering is typical for species growing beneath an open canopy (Aubin et al. 2007). Instead, woody shade- and half-shade-tolerant species with heavy seeds were favoured. According to Hermy et al. (1999) these shrub and tree species are typical for ‘true’ forest environments. Thus, also in the shrub layer, P. serotina invasion was characterized by ‘true’ forest species but also by tree and shrub regeneration in the understorey. In contrast to our results, Chabrerie et al. (2008) classified the latter as indicators for uninvaded stands, suggesting that most species establishing in disturbance-induced canopy gaps were subsequently shaded out by invasive P. serotina trees and thus less abundant in invaded stands. In addition, Chabrerie et al. (2010) revealed that the abundance of functionally similar native species (seedlings and saplings of tree and shrub species) was lower in invaded than in uninvaded stands, whereas short-living ruderals were more likely to be found. However, they assumed that dense thickets of P. serotina also promote wild boar induced soil erosion which could be a possible reason for these observations.
Similar to P. serotina, R. pseudoacacia was completely absent in the wetter parts of the study area, favouring drier stands. While it is able to grow on moist to dry soils, it avoids wet or compacted soils because of a requirement for aerated soil (Cierjacks et al. 2013). Thus, wet areas appear more resistant to the invasion of both, P. serotina and R. pseudoacacia.
While R. pseudoacacia was not important in the shrub layer due to its low abundance, its abundance in the tree layer was one of the variables showing the most significant relationships with the trait attributes. In mixed stands, R. pseudoacacia was associated with C. betulus dominance in the tree layer and decreasing light availability at the forest floor. This was in accordance to Essl and Hauser (2003) who also recorded the association of R. pseudoacacia and C. betulus for Northern Austria. There, open stands of R. pseudoacacia arose from dry oak-hornbeam forests. In the Ticino Park, the abundance of R. pseudoacacia was almost independent from the total tree layer cover but related to increasing herb-layer and decreasing shrub layer cover, and to the absence of U. minor in the canopy. Typically, R. pseudoacacia is associated with communities of species adapted to very high soil nitrogen content (Rahmonov 2009). However, such a clear pattern could not be found for the Ticino Park. Among the species that were associated with R. pseudoacacia, only a few nitrophilous species were present. Even though the ability of R. pseudoacacia to fix atmospheric nitrogen, enrich soil nitrogen, and thus cause a possible shift in vegetation composition is always quoted as one of the species’ most important impacts in newly invaded habitats (e.g., Motta et al. 2009; Rahmonov 2009), it was particularly associated to nitrogen-poor soils in the Ticino Park. However, the expression ‘nitrogen-poor’ has to be seen in relation to the generally high nitrogen input in floodplain forests due to flooding. With a mean C:N ratio <12.5, the general availability of nitrogen was more than sufficient in the investigation area.
When present in the tree layer, R. pseudoacacia was filtering for stress-tolerant graminoid species with high lateral spread, flowering start in mid spring, and long-term persistent seed-bank behaviour. In oak and hornbeam dominated stands, R. pseudoacacia was also associated with ‘true’ forest species, similar to P. serotina. Pure R. pseudoacacia stands instead were typically characterized by a dense graminoid cover in the herb-layer, often with a single dominant species, like Carex pilosa in higher and denser forests or C. brizoides in lower and lighter forests (unpublished data). In particular, the perennial sedge C. brizoides is often stand-forming due to its clonal growth type, and thereby can affect the ground-layer vegetation or displace resident communities (Chmura and Sierka 2007). These results were in accordance with forests in Poland which revealed a positive correlation between R. pseudoacacia tree height and woodland species and a negative correlation between R. pseudoacacia tree height and grassland species in the herb-layer (Dzwonko and Loster 1997). Likewise, R. pseudoacacia seems to be able to form new stable associations with a missing shrub layer but a dense herb-layer cover and thus is slowing down forest succession. This finding corresponds to other studies from Europe (e.g., Kowarik and Langer 1994; Kowarik 1995); Wilhalm et al. (2008), for example, described for Northern Italy a new and discrete association of R. pseudoacacia and almost pure stands of the perennial grass Melica ciliate, lacking as well a shrub layer, and also Essl and Hauser (2003) reported for Northern Austria stable R. pseudoacacia associations with high abundances of Bromus sterilis and Poa nemoralis.
