Abstract
Among the factors limiting species distribution, low temperatures play a key role for tropical invasive species in temperate areas. Susceptibility to cold winter conditions has been recognized as the limiting factor in Europe for Tetranychus evansi, an invasive spider mite feeding on Solanaceous plants originated from tropical South America and now present on every continent except Australia. Two genetically distinct lineages of this species were introduced to Europe; one (lineage 1) is widely distributed, while the other (lineage 2) has a limited distribution. Whether this difference corresponds to differences in cold hardiness is evaluated here by assessing phenotypic response of T. evansi to the winter conditions that the mite encounters in the coldest parts of the current invaded area. We designed the thermal regimes to mimic winter conditions, including temperature fluctuations between day and night (L:D 8:16, 12:4 °C) and exposed mites to this regime for 5, 10 or 15 weeks. We tested T. evansi from three locations, one from the tropical native area (Piracicaba, Brazil) and two, corresponding to the two introduced lineages, from the temperate invaded area (lineage 1 from Nice and lineage 2 from Perpignan, France). After 5 weeks of treatment, mites from all the locations showed high survival rates but the two introduced populations grew, producing more than one offspring per female. After 10 weeks, survival rates declined for mites from Brazil and Perpignan, but not Nice. After 15 weeks, only the mites from Nice survived and produced offspring. Thus, mites belonging to the widespread lineage 1 exhibit increased cold tolerance suggesting broader adaptability, helping to explain its current geographical distribution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding environmental factors limiting species distribution is essential for predicting current and future geographical ranges of species (Kriticos and Randall 2001; Sutherst 2003). These factors are also key elements when forecasting species’ responses to climate change (Thuiller et al. 2006; Williams and Liebhold 2002). Understanding species responses to the environment is particularly relevant in temperate agricultural systems, where many of the primary pests are alien species (Kenis et al. 2007; Roy et al. 2011; Vila et al. 2010), and it is vital to predict their final distributions in their non-native range. Given the impact that alien pests can have on human food production, distribution and security (Gaston 2009) as well as on ecological systems (Roques 2012), there is intense interest in risk analysis methods (Gilioli et al. 2014; Liu et al. 2011; Venette et al. 2010) and predicting species distributions (Kriticos et al. 2013). Most approaches are based on correlative models, using data on the environmental conditions where a species has been found to define its niche, and then projecting that onto new areas [see (Elith et al. 2006) for comparisons of these models]. While useful, this approach does not model the fundamental niche, but rather the realized niche (Hutchinson 1957), i.e. the niche due to both abiotic limitations and also biotic interactions such as predation, competition and host availability, as well as dispersal capabilities (Kearney and Porter 2004). A complementary approach focuses on the characterization of the species’ fundamental niche. This requires data on the physiological tolerances of species to key environmental parameters (Kearney and Porter 2004). A challenge in gathering the data required for mechanistic prediction of species distributions is taking into account variation within species responses, given that individuals from different environments or with different evolutionary histories often have unique tolerances to environmental factors (di Lascio et al. 2011; Sexton et al. 2002). Gathering data on different origins independently is necessary in order to capture species variability and incorporate that into mechanistic prediction of distribution.
Variation in tolerance to environmental factors can have tremendous impacts on the relative success of introduced species (Park et al. 2012). Baker (1965) proposed that successful invaders would be those harbouring “general purpose genotypes”, with the phenotypic plasticity to tolerate a broad range of environments. There is support for this idea from several systems, for instance the plants Verbascum thapsus (Parker et al. 2003) and Bromus tectorum (Richards et al. 2006). Two other phenomena associated with introductions may facilitate colonization and invasion: (1) outcrossing or hybridization (Ellstrand and Schierenbeck 2000; Lee 2002; Lee and Gelembiuk 2008; Parker et al. 2003); (2) bottlenecks that can under some circumstances lead to a rapid purge of deleterious alleles via inbreeding (Parisod et al. 2005). Given the complexity of invasions, clearly all mechanisms could be important in a single invasion.
Cold injury to arthropods is both temperature- and time- dependant (Bale 1996; Colinet et al. 2007; Nedved et al. 1998) and the combination of both parameters has been taken into consideration in some species distribution models. For example, the Climex model (Sutherst and Maywald 1985; Sutherst et al. 2004) calculates cold stress accumulation and models the lethal effects when a critical threshold is met. Additionally the effects of cold winter conditions are determined not by the mean temperature, but rather by the range of temperatures that an organism experiences. Colinet et al. (2006) have demonstrated the importance of this range of temperatures, particularly the importance of maximum temperature during the day, which limits the deleterious impact of cold on fitness and survival.
