Abstract
A particularly vexing phenomenon within invasion ecology is the occurrence of spontaneous collapses within seemingly well-established exotic populations. Here, we assess the frequency of collapses among 68 exotic bird populations established in Hawaii, Puerto Rico, Los Angeles and Miami. Following other published definitions, we define a ‘collapse’ as a decline in abundance of ≥90 % within ≤10 years that lasts for at least 3 years. We show that 44 of the 68 exotic bird populations have exhibited declines at some point within their time series. Sixteen of the populations declined sufficiently to be defined as collapsed. It took on average 3.8 ± 1.8 years for populations to decline into a collapsed state, and this state persisted on average for 7.1 ± 6.3 years across (collapsed) populations. We compared the severity and duration of declines across all 44 declining populations according to taxonomic Order and geographic region. Neither variable explained substantial variation in the metrics of collapse. Our results indicate that severe, rapid, and persistent population declines may be common among exotic populations. We suggest that incorporating the probability and persistence of collapses into management decisions can inform efforts to enact control or eradication measures. We also suggest that applying our approach to other taxa and locations is crucial for improving our understanding of when and where collapses are likely to occur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a nascent but increasing body of evidence to suggest that populations of exotic species can experience substantive declines in abundance even after they have become well established in a novel habitat, with the more dramatic of these declines often called ‘collapses’ (Sakai et al. 2001; Cassey 2002; Wolfe 2002; Colautti et al. 2004; Lockwood et al. 2005; Blackburn et al. 2009). Investigations into population declines among exotic species have generally been qualitative in nature, mostly serving to expose the range of species and situations in which collapses have occurred (Williamson 1996; Simberloff and Gibbons 2004; McDonald and Wells 2010; Burnaford et al. 2011; Cooling et al. 2012; Moore et al. 2012). Williamson (1996), for example, discusses the occurrence of population collapses for over 30 exotic species, representing half a dozen taxonomic classes. What is missing from this literature, however, is a quantitative description of exotic species population declines, and a way to rigorously define those declines that are of sufficient magnitude, longevity, and persistence to be considered collapses. Below we evaluate the prevalence of population collapses across a range of exotic bird populations using an exemplary dataset of long-term abundance records, the Audubon Christmas Bird Counts (CBC; National Audubon Society 2010). Additionally, we compare the severity and duration of declines across taxonomic groups and geographic locations to identify patterns in the occurrence of declines and collapses.
Abundance scales positively with exotic species’ impacts, although it is not clear if that relationship is always linear (Parker et al. 1999; Thomsen et al. 2009). Because of this positive relationship, abundance has become an indicator for when managers should control an exotic population, and it is used as a metric for judging the success of eradication measures (Zavaleta et al. 2001; Andersen et al. 2004; Bergstrom et al. 2009). In this context, severe, quickly developing, long-lasting declines stand apart from other population fluctuations (e.g., Simberloff and Gibbons 2004; Cooling et al. 2012). A severe decline in the abundance of an exotic population is noteworthy as it indicates that the impact of a species may be lessening and that active management may not be needed. Also, a severe and rapid population decline, even if it does not persist, may signal a moment of opportunity whereby complete eradication of a problematic invader becomes achievable (Simberloff and Gibbons 2004). Finally, persistent exotic population collapses may signal a permanent down-scaling of management concern. Thus, we suggest that our quantitative framework for describing population declines can provide insight into how such events should enter into management decisions.
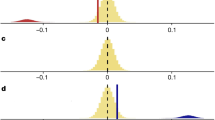
We can quantitatively define four elements of population decline: severity, duration, persistence, and probability. A population collapse is often defined as an unusually large (in terms of severity; Fig. 1a) and rapid (in terms of duration; Fig. 1a) reduction in abundance from a maximum observed population size. Collapses can also be characterized according to their persistence—defined as the length of time that the population remains in a collapsed state (Pinsky et al. 2011; Fig. 1a). We suggest that declaration of a collapse should also include an explicit statement about the certainty that a decline with a particular severity and duration occurred, given the process error and observer error inherent to time series data.