Conclusion
As P. serotina and R. pseudoacacia are important elements of the tree layer at many sites throughout the studied biosphere reserve, we propose that both species act as environmental filters to the understorey plant functional traits. While the presence of P. serotina may mitigate or even erase the effect of disturbances, acting as an overstorey-driven stabilizing force, and thus maintaining a stable forest microclimate and favouring ‘true’ forest species, R. pseudoacacia may slow down forest succession and tree regeneration by establishing new stable associations with a dense graminoid-dominated understorey. Nevertheless, as our study has only analysed correlations between macro-environmental variables, understorey species distribution, and functional traits, it is not clear if the two invaders are passengers or drivers of environmental changes. In addition, in the floodplain forests where we conducted our research, the strongest underlying environmental gradients, largely structuring the characteristics of the plots and the occurrence of the species in them, were independent of P. serotina and/or R. pseudoacacia invasion. Hence, as expected and as in accordance with other studies (Godefroid et al. 2005; Verheyen et al. 2007; Chabrerie et al. 2010; Halarewicz and Zołnierz 2014), only a weak portion of the variance may be explained by the invasion. For P. serotina, this could be related to the fact that this species is still spreading further south in the investigation area, yet not having reached its full range expansion and thus masking its impact. However, it is possible that also other factors, alone or in combination, like forest management history or small scale elevation differences (data that were not available for our study area), play an important role in the composition of the understorey species and the development of the forest community.
References
Aas G (2006) Die Schwarzpappel (Populus nigra) – zur Biologie einer bedrohten Baumart [Black Poplar (Populus nigra) – biology of a threatened tree species]. LWF Wissen 52:7–12
Annighöfer P, Schall P, Kawaletz H, Mölder I, Terwei A, Zerbe S, Ammer C (2012) Vegetative growth response of black cherry (Prunus serotina, Ehrh.) to different mechanical control methods in a biosphere reserve. Can J For Res 42(12):2037–2051
Annighöfer P, Kawaletz H, Terwei A, Mölder I, Zerbe S, Ammer C (2015) Managing an invasive tree species – silvicultural recommendations for black cherry (Prunus serotina Ehrh.). Forstarchiv 86:139–152
Aubin I, Gachet S, Messier C, Bouchard A (2007) How resilient are northern hardwood forests to human disturbance? An evaluation using a plant functional group approach. Ecoscience 14:259–271
Aubin I, Ouellette M-H, Legendre P, Messier C, Bouchard A (2009) Comparison of two plant functional approaches to evaluate natural restoration along an old-field deciduous forest chronosequence. J Veg Sci 20:185–198
Auclair AN, Cottam G (1971) Dynamics of black cherry (Prunus serotina Ehrh.) in southern Wisconsin oak forests. Ecol Monogr 41:153–177
Barbier S, Gosselin F, Balandier P (2008) Influence of tree species on understory vegetation diversity and mechanisms involved – a critical review for temperate and boreal forests. For Ecol Manage 254:1–15
Battaglia LL, Sharitz RR (2006) Responses of floodplain forest species to spatially condensed gradients: a test of the flood-shade tolerance tradeoff hypothesis. Oecologia 147:108–118
Bazzaz FA (1979) Physiological ecology of plant succession. Annu Rev Ecol Syst 10:351–371
Bazzaz FA (1996) Plants in changing environments: linking physiological, population, and community ecology. Cambridge University Press, New York