Temperature regimes are likely to play a particularly large role in determining invasion success of tropical species introduced to temperate environments. Numerous alien species of tetranychids have recently established in Europe (Navajas et al. 2010), a large proportion of them originates from tropical or sub-tropical areas, making an excellent system to evaluate how temperature regimes influence the distribution and invasion success of introduced tropical species. For such tropical species, low winter temperatures appear to be crucial in limiting their distribution to more temperate regions (Migeon et al. 2009). In this study we focus on the red tomato spider mite, Tetranychus evansi Baker & Pritchard 1960, an invasive pest of Solanaceous crops distributed from South to North America, Africa, Eastern Asia and the Mediterranean basin [see (Migeon and Dorkeld 2006–2013; Navajas et al. 2013) for a complete review]. Tetranychus evansi was first reported from Brazil in 1915 (Caldas 1915; Paschoal 1971) and genetic studies show that this species originates in South America (Boubou et al. 2011, 2012). As an invasive species of tropical origin, the wide geographical area colonized by T. evansi is striking. It is unclear how it survives the winter in the coldest parts of the newly invaded area. Interestingly, unlike many spider mites, T. evansi does not diapause (Migeon 2007; Navajas et al. 2013; Ohashi et al. 2003), and thus is not able to avoid the cold by going dormant. Like other tetranychids mites, it has an arrhenotokous reproductive system: diploid females produce haploid males with unfertilized eggs (Helle and Pijnacker 1985). In this reproductive system, male haploidy allows rapid purge of deleterious alleles but also the rapid expansion of favourable ones. Mites populations often exhibit high inbreeding rate (Bailly et al. 2004; Boubou et al. 2012; Carbonnelle et al. 2007; Navajas et al. 2002). A similar phenomenon in found in clonal weeds, and that is what led Baker (Baker 1965) to hypothesize that clonal reproduction, as observed in weeds, could favour the spread of a general-purpose genotype should one exist.
Two independent introduction events, corresponding to two well-separated genetic lineages (Boubou et al. 2011, 2012), have occurred during the colonisation of the mite outside its area of origin in South America. Mites belonging to one of them, hereafter lineage 1, are the most invasive, having been recorded from almost all parts of the world where the species is established. Mites belonging to the second lineage, hereafter lineage 2, are found out of the species native area, only in limited parts of Southern Europe: in Portugal, and the Catalonia region located from Barcelona (North-Eastern Spain) to Narbonne (Southern France) (Boubou et al. 2011, 2012; Migeon et al. 2009; Navajas et al. 2013; Migeon unpub. data). When using Approximate Bayesian Computation (ABC) approaches to test alternative colonisation pathways, the most likely invasion scenario involved a first introduction of mites belonging to lineage 1 from Brazil into Africa, and from there to Europe, with a later introduction into some parts of Asia (Boubou et al. 2012). Mites from lineage 2 appear to have been independently introduced from Brazil to Portugal and from there to Catalonia. Whether the marked difference in the range sizes of the widespread lineage 1 and the more narrowly distributed lineage 2 results from the historical colonization pathways (and time since introduction) or is due to different life history traits of two types of mites making them more or less prone to colonize new environments remains unknown. We modelled the potential distribution of the two T. evansi lineages and found that mites from lineage 2 tend to occupy warmer environments with narrower annual temperature ranges than mites from lineage 1 (in both the native area and the invaded area) (Meynard et al. 2013). Whether this difference in the realized niche corresponds to different cold hardiness between the two types of mites (lineage 1 and 2) warrants investigation.
In this work, we evaluate the response of T. evansi to the winter conditions that the mite encounters in the coldest parts of the invaded area. Thermal fluctuations are the rule in nature and thus in understanding temperature tolerances meaningful experimental conditions must include an ecologically defensible thermal regime. We evaluate the impact of winter conditions, simulated in the laboratory using a fluctuating thermal regime, to test the cold tolerance of mites from both lineages introduced to Europe. We also compare whether mites from lineage 2 in Europe experienced an evolutionary shift in cold tolerance relative to mites from lineage 2 in the native range. In evaluating the role that cold hardiness has had in T. evansi invasion success and its resulting actual distribution, this study aims at understanding key traits that allow a species or a population to invade once a propagule arrives to a new geographical area, and to address Baker (1965) general-purpose genotype hypothesis.