a Graphical depiction of the four elements of collapse evaluated here. Duration (the time between the maximum abundance and the threshold for collapse) and severity (the percent of the decline from the maximum) are used to distinguish between generic declines and collapses. Persistence [the amount of time the population spends in a collapsed state (i.e., below the collapse threshold)] and probability (the likelihood that a population has collapsed, given uncertainty about observed abundances (error bars around points), calculated as the proportion of simulations of a population’s time series that result in a collapse) are used to further rank collapses for prioritization (longer persistence and greater probability equate to “worse” collapses). b Graphical representation of the aspects of a population’s time series that we monitor for every run of the model from Aagaard et al. (in press). We use these five aspects to calculate the four main elements of population collapse. The horizontal dashed line represents our collapse threshold (90 % reduction of the maximum abundance for a given simulation)
As an illustrative example of how to implement this framework, we adopt a relatively strict set of quantitative thresholds for these decline elements, only classifying reductions in abundance of ≥90 %, within (at most) 10 years and that last for 3 years or more as collapses. Although these thresholds are arbitrary, and thus can easily be changed to fit any user-specified objectives (see below), we have set them here to be consistent with definitions of collapse previously used in invasion ecology (e.g., Simberloff and Gibbons 2004), and with definitions of collapse developed within the conservation and management literature for native species (e.g., IUCN World Conservation Union 2001; Pinsky et al. 2011). This allows us to compare our results with previous findings in the invasion and conservation literature.
Methods
First, we provide background on the data used in this study and include a brief discussion of the constraints imposed on the data prior to applying the method. We then explain the salient components of the method for the current investigation, which are primarily concerned with elucidating the uncertainty in each of the elements of collapses. For a complete statistical elaboration of this method see Aagaard et al. (in press).
CBC data
Christmas Bird Counts involve searches for all species seen within a fixed location of known size (15 mile diameter circle) during 1 day, with the number of individuals observed (seen or heard) per species recorded. These counts are temporally and spatially standardized and are reported in terms of effort per party hour to account for error relating to differing observer effort (e.g., one person counting for 10 h equates to five people counting for two). We downloaded CBC data from the Audubon website for 68 exotic bird populations from 11 count circles; seven across the five main Hawaiian Islands, and one each in Cabo Rojo (Puerto Rico), Los Angeles (California), and Miami (Florida; see Table 1 for common and scientific names for species included, and Table 2 for CBC codes of circles used). We chose these sites for two reasons. First, these locations have all been included in the CBC for a sufficient amount of time to compile time series for evaluation of population collapses. Second, they are all North American avian invasion ‘hot spots’. Given their geographic locations and mild climates, many exotic birds have established in this locations as cage escapees via the pet trade or through intentional release for aesthetics or biocontrol (Blackburn et al. 2009).
We note that while the island CBC circles (Hawaii and Puerto Rico) cover most of the terrestrial extent of these locations and are thus reasonable representations of the exotic bird populations there, the count circles used for Los Angeles and Miami are likely only spatial subsets of larger populations. However, exotic birds are largely confined to the two urban centers in these cities and our circles capture these urban subpopulations.
Methodological requirements
There is a set of requirements that data must meet to be included in our analysis. We detail these requirements in Appendix 1 of Electronic Supplementary Material. In brief, we are only concerned with declining populations, so those species with time series that do not exhibit a decline are ignored (by definition, an increasing population cannot have collapsed). We only evaluate declines that are clearly not caused by hunting or management efforts. This requirement largely excludes consideration of exotic game birds in this analysis since they are (or have been) regularly hunted. We only include species where the available time series in the CBC is at least 10 years long because our definition of collapse specifies a 10-year decline period. And finally, we require that each population is considered established and self-sustaining. Imposing these requirements on the suite of exotic birds present in the CBC records of our four locations reduced our list of possible populations from 68 to 44.
Quantifying population collapses
Quantifying each of the four elements of decline depends critically on identifying the maximum observed abundance in the time series. As with any observation of a population’s abundance, the maximum observed abundance carries with it associated uncertainty stemming from observer bias (false positives, false negatives) and natural stochastic changes in population size. For example, imagine a time series with three successive abundance records in excess of all others (a, b, c) in which a is marginally <b, which is marginally <c. Allowing for both observation and process error about these annual abundance estimates, it is conceivable that any of these three records could be the true maximum abundance of the time series. This means that the true maximum abundance could have occurred in any of these 3 years, and could have been some value within the range of variances of a, b, and c. The method of Aagaard et al. (in press) accounts for observation and process error, and by simulating a range of possible maximum abundance values (as well as a range of possible values for all other data points in the time series) it quantifies the uncertainty around the elements of collapses that we seek to estimate. We provide an overview of this method below.