Bello F, Lavorel S, Díaz S, Harrington R, Cornelissen JHC, Bardgett RD, Berg MP, Cipriotti P, Feld CK, Hering D, Martins da Silva P, Potts SG, Sandin L, Paulo Sousa J, Storkey J, Wardle DA, Harrison PA (2010) Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers Conserv 19:2873–2893
Bemmerlein-Lux FA, Fischer HS, Lindacher R (1994) Umwandlung von Artmächtigkeitsskalen und Bedeutung skalarer Transformationen in der Vegetationskunde [Conversion of abundance scales and significance of scalar transformation in vegetation science]. Hoppea 55:645–656
Bernhardt-Römermann M, Römermann C, Nuske R, Parth A, Klotz S, Schmidt W, Stadler J (2008) On the identification of the most suitable traits for plant functional trait analyses. Oikos 117:1533–1541
Blank L, Carmel Y (2012) Woody vegetation patch types affect herbaceous species richness and composition in a Mediterranean ecosystem. Community Ecol 13:72–81
Boring LR, Swank WT (1984) The role of Black Locust (Robinia pseudoacacia) in forest succession. J Ecol 72:749–766
Bornette G, Tabacchi E, Hupp C, Puijalon S, Rostan JC (2008) A model of plant strategies in fluvial hydrosystems. Freshw Biol 53:1692–1705
Boschetti M, Canova I, Casati L, Oliveri S (2005) Mappatura delle specie arboree del Parco del Ticino mediante Telerilevamento iperspettrale [Mapping of tree species in the Ticino Park by hyperspectral remote sensing]. Consorzio Parco Lombardo della Valle del Ticino, Milano
Bourtsoukidis E, Kawaletz H, Radacki D, Schütz S, Hakola H, Hellén H, Noe S, Mölder I, Ammer C, Bonn B (2014) Impact of flooding and drought conditions on the emission of volatile organic compounds of Quercus robur and Prunus serotina. Trees Struct Funct 28:193–204
Braun-Blanquet J (1964) Pflanzensoziologie. Grundzüge der Vegetationskunde [Plant sociology. Main features of vegetation science], 3rd edn. Springer, Berlin
Brown CD, Boutin C (2009) Linking past land use, recent disturbance, and dispersal mechanism to forest composition. Biol Conserv 142:1647–1656
Brym ZT, Lake JK, Allen D, Ostling A (2011) Plant functional traits suggest novel ecological strategy for an invasive shrub in an understorey woody plant community. J Appl Ecol 48:1098–1106
Byun C, de Blois S, Brisson J (2013) Plant functional group identity and diversity determine biotic resistance to invasion by an exotic grass. J Ecol 101:128–139
Caliński T, Harabasz J (1974) A dendrite method for cluster analysis. Commun Stat 3:1–27
Caronni FE (2010) Il caso del ciliegio tardivo (Prunus serotina Ehrh.) al Parco lombardo della Valle del Ticino [The case of Black Cherry (Prunus serotina Ehrh.) at ´Parco lombardo della Valle del Ticino´]. In: Le specie alloctone in Italia: censimenti, invasività e piani di azione [Non-native species in Italy: survey, invasivness and management plans], Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano, Milano, 36 (1):37–38
Catorci A, Vitanzi A, Tardella FM, Hršak V (2012) Trait variations along a regenerative chronosequence in the herb-layer of submediterranean forests. Acta Oecol 43:29–41
Catorci A, Tardella FM, Cutini M, Luchetti L, Paura B, Vitanzi A (2013) Reproductive traits variation in the herb-layer of a submediterranean deciduous forest landscape. Plant Ecol 214:737–749
Centro Nazionale di Meteorologia e Climatologia Aeronautica (CNMCA) (2009) Atlante Climatico d´Italia 1971–2000 del Servizio Meteorologico dell’Aeronautica Militare [Climate atlas of Italy 1971-2000 of the Military Aeronautics Meterological Service]. http://clima.meteoam.it/AtlanteClimatico/pdf/%28066%29Milano%20Malpensa.pdf. Accessed 8 Aug 2012