Materials and methods
Origin of mites
Mites originating from three different locations were used to assess cold hardiness: two from France (Nice and Perpignan) and one from Brazil (Piracicaba). The French locations correspond to the northern distribution limit of the species as defined in Migeon et al. (2009), with Nice harbouring lineage 1, and Perpignan lineage 2 (Table 1) (Boubou et al. 2011, 2012). The Brazilian location corresponds to the native subtropical habitat and comprises mites of lineage 2. Table 1 reports minimal and maximal temperature of the coldest month for each location obtained from WorldClim (Hijmans et al. 2005).
Experimental design
We evaluated the winter tolerance of mites from the three locations in a two-step procedure. First we exposed mites to 5, 10, and 15 weeks of temperate winter conditions in the laboratory (experiment 1). Second, to assess recovery after cold exposure, surviving females were placed for 2 weeks in conditions of temperature that allow development (experiment 2).
Experiment 1
Experiments were conducted in a climate room set to simulate winter conditions in southern France (see Table 1) with diurnal (12 °C) and nocturnal (4 °C) temperatures and using a light cycle of L/D 8/16 h. Relative humidity was not regulated and was about 60 ± 20 % RH. Mites were reared on black night shade (Solanum nigrum), which is a frequent host for T. evansi worldwide (Migeon and Dorkeld 2006–2013; Navajas et al. 2013). Sixty potted black nightshade seedlings (about 25 days old, with 5–7 well developed leaves) were used, 20 for each of the three origins of mites. Plants were cultivated in a regulated greenhouse with additional light in 8 × 8 × 8 cm pots filled with growing substrate (Neuhauss Humin-substrat N2, Klasmann-Deilmann, Germany). The day before mites were introduced to the plants, the plants were moved to the climate room and trimmed to four leaves. Mite stock populations were reared at 25 ± 1 °C, LD 16/8 h, on detached leaves of black nightshade in 13 × 9 × 5 cm double bottom plastic boxes with a moist cotton pad and a water reservoir. To obtain cohorts of females of similar age, 2 weeks before the experiment one hundred females of each population were put on detached leaves, allowed to lay eggs for 24 h and then removed. These offspring were used in the experiment. Because tetranychid females are fertilized just after emergence by males that guard the teleiochrysalis (Cone 1985), likely all females were mated. Three day old adult females were kept in the experimental climate room for 1 day prior to the start of the experiment.
Ten females were placed on each plant (one on the smaller top leaf, three on each of the three remaining leaves) for a total of 200 females tested per origin. Five plants were dissected 5, 10 and 15 weeks after initiation of the experiment. As the mites are minute (300 µm) and the eggs are even smaller, all leaves and twigs were carefully examined under a stereomicroscope. Living and dead females were recorded. If one or more female were not found, one supplementary plant was dissected (up to two maximum). Eggs laid during the experiment were also counted. We considered spherical eggs without any distortion to be living. Eggs that were crumpled, distorted or shrivelled were recorded as dead. No immature stages (larvae or nymphs) were found. To assess egg viability and hatching rate, all the pieces of leaves bearing living eggs were kept in rearing boxes at 25 ± 1 °C, LD 16/8 h. Larvae (hatched eggs) and dead eggs were counted 1 week later.
Experiment 2
To assess the longevity and fecundity of the females after each period of cold exposure in experiment 1, living females were reared individually on small detached leaves at 25 ± 1 °C, LD 16/8 h and checked at 4, 7, 10 and 14 days. Surviving females were transferred to a new leaf after 1 week. Eggs laid during the first week were counted and hatching rate was evaluated by counting the number of newly emerged larvae.
Statistical analysis
Statistical analysis was performed using R (R Core Team 2013). We used non-parametric tests to offset the absence of normality for small and limited range sample. Kruskal–Wallis rank sum test was used and completed by post hoc pair rank comparisons when differences were significant.
Because in some cases one or more females were not found (see experimental design), we conducted two parallel analyses, one using only plants with all ten females (dead or alive) and the other using all the plants, scoring missing females as dead. As the results for both analyses were equivalent, we report here the analysis performed with all the plants together.