Aagaard et al. (in press) use a statistical simulation model to generate 95 % posterior credible intervals (CIs) about every abundance record within a time series. They accomplish this by applying a moving average function to an observed time series to produce a large number of simulated abundance estimates at each time step in a population trajectory (see below for specifics of this model). Using this method, we created 1.0E9 estimated abundance values around each recorded observation within all 44 population’s time series we considered. For example, if a population was recorded in the CBC for 20 years, we created 1.0E9 estimated abundances for each of those 20 annual observations. From this, we derived a 95 % posterior CI about each year’s abundance observation within each of the 44 population trajectories we considered. The model of Aagaard et al. (in press) has the following form:
Here, Y i is the observed abundance in year i (updated as j in the summation on the right-hand side of the equation as i is already set on the left-hand side); μ i+1 is the abundance being simulated; β is the influence of each of the previous year’s (j’s) observed abundances on the current year’s (i’s) simulated abundance; L is the length of the longest consecutive string of observed ‘0’ abundance records in the time series; and σ 2 is the variance about the simulated abundances. This formulation allows each year’s simulated abundance to be influenced by all previous year’s observed abundance estimates, thereby accounting for potential temporal autocorrelation in the data.
We include the ‘L + 1’ term to avoid the condition in which the model simulates extinction for a population that has several consecutive observed ‘0’ abundance records in its time series followed by observed positive abundance records (indicating that the population is persistent and is not truly extinct). In this way, the model can approximate a population with, say, five consecutive years of observed ‘0’ abundance (L = 5) followed by several years of observed positive abundance by drawing on at least one positive value when the moving average period encompasses the string of observed ‘0’ abundances (L + 1 = the observed abundance from 6 years prior; see Fig. 2). We apply a vague normal prior distribution to the set of β’s estimated over the 6-year window to inform the influence of prior observations on the abundance of the year being simulated.
We applied this model in OpenBUGS v3.2.1 (Lunn et al. 2009), for 1.0E9 iterations, using 1 chain and a burn-in of 1000. We used R v3.0.2 (R Core Team 2012) to generate design matrices [with m rows (m = length of record) and L + 1 columns] for the β j * μ j terms.
In every iteration of the model we monitored five values to calculate our four elements of decline (see Fig. 1b for graphical overview): (A) the simulated maximum abundance; (B) the year in which this maximum occurred; (C) the year in which the first simulated abundance is below our collapse threshold (90 % of the given run’s simulated maximum abundance); (D) the simulated abundance value of this year; and (E) the number of consecutive years of simulated abundances below our collapse threshold. With these five values, and for each iteration of the model, we calculated the percent decline of the population (1 − [value D/value A], ‘severity’), the time between a population’s simulated maximum abundance and the first year it can be considered collapsed (value C − value B, ‘duration’), and the length of time the population was simulated to have spent in a collapsed state, if any (value E, ‘persistence’). Finally, we monitored the percent of iterations (i.e. out of 1.0E9) in which the population was simulated to have declined sufficiently to be considered collapsed by our definition, if at all (the number of iterations in which a collapse occurred divided by the total number of iterations, ‘probability’).
Interspecific comparisons
Although our primary goal is to define and assess the prevalence of population collapse in exotic bird populations, the derivation of our metrics also allows for quantitative comparisons across categories that may be relevant to explain differences in trajectories across populations. To this end, we assessed the degree to which taxonomic affiliation and geographic location could explain observed variance in severity and duration of population declines across all 44 exotic bird populations. To the degree that taxonomic classification encapsulates the slow to fast life history in birds (Bennett and Owens 2002), our analysis looks to see if such differences can explain variation in the severity and duration of population declines in our data. Similarly, our locations include both islands and continents, and a range of climatic conditions (e.g., seasonal precipitation ranges from “15 in Los Angeles to >60” in Miami). Comparison between locations thus allows a broad-stroke way to assess the extent to which these conditions explain variance in decline metrics.