Chabrerie O, Roulier F, Hoeblich H, Sebert-Cuvillier E, Closset-Kopp D, Leblanc I, Jaminon J, Decocq G (2007) Defining patch mosaic functional types to predict invasion patterns in a forest landscape. Ecol Appl 17:464–481
Chabrerie O, Verheyen K, Saguez R, Decocq G (2008) Disentangling relationships between habitat conditions, disturbance history, plant diversity, and American black cherry (Prunus serotina Ehrh.) invasion in a European temperate forest. Divers Distrib 14:204–212
Chabrerie O, Loinard J, Perrin S, Saguez R, Decocq G (2010) Impact of Prunus serotina invasion on understory functional diversity in a European temperate forest. Biol Invasions 12:1891–1907
Chapin FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC, Diaz S (2000) Consequences of changing biodiversity. Nature 405:234–242
Chmura D, Sierka E (2007) The invasibility of deciduous forest communities after disturbance: a case study of Carex brizoides and Impatiens parviflora invasion. For Ecol Manage 242:487–495
Cierjacks A, Kowarik I, Joshi J, Hempel S, Ristow M, von der Lippe M, Weber E (2013) Biological flora of the British Isles: Robinia pseudoacacia. J Ecol 101:1623–1640
Closset-Kopp D, Chabrerie O, Valentin B, Delachapelle H, Decocq G (2007) When Oskar meets Alice: does a lack of trade-off in r/K-strategies make Prunus serotina a successful invader of European forests? For Ecol Manage 247:120–130
Crooks JA (2005) Lag times and exotic species: the ecology and management of biological invasions in slow-motion. Ecoscience 12:316–329
Decocq G, Hermy M (2003) Are there herbaceous dryads in temperate deciduous forests? Acta Bot Gallica 150:373–382
Decocq G, Valentin B, Toussaint B, Hendoux R, Saguez R et al (2004) Soil seed bank composition and diversity in a managed temperate deciduous forest. Biodivers Conserv 13:2485–2509
Diaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
DIN – Deutsches Institut für Normung e.V. (1996) DIN ISO 10694. Bodenbeschaffenheit – Bestimmung von organischem Kohlenstoff und Gesamtkohlenstoff nach trockener Verbrennung (Elementaranalyse) [Soil condition – determination of organic and total carbon after dry burning (elementary analysis)] (ISO 10694:1995). Beuth, Berlin, Germany
Dolédec S, Chessel D, TerBraak CJF, Champely S (1996) Matching species traits to environmental variables: a new three-table ordination method. Environ Ecol Stat 3:143–166
Douma J, De Haan M, Aerts R, Witte J, Van Bodego P (2012) Succession-induced trait shifts across a wide range of NW European ecosystems are driven by light and modulated by initial abiotic conditions. J Ecol 100:366–380
Dray S, Dufour AB (2007) The ade4 Package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20
Dray S, Legendre P (2008) Testing the species traits-environment relationships: the fourth-corner problem revisited. Ecology 89:3400–3412
Dray S, Chessel D, Thioulouse J (2003) Co-inertia analysis and the linking of ecological data tables. Ecology 84:3078–3089
Dray S, Choler P, Dolédec S, Peres-Neto PR, Thuiller W, Pavoine S, ter Braak CJF (2014) Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 95(1):14–21
Drenovsky RE, Grewell BJ, D’Antonio CM, Funk JL, James JJ, Molinari N, Parker IM, Richards CL (2012) A functional trait perspective on plant invasions. Ann Bot 110:141–153
Dzwonko Z, Loster S (1997) Effects of dominant trees and anthropogenic disturbances on species richness and floristic composition of secondary communities in Southern Poland. J Appl Ecol 34:861
Ellenberg H, Weber HE, Düll R, Wirth V, Beck W (2001) Zeigerwerte von Pflanzen in Mitteleuropa [Indicator values of plants in Central Europe], 3rd edn. Erich Goltze, Göttingen