Modelling cold stress using Climex
Climex (Sutherst and Maywald 1985) is a mechanistic modelling software allowing the calculation of several indices describing organismal responses to environmental variables. We used Climex V2 (Sutherst et al. 2004) with parameters found by Migeon et al. (2009), i.e. 10 °C as cold stress accumulation threshold, to calculate cold stress values for the French sampling locations. Stress values are scaled from 0 to 100, with 0 corresponding to an absence of stress and 100 being lethal, even if annual temperatures allow the development of populations. Stress values are calculated monthly and added. Climate data are provided by Climex for Nice and Perpignan, locations very close to our sampling sites. Cold stress was also calculated from the experimental conditions (4 °C minimum and 12 °C maximum temperature) experienced during 1, 2 or 3 months by the mites.
Results
Experiment 1: female survival
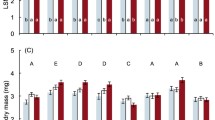
The numbers of surviving females per plant for the three time periods tested (5, 10 and 15 weeks) are reported Fig. 1 and Table 2. As expected, the longer the mites were exposed to cold, the fewer survived. Kruskal–Wallis tests show significant differences between mites of different origins, at 5 and 10 weeks but not at 15 weeks. Mites from Perpignan (lineage 2) had the highest survival at 5 weeks with 7.4 ± 1.1 females alive/plant. Mites from Nice (lineage 1) had a comparable survival—5.6 ± 1.6 females—alive/plant as mites from Piracicaba—5.4 ± 1.3 females alive/plant—(native range, lineage 2). Survival declined sharply after 10 and 15 weeks of cold exposure. Mites from Nice (lineage 1) exhibit survival rates comparable to mites from the two other origins at 5 weeks but the highest survival rate at 10 weeks with 3.5 ± 1.8 females alive/plant and even at 15 weeks with almost one female surviving per plant (0.9 ± 1.1 females alive/plant).
Boxplots showing the number of living females per plant after 5, 10 and 15 weeks of exposure to cold (experiment 1) comparing Tetranychus evansi mites of three different origins (NI Nice, PE Perpignan, and PI Piracicaba). The bold line represents median, shaded box range from first to third quartile and dashed lines 1.5 interquartile distances. Outliers are represented by isolated dots. Letters gather the same group when Kruskal–Wallis test shows significant differences: 5 weeks (H = 6.37, N = 19, d.f. = 2, *p = 0.041), 10 weeks (H = 10.18, N = 17, d.f. = 2, **p = 0.006) and 15 weeks (H = 4.54; N = 23, d.f. = 2, p = 0.103)
Experiment 1: fecundity
The numbers of eggs laid during cold exposure were low and varied with cold duration and with mite’s origin (Fig. 2a). The number of eggs laid by the females from Nice was higher than for the two other studied locations after 5 and 10 weeks of cold exposure. A reduction of the total number of eggs per plant after 15 weeks of cold exposure was observed for the mites from Perpignan and was even more marked for the mites from Nice. A growing number of eggs, or at least steady production, was expected between 10 and 15 weeks. The reduction of the total number of eggs over time could be due to the difficulty of finding dead eggs. While living eggs are relatively easy to find, dead eggs, especially old ones, can be shrunk and thus remain undetected. Alternatively, some eggs may have hatched.