We performed an ordinal rank regression of the severity and duration of decline across all 44 exotic bird populations using OpenBUGS v3.2.1 (Table 1, A-D; Lunn et al. 2009). We first adjusted the decline duration to be on the same scale as decline severity by calculating 1 − (x/y), where x is the length of the decline (duration) for a given species, and y is the maximum decline length for all species. Values closer to 1 represent collapses of shorter duration, and values closer to 0 represent longer collapses. We ranked the resulting product of the severity (percent decline) and duration (decline length) to generate a collapse ranking of all populations (P). We then fit the following model to identify the importance of Order and location as predictors of population collapses:
where α is the intercept (a random effect), β 1 is the location effect, β 2 is the Order effect, and ε is a random effect error term. We ran one chain of the model for 1.0E5 iterations, with a burn in of 1.0E4 to allow for convergence. We evaluated the posterior 95 % CI for β 1 and β 2. If the CIs overlapped zero, then we determined that the parameter had no influence on the model. If the CIs did not overlap zero then the parameter did have an effect, and the effect was commensurate with the magnitude of its sign (i.e., a positive effect if the CI was entirely above zero, negative if entirely below).
Results
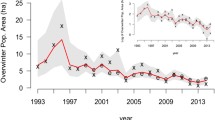
Out of the 68 exotic bird populations established at our four locations, 44 showed a decline at some point over a 10-year period in their CBC record. Thirty-five of these 44 populations showed reductions of ≥90 % at least once during their time series, and 24 declined by at least this much in fewer than 10 years. Of these 24, 16 remained in a collapsed state (<90 % of maximum abundance) for over 3 years and qualified as ‘collapsed’ under our definition (Fig. 3). Considering only those 16 collapsed populations, the average decline duration was 3.8 ± 1.8 years, the average severity was 99.8 ± 0.3 % (range of 98.9–100 %) and the average persistence in the collapsed state was 7.1 ± 6.3 years (with a range of 3–26 years; Table 2). For collapsed populations, maximum abundance ranged from low (yellow-faced grassquit, 0.06 individuals/party hour, or i/ph) up to the highest abundance recoded (common myna, 135 i/ph; Table 2). Among those populations not exhibiting collapses, maximum abundance estimates also ranged from low (red-whiskered bulbul, 0.57 i/ph) to high (zebra dove, 57 i/ph; Table 2). Only one population, the red-whiskered bulbul in Hawaii, had a probability of having collapsed of <90 % (15–16 %; Table 2). The skylark and saffron finch in Hawaii (91–91 and 98–99 % respectively) are the only other two collapsed populations that had less than a 100 % probability of having collapsed, according to our model (Table 2).
(top) Length of collapse (from maximum to <10 %). Only populations that decline by at least 90 % of the lower bound of the 95 % credible interval of their maximum abundance estimates within 10 years, and hold this condition for 3 years or more, are included, with declines longer than 10 years or of <90 % severity or persisting for <3 years indicated by white bars. (bottom) Results in terms of the percent decline from estimated maximum abundance. Bar colors correspond to the collapse classification (white = no collapse, black = probable collapse). The dashed line marks the 90 % threshold marking a collapsed population. Whiskers represent the upper bounds for the estimated maximum abundance, which approximates uncertainty in the observed maximum value (see text for details). Species codes (HI: Hawaii, LA: Los Angeles, MI: Miami, CR: Cabo Rojo)—1: LA house sparrow; 2: LA rock pigeon; 3: LA spotted dove; 4: LA yellow-chevroned parakeet; 5: HI chestnut mannikin; 6: HI common myna; 7: CR white-winged parakeet; 8: CR chestnut manakin; 9: CR Indian silverbill; 10: CR nutmeg manniking; 11: CR orange-cheeked waxbill; 12: CR rock pigeon; 13: MI house sparrow; 14: MI mitred parakeet; 15: MI monk parakeet; 16: MI red-masked parakeet; 17: MI rose-ringed parakeet; 18: MI spot-breasted oriole; 19: HI greater-necklaced laughingthrush; 20: HI house finch; 21: HI house sparrow; 22: HI hwamei; 23: HI Indian silverbill; 24: HI Japanese bush warbler; 25: HI Japanese white-eye; 26: HI Java sparrow; 27: HI lavender waxbill; 28: HI northern cardinal; 29: HI northern mockingbird; 30: HI nutmeg mannikin; 31: HI orange-checked waxbill; 32: HI red-billed leiothrix; 33: HI red-crested cardinal; 34: HI red-vented bulbul; 35: HI red-whiskered bulbul; 36: HI red avadavat; 37: HI saffron finch; 38: HI skylark; 39: HI spotted dove; 40: HI western meadowlark; 41: HI white-rumped shama; 42: HI yellow-faced grassquit; 43: HI yellow-fronted canary; 44: HI zebra dove
Three groups of collapses were evident among these 16 populations (Appendix 2 of Electronic Supplementary Material). One group of four species showed evidence of recovery to above-collapse-threshold levels subsequent to the collapse (red-whiskered bulbul and saffron finch in Hawaii; red-masked parakeet in Miami; white-winged parakeet in Puerto Rico). Another group of six species maintained abundance at, or below, the collapse threshold throughout the remainder of their time series (common myna, nutmeg mannikin, and orange-cheeked waxbill in Hawaii; chestnut mannikin and orange-cheeked waxbill in Puerto Rico; and rose-ringed parakeet in Miami). A final group of six species showed some evidence of recovery out of a collapsed state, followed by either extinction or a return to below-collapse-threshold abundance until the end of the time series (yellow-fronted canary, greater-necklaced laughingthrush, Indian silverbill, lavender waxbill, and yellow-faced grassquit in Hawaii; nutmeg mannikin in Puerto Rico).