Endels P, Adriaens D, Bekker RM, Knevel IC, Decocq G, Hermy M (2007) Groupings of life-history traits are associated with distribution of forest plant species in a fragmented landscape. J Veg Sci 18:499–508
Eriksson O (1997) Clonal life histories and the evolution of seed recruitment. In: van Groenendael JM, de Kroon H (eds) The ecology and evolution of clonal plants. Backhuys Publishers, Leiden, pp 211–226
Essl F, Hauser E (2003) Verbreitung, Lebensraumbindung und Managementkonzept ausgewählter invasiver Neophyten im Nationalpark Thayatal und Umgebung (Österreich) [Distribution, habitat preference and management concept of selected invasive neophytes in the national park Thayatal and the adjacent area (Austria)]. Linzer Biol Beitr 35(1):75–101
Everitt BS, Landau S, Leese M, Stahl D (2011) Cluster analysis, 5th edn. Wiley, Chichester
FAO (1998) World reference base of soil resources. Food and Agriculture Organization of the United Nations, Rome
Fischer MA, Oswald K, Adler W (2008) Exkursionsflora für Österreich, Liechtenstein und Südtirol [Excursion flora of Austria, Liechtenstein, and South Tyrol], 3rd edn. Biologiezentrum der Oberösterreichischen Landesmuseen, Linz
Fischer LK, von der Lippe M, Kowarik I (2013) Urban grassland restoration: which plant traits make desired species successful colonizers? Appl Veg Sci 16:272–285
Gallardo B, Gascon S, Garcia M, Comin FA (2009) Testing the response of macroinvertebrate functional structure and biodiversity to flooding and confinement. J Limnol 68(2):315–326
Garnier E, Cortez J, Billès G, Navas M-L, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C, Toussaint J-P (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85:2630–2637
Gilliam FS (2007) The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 57:845–858
Godefroid S, Phartyal SS, Weyembergh G, Koedam N (2005) Ecological factors controlling the abundance of non-native invasive black cherry (Prunus serotina) in deciduous forest understory in Belgium. For Ecol Manage 210:91–105
Graae BJ, Sunde PB (2000) The impact of forest continuity and management on forest floor vegetation evaluated by species traits. Ecography 23:720–731
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194
Grime JP, Thompson K, Hunt R, Hodgson JG, Cornelissen JHC, Rorison IH, Hendry GAF, Ashenden TW, Askew AP, Band SR, Booth RE, Bossard CC, Campbell BD, Cooper JEL, Davison AW, Gupta PL, Hall W, Hand DW, Hannah MA, Hillier SH, Hodkinson DJ, Jalili A, Liu Z, Mackey JML, Matthews N, Mowforth MA, Neal AM, Reader RJ, Reiling K, Ross-Fraser W, Spencer RE, Sutton F, Tasker DE, Thorpe PC, Whitehouse J (1997) Integrated screening validates primary axes of specialisation in plants. Oikos 79:259
Halarewicz A, Zołnierz L (2014) Changes in the understorey of mixed coniferous forest plant communities dominated by the American black cherry (Prunus serotina Ehrh.). For Ecol Manage 313:91–97
Hejda M, Pyšek P, Jarošík V (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97:393–403
Hermy M, Honnay O, Firbank L, Grashof-Bokdam C, Lawesson JE (1999) An ecological comparison between ancient and other forest plant species of Europe, and the implications for forest conservation. Biol Conserv 91:9–22
Hill MO, Smith AJE (1976) Principal component analysis of taxonomic data with multi-state discrete characters. Taxon 25:249
Hodgson JG, Grime JP, Hunt R, Thompson K (1995) The electronic comparative plant ecology. Chapman & Hall, London
Honnay O, Degroote B, Hermy M (1998) Ancient-forest plant species in Western Belgium: a species list and possible ecological mechanisms. Belg J Bot 130:139–154