Boxplots showing a the total number of eggs, b living eggs and c hatched eggs per plant after 5, 10 and 15 weeks of cold exposure (experiment 1) comparing Tetranychus evansi mites of three different origins (NI Nice, PE Perpignan and PI Piracicaba). The bold line represents median, shaded box range from first to third quartile and dashed lines 1.5 interquartile distances. Outliers are represented by isolated dots. Letters gather same group when Kruskal–Wallis test shows significant differences. a eggs total: 5 weeks (H = 14.23, N = 19, d.f. = 2, ***p < 0.001), 10 weeks (H = 12.56, N = 17, d.f. = 2, **p = 0.002) and 15 weeks (H = 1.94, N = 23, d.f. = 2, p = 0.377). b alive eggs: 5 weeks (H = 5.90, N = 17, d.f. = 2, p = 0.052), 10 weeks (H = 12.38, N = 17, d.f. = 2, **p = 0.002) and 15 weeks (H = 0.04, N = 23, d.f. = 2, p = 0.979). c hatched eggs: 5 weeks (H = 7.91, N = 19, d.f. = 2, *p = 0.019), 10 weeks (H = 12.40, N = 17, d.f. = 2, **p = 0.002) and 15 weeks (H = 4.77, N = 23, d.f. = 2, p = 0.0929)
Eggs from the mites from Piracicaba did not hatch after exposure to cold (Fig. 2b, c). A few egg from the mites from Perpignan hatched after 5 weeks (5 on 7 plants) and 10 weeks (5 on 6 plants) of cold exposure. Only mites from Nice produced eggs that hatched with any consistency, with the greatest number at 10 weeks (48 on 6 plants) and a decrease at 15 weeks (13 on 7 plants), perhaps due to the death of the eggs after the long exposure to cold. Even for the mites from Nice, hatching rates were low with a global mean of 11 % (6 plants: 48/426) and a maximum of 16 % for 2 plants (16/101 and 11/70) for eggs laid during 10 weeks of cold exposure.
Experiment 2: female survival
Female survival after cold exposure is presented in Fig. 3. Females from Nice survived better than the mites from the other two locations for each of the three lengths of cold exposure. After 5 weeks of exposure to cold, the rate of survival after 7 days at 25 °C was 22 % (12/55) for mites from Perpignan, 26 % (7/27) for mites from Piracicaba, and reaches 55 % (22/40) for mites from Nice. By 14 days, only one mite from Perpignan was alive whereas 23 % (9/40) of the mites from Nice were alive. The same pattern was observed after 10 weeks of cold exposure: the survival rate after 7 days at 25 °C was nil for mites from Piracicaba and Perpignan whereas 40 % (8/20) of the mites from Nice were alive. Even after 14 days 20 % (4/20) of the mites from Nice were alive. After 15 weeks of cold exposure, the number of females tested (females remaining alive) was so low that no general pattern could be discerned.
Experiment 2: fecundity
Fecundity of females after cold exposure and transfer at 25 °C is reported Table 2 and Fig. 4. Whereas 60 % (24/40) of the females from Nice laid eggs after 5 weeks of cold exposure, only 18 % (10/55) of the mites from Perpignan and 15 % (4/27) of mites from Piracicaba did. Significant differences between mites from Nice and the two other origins could be detected after 5 weeks of cold exposure, both in the number of eggs laid and in the number of hatched eggs per female. After 10 and 15 weeks of cold exposure, as only females from Nice laid eggs, no further analysis was performed. The hatching rate of eggs laid by the females from Nice decreased from 94 % (251/266) after 5 weeks of cold exposure to 81 % (110/135) after 10 weeks and to 20 % (1/5) after 15 weeks.
Boxplots showing a the total number of eggs laid per female and b the hatched eggs during 1 week at 25 °C by the surviving females that experienced 5, 10 and 15 weeks of cold exposure (experiment 2) comparing Tetranychus evansi of three different origins (NI Nice, PE Perpignan, and PI Piracicaba). The bold line represents median, shaded box range from first to third quartile and dashed lines 1.5 interquartile distances. Outliers are represented by isolated dots. Letters gather same group when Kruskal–Wallis test shows significant differences. a eggs total: 5 weeks (Kruskal–Wallis test: H = 22.71, N = 122, d.f. = 2, ***p < 0.0001). b hatched eggs: 5 weeks (Kruskal–Wallis test: H = 24.22, N = 122, d.f. = 2, ***p < 0.0001). No test was performed after 10 and 15 weeks of cold exposure
The contribution of each female to the next generation is reported Table 2 (larvae/female). After 5 weeks of cold exposure, only mites from Perpignan and Nice gave rise to more than 1 larva per female (1.5 and 3.6 respectively). After 10 weeks only mites from Nice had more than 1 offspring (2.6 larvae/female). After 15 weeks, females from Perpignan and Piracicaba produce no offspring and females from Nice produced only 0.2 larvae/female on average.
Climex cold stress values
Cold stress values calculated for Nice and Perpignan locations are respectively 32 and 57. Accordingly the values calculated with laboratory conditions are 11, 28 and 53, respectively for 1, 2 and 3 months.