Seven species have populations that occur in multiple locations (Table 2). Three species with multiple exotic populations in our dataset (house sparrow, spotted dove, and rock pigeon) have not experienced collapses in any of the locations in which they occur. Two species with two populations have collapsed in only one location (chestnut mannikin and Indian silverbill), whereas two other species with two populations collapsed in both locations in which they occur (nutmeg mannikin and orange-cheeked waxbill both collapsed in Hawaii and in Puerto Rico; Table 2).
We found that four out of six exotic bird populations in Puerto Rico collapsed, while no populations did so in Los Angeles. Exotic bird populations in Miami and Hawaii demonstrated roughly equivalent numbers of collapsed versus non-collapsed populations (two out of six and 10 out of 28, respectively; Table 2). In terms of Order, 13 out of 28 Passeriformes collapsed, and three out five Psittaciformes collapsed. We found no evidence of any of the five Columbiformes species having collapsed. Geographical location and taxonomic affiliation, however, did not explain much variation in the severity and duration of declines across populations (posterior 95 % CI for β 1 = −0.12 to 0.04 and for β 2 = −0.15 to 0.08). The majority of the error in this model was accounted for by the random effect term, ε, which had a posterior 95 % CI of −56.51 to −33.62, emphasizing the degree to which variation in declines is not explained by either Order or location. We suspect that the limited number of locations and Orders included in our analysis precluded meaningful partitioning of observed variation between groups; for instance, most Psittaciformes (four out of six) occurred in Miami. Including a more diverse combination of locations and taxonomic groups would help elucidate the interaction of Order and geography.
Discussion
There have been repeated calls to better understand the long-term dynamics of exotic populations so that we can improve our knowledge base of biological invasions (Drake et al. 1989; Sakai et al. 2001). Our approach for quantifying population declines advances that agenda by providing a transparent and repeatable method for identifying population collapses, while allowing the definition of collapse to be user-generated. Following previously published research on collapsed populations, we set our definition to be relatively conservative in the sense that only rapid, severe, and persistent declines qualify. Our survey of 68 exotic bird populations revealed declines among 44 populations (65 %), with 16 of these 44 showing declines of such severity, magnitude and persistence that they satisfy our definition of collapse (>23 % of all species, >36 % of declining species). These results, combined with the qualitative reviews of exotic population collapses using similar definitions (Williamson 1996; Simberloff and Gibbons 2004), suggest that rapid and severe exotic population declines may be a relatively common phenomenon within exotic populations.
Are the population dynamics of exotic birds qualitatively different from that of native birds? Without the availability of analyses for native species that use a similar definition of collapse and methods as we do here, this question is difficult to fully address. However, Aagaard et al. (in press) did evaluate declines among endemic Hawaiian bird species using the same data source (CBC) and methods we used here, thus providing a limited but sound comparison. Results from Aagaard et al. (in press) revealed that six of 12 (50 %) declining native Hawaiian bird populations have suffered >90 % reductions in abundance in <10 years, with three populations (25 %) satisfying our definition of collapse. These proportions are similar to those found in our survey of exotic populations in Hawaii (50 % with declines of 90 % or more in <10 years, and 36 % collapsed). This evidence suggests that exotic bird populations are not collapsing any more commonly than are native bird populations in Hawaii, but also not any less so.