Jarvis CE, Cafferty S (2005) The Linnaean plant name typification project: Robinia pseudoacacia. http://www.nhm.ac.uk/research-curation/research/projects/linnaean-typification/database/index.dsml. Accessed 25 Sept 2012
Jauni M, Hyvönen T (2012) Interactions between alien plant species traits and habitat characteristics in agricultural landscapes in Finland. Biol Invasions 14:47–63
Kleyer M, Bekker RM, Knevel IC, Bakker JP, Thompson K, Sonnenschein M, Poschlod P, Van Groenendael JM, Klimeš L, Klimešová J, Klotz S, Rusch GM, Hermy M, Adriaens D, Boedeltje G, Bossuyt B, Dannemann A, Endels P, Götzenberger L, Hodgson JG, Jackel A-K, Kühn I, Kunzmann D, Ozinga WA, Römermann C, Stadler M, Schlegelmilch J, Steendam HJ, Tackenberg O, Wilmann B, Cornelissen JHC, Eriksson O, Garnier E, Peco B (2008) The LEDA Traitbase: a database of life-history traits of the Northwest European flora. J Ecol 96:1266–1274
Kleyer M, Dray S, Bello F, Lepš J, Pakeman RJ, Strauss B, Thuiller W, Lavorel S (2012) Assessing species and community functional responses to environmental gradients: which multivariate methods? J Veg Sci 23:805–821
Klotz S, Kühn I, Durka W (eds) (2002) BIOLFLOR – Eine Datenbank zu biologisch-ökologischen Merkmalen der Gefäßpflanzen in Deutschland [BIOLFLOR – a database of biological-ecological traits of the German flora]. Schriftenreihe für Vegetationskunde, Bundesamt für Naturschutz, Bonn
Kowarik I (1995) Zur Gliederung anthropogener Gehölzbestände unter Beachtung urban-industrieller Standorte [Classification of anthropogenic tree stands in consideration of urban-industrial sites]. Verh Ges Ökol 24:411–421
Kowarik I, Langer A (1994) Vegetation einer Berliner Eisenbahnfläche (Schöneberger Südgelände) im vierten Jahrzehnt der Sukzession [Vegetation of a former railroad area in Berlin (Schöneberger Südgelände) in the fourth decade of succession]. Verh Bot Ver Berlin Brandenburg 127:5–43
Lacourse T (2009) Environmental change controls postglacial forest dynamics through interspecific differences in life-history traits. Ecology 90:2149–2160
Lamarque LJ, Delzon S, Lortie CJ (2011) Tree invasions: a comparative test of the dominant hypotheses and functional traits. Biol Invasions 13(9):1969–1989
Lavorel S, McIntyre S, Landsberg J, Forbes TD (1997) Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends Ecol Evol 12:474–478
Lavorel S, Storkey J, Bardgett RD, Bello F, Berg MP, Roux X, Moretti M, Mulder C, Pakeman C, Díaz S, Harrington R (2013) A novel framework for linking functional diversity of plants with other trophic levels for the quantification of ecosystem services. J Veg Sci 24:942–948
Legendre P, Galzin R, Harmelin-Vivien ML (1997) Relating behavior to habitat: solutions to the fourth-corner problem. Ecology 78:547–562
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond B 270:775–781
MacDougall AS, Turkington R (2005) Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86:42–55
Mann LE, Harcombe PA, Elsik IS, Hall RBW (2008) The trade-off between flood-and shade-tolerance: a mortality episode in Carpinus caroliniana in a floodplain forest, Texas. J Veg Sci 19:739–746
Martin FN, Loper JE (1999) Soilborne plant diseases caused by Pythium spp.: ecology, epidemiology, and prospects for biological control. Crit Rev Plant Sci 18:111–181
Mason TJ, French K, Lonsdale WM (2009) Do graminoid and woody invaders have different effects on native plant functional groups? J Appl Ecol 46:426–433
McGill B, Enquist BJ, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends Ecol Evol 21:178–185
McLean EO (1982) Soil pH and lime requirement. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2: chemical and microbiological properties. Agronomy Monograph ASA–SSSA, Madison, WI, USA, pp 199–224