Discussion
The experimental conditions here used correspond to the expected winter duration in the two studied localities. The cold stress values calculated with Climex illustrate the accumulation of cold injury during the cold season in the two French localities in laboratory conditions. As the climatic conditions experienced by the mites during this study are very close to the natural conditions observed in the sampling locations, thus we were able to realistically simulate winter conditions in the Northern margin of the current distribution of T. evansi. The problems caused by cold include an increase of female mortality during the winter, a decrease in egg viability and a decline in the ability of females to recover after cold. These occurred in the mites from each of the tested origins with the mites from Nice best able to recover from cold.
The lower development temperature threshold for T. evansi has been calculated at 12.1 °C and the optimal temperature at 37.9 °C (Navajas et al. 2013). Therefore, the winter temperatures recorded in the two French localities where the mites were collected for this study, between 3 and 5 °C (minimum) and 11–13 °C (maximum) for a period of 8–12 weeks in Nice and Perpignan respectively, do not allow mite population growth but only survival. A decline in the number of living females was observed in all the situations tested regardless the origin of the mites, which coincided with a decline in recovery after cold exposure and a decline of egg hatching rates. In a study conducted on the same species in Japan during winter, near Kyoto with same climatic conditions as in the French localities studied here, survival of eggs collected in the field decreased from 70 % in December to 30 % in February and <10 % in April (Ohashi et al. 2003). The authors also estimated cold resistance by storing individuals collected in the field at 5 and 0 °C during 1 week to 1 month. Good survival rates were observed for all stages at both temperatures for 1 week (95 to 80 % survival rate), but none of the individuals survived after 1 month. The results are particularly relevant when assessing the potential distribution of tropical species in temperate regions as a steadily increasing proportion of the alien invasive mites have a tropical origin, especially in the Mediterranean Europe (Navajas et al. 2010, 2013). Clearly the ability of tropical arthropods to colonize and settle in temperate regions is due to a complex set of reasons. This study shed some light on the impact of winter conditions on a non-diapausing tropical species as T. evansi when it moves to temperate areas. The high mortality observed during the experiments miming local winter conditions, is offset by a high intrinsic rate of increase of this mite (Bonato 1999; Gotoh et al. 2010; Moraes and McMurtry 1985). This high rate of increase allows mite populations to reach very high densities in late summer, which may be necessary for the species to persist (Migeon et al. 2009). Our results show that the length of the cold is critical. Females are able to recover from 5 weeks of cold and even to lay viable eggs (Table 2). Short winter conditions, as may happen with global warming, could increase the number of viable females in spring. This would enable a more rapid increase of mite densities and consequently an increase of agricultural impact of pest which previously had been considered of minor importance.
The two distinct lineages of T. evansi studied here exhibit different life history traits. Although the link between neutral markers, as microsatellites, and phenotypic traits is often difficult to make (Hufbauer 2004), neutral markers are a powerful means of delineate populations or taxa. Here we showed that mites from one lineage (lineage 1), represented by the Nice sample, perform better when they are challenged to cold conditions than the mites from lineage 2, exhibiting: (1) generally highest survival of females exposed to cold (except for 5 weeks of cold exposure); (2) highest fecundity during cold exposure; (3) best recovery and survival after cold exposure; (4) highest fecundity after cold exposure; (5) highest global production of offspring. When assessing global fitness in parameters as fecundity or rate of development, Gotoh et al. (2010) did not noticed any difference between the two major lineages previously recognised (Boubou et al. 2011, 2012).
The best performing mites (Nice) also belong to the wider spread and the most invasive lineage. Likewise mites belonging to this most invasive lineage (lineage 1) also exhibit the highest tolerance to temperature range with lower minimal temperatures and higher maximal temperatures when modelling its potential distribution (Meynard et al. 2013). Such plasticity in phenotypic traits is hypothesized to be an important characteristic for invasive species (Baker 1965; Chen et al. 2006; Valiente et al. 2010). The results obtained here confirm the phenotypic differences previously observed (Meynard et al. 2013). We also observe greater cold hardiness in the mites collected in the invaded area than in those originating from the native area, a trend observed in other invasive species (Blair et al. 2012), suggesting a shift in cold hardiness during the invasion process.