There is a clear need to identify patterns and predictors of collapse within native and exotic species’ conservation (Lande 1987; Bascompte and Solé 1996; Williamson 1996; Simberloff and Gibbons 2004), and our (albeit limited) comparison between native and exotic species suggests that research on either group will inform this quest. Using our methods we illustrate how larger-scale comparative approaches to these questions can be addressed. Our results here are narrow in this context due to data limitations. However we show that it is rare that species with more than one exotic population collapse in all locations, that all the taxonomic Orders we evaluated are about as equally likely to have collapsed populations as not, and that there are no locations that we examined that can be called collapse ‘hotspots’. We suggest that as more surveys of native or exotic population collapses surface, or data availability increases, our ability to more rigorously test for such patterns will grow.
For consistency and comparative purposes we set our threshold values for severity, duration, and persistence of decline so that they followed previous collapse definitions published in the invasion biology and conservation literature. However, the methods of Aagaard et al. (in press) allow thresholds to be altered to fit the aims of any user. If warranted, the thresholds we set could be altered with such changes simply increasing or decreasing the number of populations we declared as collapsed. Such changes would make it impossible to compare our results to previous research; however, if our approach is used in the context of exotic species management, such changes may be well-warranted.
We suggest that the choice in where to set decline thresholds should be driven by the risk that managers and society are willing to accept relative to the outcome of their decisions. For example, managers may choose not to invest in control efforts when an exotic population demonstrates evidence of only a moderate decline. However, doing so risks the population rebounding back to high(er) abundance and thus the manager will have lost an opportunity to implement more efficient and effective control/eradication efforts. In addition, we suggest that the production of probability estimates for collapse, as done here, provides managers a transparent way to convey their confidence that withdrawing or re-allocating control/eradication efforts away from declining populations is the most effective use of limited resources. One could argue that resources should be diverted only in cases where a collapse is certain to have occurred, for example.
A similar argument can be made relative to setting the persistence threshold of collapse. By including the 3-year persistence criterion in our collapse definition, we can differentiate between declines that persist from those that may be better described as natural (although extreme) fluctuations. It is easy to envision situations in which managers might alter the persistence criterion to fit their goals. Managers trying to identify when to enact eradication measures, which are typically much more successful when population size is low (Bomford and O’Brien 1995; Myers et al. 2000; Zavaleta et al. 2001; Clout and Veitch 2002; Rejmánek and Pitcarin 2002; McDonald and Wells 2010), might be inclined to invoke a shorter persistence rule. In this case, if the population reaches a substantially lower abundance it is always beneficial to act on eradication measures no matter how long that lower abundance would ‘normally’ persist. In contrast, if there is no evidence that an exotic species will be harmful to co-occurring native species, a manager may relax the persistence rule to span more years, especially if resources to manage the exotic are limited. It is also necessary to change the duration and persistence of thresholds to match the life history of the species evaluated so that it is longer for longer-lived species and vice versa. The important point is that the collapse definitional thresholds adopted are at the user’s discretion, and should be informed by the target species’ impacts and intrinsic biology as well as perceived management gains versus risks.
Concern over biological invasions is often driven by rapid population growth after establishment and the attainment of high abundance in particular locations. These two factors do portend substantial ecological and economic impacts (Parker et al. 1999). However, the longer-term population dynamics of exotic species can be quite complex, with our results adding to growing evidence that exotic populations can show substantial declines in abundance through time (Strayer et al. 2006). Perhaps more germane to the growth in understanding the management of harmful exotic species, we illustrate the application of a method to produce quantitative assessments of population collapse among exotic species that can help managers understand and evaluate uncertainty in their decision as to how to respond to such declines.