Minden V, Andratschke S, Spalke J, Timmermann H, Kleyer M (2012) Plant trait-environment relationships in salt marshes: deviations from predictions by ecological concepts. Perspect Plant Ecol Evol Syst 14:183–192
Motta R, Nola P, Berretti R (2009) The rise and fall of the black locust (Robinia pseudoacacia L.) in the “Siro Negri” Forest Reserve (Lombardy, Italy): lessons learned and future uncertainties. Ann For Sci 66:410
Naeem S, Bunker DE, Hector A (2009) Biodiversity, ecosystem functioning, and human wellbeing: an ecological and economic perspective. Oxford University Press, Oxford
Ordonez A, Wright IJ, Olff H (2010) Functional differences between native and alien species: a global-scale comparison. Funct Ecol 24:1353–1361
Packer A, Clay K (2003) Soil pathogens and Prunus serotina seedling and sapling growth near conspecific trees. Ecology 84:108–119
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Petchey OL, Gaston KJ (2006) Functional diversity: back to basics and looking forward. Ecol Lett 9:741–758
Pignatti S, Menegoni P, Pietrosanti S (2005) Valori di bioindicazione delle piante vascolari della Flora d’Italia. Bioindicator values of vascular plants of the Flora of Italy. Braun-Blanquetia 39:3–95
Prach K, Pyšek P (1994) Clonal plants – what is their role in succession? Folia Geobot 29:307–320
Prach K, Pyšek P, Šmilauer P (1997) Changes in species traits during succession: a search for pattern. Oikos 79:201–205
Pyšek P, Jarošík V, Hulme P, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Change Biol 18:1725–1737
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rahmonov O (2009) The chemical composition of plant litter of black locust (Robinia pseudoacacia L.) and its ecological role in sandy ecosystems. Acta Ecol Sin 29:237–243
Reichelt G, Wilmanns O (1973) Vegetationsgeographie [Phytogeography]. Georg Westermann, Braunschweig
Roloff A, Weisgerber H, Lang U, Stimm B, Schutt B (1994) Enzyklopädie der Holzgewächse. Handbuch und Atlas der Dendrologie [Encyclopaedia of woody species. Handbook and atlas of dendrology]. Wiley, Weinheim
Rostan JC, Amoros C, Juget J (1987) The organic content of the surficial sediment: a method for the study of ecosystems development in abandoned river channels. Hydrobiologia 148:45–62
Royal Botanic Gardens Kew (2008) Seed information database (SID). Version 7.1. http://data.kew.org/sid/. Accessed 9 Sept 2012
Saar L, Takkis K, Pärtel M, Helm A (2012) Which plant traits predict species loss in calcareous grasslands with extinction debt? Divers Distrib 18:808–817
Sartori F (1980) Les forêts alluviales de la basse vallée du Tessin (Italie du nord). Colloques phytosociologiques IX. Les forêts alluviales, Strasbourg, pp 201–216
Sartori F, Bracco F (1996) Present vegetation of the Po plain in Lombardy. Alliona 34:112–135
Savage VM, Webba CT, Norberg J (2007) A general multi-trait-based framework for studying the effects of biodiversity on ecosystem functioning. J Theor Biol 247:213–229
Schleicher A, Peppler-Lisbach C, Kleyer M (2011) Functional traits during succession: is plant community assembly trait-driven? Preslia 83:347–370
Schnitzler A (1995) Successional status of trees in gallery forest along the river Rhine. J Veg Sci 6:479–486
Schüller H (1969) Die CAL-Methode, eine neue Methode zur Bestimmung des pflanzenverfügbaren Phosphates in Böden [The CAL method, a new method for determination of plant available phosphate in soils]. Z Pflanzenernähr Bodenkde 123:48–63
Schwarz WL, Malanson GP, Weirich FH (1996) Effect of landscape position on the sediment chemistry of abandoned-channel wetlands. Landscape Ecol 11:27–38