In the case of T. evansi the invasive lineage also displays a wider host plant range (Navajas et al. 2013). Together, these data fit well with the occurrence records and support the modelling results, giving evidence for greater invasive potential of the wider spread of mites of T. evansi lineage 1. It is now clear from well-supported genetic studies on the pattern and history of the T. evansi invasion (Boubou et al. 2012), that at least three different colonization events have taken place since the middle of the last century. Interestingly, not all of the newly introduced mites gave rise to a global invasion. These results are of great interest as they are in line with Baker’s assertion (1965) linking invasiveness to clonal reproduction and physiological plasticity. Parker et al. (2003) described two different pathways leading to a widespread invasion. Besides the general-purpose genotype, the other pathway is characterized by rapid adaptation, which is favoured by an open breeding system and outcrossing, coupled to multiple independent introductions facilitating gene flow and genetic diversity. Boubou et al. (2011, 2012) have highlighted that the multiple introductions of T evansi that occurred in Europe were followed by hybridization events that could have favoured rapid adaptation and invasion, but none of these hybrids has shown invasive potential.
The red tomato spider mite supports the idea that invasiveness can be linked to having a general purpose genotype, and it also demonstrates the importance of the link between reproductive system and characteristics that may give rise to invasion. Tetranychus evansi has a reproductive system, characterized by arrhenotokous parthenogenesis, which leads to strong founder effects and low genetic variability (Boubou et al. 2012). Despite its low genetic variability it has become widespread, supporting the idea (Baker 1965) that a generalist genotype tolerant to a wide range of environments can successfully invade.
References
Bailly X, Migeon A, Navajas M (2004) Analysis of microsatellite variation in the spider mite pest Tetranychus turkestani (Acari: Tetranychidae) reveals population genetic structure and raises questions about related ecological factors. Biol J Linn Soc 82:69–78
Baker HG (1965) Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL (eds) The genetics of colonizing species, Asilomar, California, USA, 1965. Academic Press, New York, pp 147–172
Bale JS (1996) Insect cold hardiness: a matter of life and death. Eur J Entomol 93:369–382
Blair AC, Blumenthal D, Hufbauer RA (2012) Hybridization and invasion: an experimental test with diffuse knapweed (Centaurea diffusa Lam.). Evol Appl 5:17–28. doi:10.1111/j.1752-4571.2011.00203.x
Bonato O (1999) The effect of temperature on life history parameters of Tetranychus evansi (Acari: Tetranychidae). Exp Appl Acarol 23:11–19
Boubou A, Migeon A, Roderick G, Navajas M (2011) Recent emergence and worldwide spread of the red tomato spider mite, Tetranychus evansi: genetic variation and multiple cryptic invasions. Biol Invasions 13:81–92. doi:10.1007/s10530-010-9791-y
Boubou A, Migeon A, Roderick GK, Auger P, Cornuet J-M, Magalhães S, Navajas M (2012) Test of colonisation scenarios reveals complex invasion history of the red tomato spider mite Tetranychus evansi. PLoS ONE 7:e35601. doi:10.1371/journal.pone.0035601
Caldas D (1915) Um acarino parasita de batata. Chacaras e Quintais 12:434
Carbonnelle S, Hance T, Migeon A, Baret P, Cros-Arteil S, Navajas M (2007) Microsatellite markers reveal spatial genetic structure of Tetranychus urticae (Acari: Tetranychidae) populations along a latitudinal gradient in Europe. Exp Appl Acarol 41:225–241
Chen YH, Opp SB, Berlocher SH, Roderick GK (2006) Are bottlenecks associated with colonization? Genetic diversity and diapause variation of native and introduced Rhagoletis completa populations. Oecologia 149:656–667. doi:10.1007/s00442-006-0482-4
Colinet H, Renault D, Hance T, Vernon P (2006) The impact of fluctuating thermal regimes on the survival of a cold-exposed parasitic wasp, Aphidius colemani. Physiol Entomol 31:234–240. doi:10.1111/j.1365-3032.2006.00511.x