References
Aagaard K, Lockwood JL, Green EJ (in press) A Bayesian approach for characterizing uncertainty in declaring a population collapse. Ecol Model. doi:10.1016/j.ecolmodel.2016.02.014
Andersen MC, Adams H, Hope B, Powell M (2004) Risk assessment of invasive species. Risk Anal 24:787–793
Bascompte J, Solé RV (1996) Habitat fragmentation and extinction thresholds in spatially explicit models. J Anim Ecol 65:465–473
Bennett PM, Owens PF (2002) Evolutionary ecology of birds: life histories, mating systems and extinction. Oxford University Press, Oxford
Bergstrom DM, Lucieer A, Keifer K, Wasley J, Belbin L, Pedersen TK, Chown SL (2009) Indirect effects of invasive species removal devastate World Heritage Island. J Appl Ecol 46:73–81
Blackburn TM, Lockwood JL, Cassey P (2009) Avian invasions: the ecology and evolution of exotic birds. Oxford University Press, Oxford
Bomford M, O’Brien P (1995) Eradication or control for vertebrate pests? Wildl Soc Bull 23:249–255
Burnaford JL, Henderson SY, Pernet B (2011) Assemblage shift following population collapse of a non-indigenous bivalve in an urban lagoon. Mar Biol 158:1915–1927
Cassey P (2002) Life history and ecology influences establishment success of introduced land birds. Biol J Linnaean Soc 76:465–482
Clout MN, Veitch CR (2002) Turning the tide of biological invasion: the potential for eradicating invasive species. In: Clout MN, Veitch CR (eds) Turning the tide: the eradication of invasive species. IUCN SSC Invasive Species Specialist Group. IUCN, Gland, Switzerland and Cambridge, UK, pp 1–4
Colautti R, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Cooling M, Hartley S, Sim DA, Lester PJ (2012) The widespread collapse of an invasive species: Argentine ants (Linepithema humile) in New Zealand. Biol Lett. doi:10.1098/rsbl.2011.1014
Drake JA, Mooney HA, di Castri F, Groves RH, Kruger FJ, Rejmánek M, Williamson M (eds) (1989) Biological invasions, a global perspective. Wiley, Chichester
IUCN (World Conservation Union) (2001) IUCN Red List categories and criteria. Version 3.1. IUCN Species Survival Commission, IUCN, Gland, Switzerland and Cambridge, United Kingdom. http://www.redlist.org/info/categoriescriteria2001.html. Accessed March 2013
Lande R (1987) Extinction thresholds in demographic models of territorial populations. Am Nat 130:624–635
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Lunn D, Spiegelhalter D, Thomas A, Best N (2009) The BUGS project: evolution, critique, and future directions. Stat Med 28:3049–3067
McDonald JI, Wells FE (2010) The apparent demise of the Asian date mussel Musculista senhousia in Western Australia: or using acts of god as an eradication tool. Biol Invasions 12:715–719
Moore JW, Herbst DB, Heady WN, Carlson SM (2012) Stream community and ecosystem responses to the boom and bust of an invading snail. Biol Invasions 14:2435–2446
Myers JH, Simberloff D, Kuris AM, Carey JR (2000) Eradication revisited: dealing with exotic species. Trends Ecol Evol 15:316–320
National Audubon Society (2010) The Christmas Bird Count historical results. http://netapp.audubon.org/cbcobservation/. Accessed Nov 2011
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Pinsky ML, Jensen OP, Ricard D, Palumbi SR (2011) Unexpected patterns of fisheries collapse in the world’s oceans. PNAS 108:8317–8322
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/
Rejmánek M, Pitcairn MJ (2002) When is eradication of exotic pest plants a realistic goal? In: Clout MN, Veitch CR (eds) Turning the tide: the eradication of invasive species. IUCN SSC Invasive Species Specialist Group. IUCN, Gland, Switzerland and Cambridge, UK, pp 249–253
Sakai AM, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Evol Syst 32:305–332
Simberloff D, Gibbons L (2004) Now you see them, now you don’t!—population crashes of established introduced species. Biol Invasions 6:161–172
Strayer DL, Eviner VT, Jeschke JM, Pace ML (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21:645–651
Thomsen MS, Wernberg T, Tuya F, Silliman BR (2009) Evidence for impacts of nonindigenous macroalgae: a meta-analysis of experimental field studies. J Phycol 45:812–819
Williamson M (1996) Biological invasions. Chapman-Hall, London
Wolfe LM (2002) Why alien invaders succeed: support for the escape-from-enemy hypothesis. Am Nat 160:705–711
Zavaleta ES, Hobbs RJ, Mooney HA (2001) Viewing invasive species removal in a whole ecosystem context. Trends Ecol Evol 16:454–459
Acknowledgments
We thank E. Green, N. Fefferman, D. Simberloff, J. Burkhalter, O. Robinson, and several anonymous reviewers for their methodological advice and feedback of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aagaard, K., Lockwood, J.L. Severe and rapid population declines in exotic birds. Biol Invasions 18, 1667–1678 (2016). https://doi.org/10.1007/s10530-016-1109-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1109-2