Siebel HN, Bouwma IM (1998) The occurrence of herbs and woody juveniles in a hardwood floodplain forest in relation to flooding and light. J Veg Sci 9:623–630
Sterk M, Gort G, Klimkowska A, van Ruijven J, van Teeffelen AJA, Wamelink GWW (2013) Assess ecosystem resilience: linking response and effect traits to environmental variability. Ecol Indic 30:21–27
Strayer DL, Eviner VT, Jeschke JM, Pace ML (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21:645–651
Terwei A, Zerbe S, Zeileis A, Annighöfer P, Kawaletz H et al (2013) Which are the factors controlling tree seedling establishment in North Italian floodplain forests invaded by non-native tree species? For Ecol Manage 304:192–203
Thompson K (1987) Seeds and seed banks. New Phytol 106:23–34
Thuiller W, Richardson DM, Rouget M, Proches S, Wilson JRU (2006) Interactions between environment, species traits, and human uses describe patterns of plant invasions. Ecology 87:1755–1769
Thuiller W, Gasso N, Pino J, Vilà M (2012) Ecological niche and species traits: key drivers of regional plant invader assemblages. Biol Invasions 14:1963–1980
UNESCO (2005) MAB biosphere reserves directory. Biosphere reserve information: Italy, Valle del Ticino. http://www.unesco.org/mabdb/br/brdir/directory/biores.asp?code=ITA+06&mode=all. Accessed 12 May 2013
Verheyen K, Honnay O, Motzkin G, Hermy M, Foster DR (2003) Response of forest plant species to land-use change: a life-history trait-based approach. J Ecol 91:563–577
Verheyen K, Vanhellemont M, Stock T, Hermy M (2007) Predicting patterns of invasion by black cherry (Prunus serotina Ehrh.) in Flanders (Belgium) and its impact on the forest understorey community. Divers Distrib 13:487–497
Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116:882–892
Weiher E, van der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O (1999) Challenging Theophrastus: a common core list of plant traits for functional ecology. J Veg Sci 10:609–620
Wesuls D, Oldeland J, Dray S (2012) Disentangling plant trait responses to livestock grazing from spatio-temporal variation: the partial RLQ approach. J Veg Sci 23:98–113
Wilhalm T, Staffler H, Wallnöfer S (2008) Das Melico ciliatae-Robinietum pseudacaciae, eine neue Robinienwald-Assoziation in der inneralpinen Trockenvegetation des Vinschgaues (Südtirol, Italien) [The Melico ciliatae-Robinietum pseudacaciae, a new black locust forest association in the xerothermic vegetation of the Vinschgau Valley (South Tyrol, Italy)]. Verh Zool-Bot Ges Österreich 145:65–81
Acknowledgments
We would like to thank the Marianne and Dr. Fritz-Walter Fischer Foundation within the Stifterverband für die Deutsche Wissenschaft and the Foundation of the Free University Bozen-Bolzano for funding our research and the Ateneo Italo-Tedesco for providing the VIGONI program for supporting project based exchange of staff. Many thanks to Thomas Wilhalm and Francesco Sartori for help and support with the determination of the plant species, to Fulvio Caronni, Valentina Parco, and the park administration for useful information about the biosphere reserve and providing free accommodation during field work, and to Anna Radtke for valuable comments on former versions of the manuscript. We also thank Jeff Wright and four anonymous reviewers for many constructive comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Terwei, A., Zerbe, S., Mölder, I. et al. Response of floodplain understorey species to environmental gradients and tree invasion: a functional trait perspective. Biol Invasions 18, 2951–2973 (2016). https://doi.org/10.1007/s10530-016-1188-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1188-0