Colinet H, Hance T, Vernon P, Bouchereau A, Renault D (2007) Does fluctuating thermal regime trigger free amino acid production in the parasitic wasp Aphidius colemani (Hymenoptera : Aphidiinae)? Comp Biochem Physiol A: Mol Integr Physiol 147:484–492. doi:10.1016/j.cbpa.2007.01.030
Cone WW (1985) Mating and chemical communication. In: Helle W, Sabelis MW (eds) Spider mites. Their biology, natural enemies and control, vol 1A. Elsevier, Amsterdam, pp 243–251
di Lascio A, Rossi L, Costantini ML (2011) Different temperature tolerance of northern and southern European populations of a freshwater Isopod Crustacean species (Asellus aquaticus L.). Fundam Appl Limnol 179:193–201. doi:10.1127/1863-9135/2011/0179-0193
Elith J et al (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29:129–151
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci 97:7043–7050. doi:10.1073/pnas.97.13.7043
Gaston KJ (2009) Geographic range limits of species. Proc R Soc B Biol Sci 276:1391–1393. doi:10.1098/rspb.2009.0100
Gilioli G et al (2014) Environmental risk assessment for plant pests: a procedure to evaluate their impacts on ecosystem services. Sci Total Environ 468–469:475–486. doi:10.1016/j.scitotenv.2013.08.068
Gotoh T et al (2010) Reproductive performance of seven strains of the tomato red spider mite Tetranychus evansi (Acari: Tetranychidae) at five temperatures. Exp Appl Acarol 52:239–259
Helle W, Pijnacker LP (1985) Parthenogeneis, chromosomes and sex. In: Helle W, Sabelis MW (eds) Spider mites their biology, natural enemies and control, vol 1A., World Crop PestsElsevier Science Publisher B. V, Amsterdam, pp 129–139
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hufbauer RA (2004) Population genetics of invasions: can we link neutral markers to management? Weed Technol 18:1522–1527. doi:10.1614/0890-037x(2004)018[1522:pgoicw]2.0.co;2
Hutchinson GE (1957) Concluding remarks. In: Cold Spring Harbour Symposium on Quantitative Biology, vol 22. pp 415–427. doi:10.1101/SQB.1957.022.01.039
Kearney M, Porter WP (2004) Mapping the fundamental niche: physiology, climate, and the distribution of a nocturnal lizard. Ecology 85:3119–3131. doi:10.1890/03-0820
Kenis M, Rabitsch W, Auger-Rozenberg MA, Roques A (2007) How can alien species inventories and interception data help us prevent insect invasions? Bull Entomol Res 97:489–502. doi:10.1017/s0007485307005184
Kriticos DJ, Randall RP (2001) A comparison of systems to analyze potential weed distributions. In: Groves RH, Panetta FD, Virtue JG (eds) Weed risk assessment. pp 61–79
Kriticos DJ, Le Maitre DC, Webber BL (2013) Essential elements of discourse for advancing the modelling of species’ current and potential distributions. J Biogeogr 40:608–611. doi:10.1111/j.1365-2699.2012.02791.x
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391. doi:10.1016/s0169-5347(02)02554-5
Lee CE, Gelembiuk GW (2008) Evolutionary origins of invasive populations. Evol Appl 1:427–448. doi:10.1111/j.1752-4571.2008.00039.x
Liu S, Sheppard A, Kriticos D, Cook D (2011) Incorporating uncertainty and social values in managing invasive alien species: a deliberative multi-criteria evaluation approach. Biol Invasions 13:2323–2337. doi:10.1007/s10530-011-0045-4
Meynard CN, Migeon A, Navajas M (2013) Uncertainties in predicting species distributions under climate change: a case study using Tetranychus evansi (Acari: Tetranychidae), a widespread agricultural pest. PLoS ONE 8:e66445. doi:10.1371/journal.pone.0066445
Migeon A (2007) Acarien rouge de la tomate: nouvelles observations et perspectives. PHM Revue Horticole 488:20–24
Migeon A, Dorkeld F (2006–2013) Spider mites web: a comprehensive database for the Tetranychidae. INRA. http://www.montpellier.inra.fr/CBGP/spmweb. Accessed 2014-06-25 2014
Migeon A et al (2009) Modelling the potential distribution of the invasive tomato red spider mite, Tetranychus evansi (Acari: Tetranychidae). Exp Appl Acarol 48:199–212
Moraes GJ de, McMurtry JA (1985) Comparison of Tetranychus evansi and T. urticae (Acari: Tetranychidae) as prey for eight species of phytoseiid mites. Entomophaga 30:393–397
Navajas M, Perrot-Minnot MJ, Lagnel J, Migeon A, Bourse T, Cornuet JM (2002) Genetic structure of a greenhouse population of the spider mite Tetranychus urticae: spatio-temporal analysis with microsatellite markers. Insect Mol Biol 11:157–165
Navajas M, Migeon A, Estrada-Pena A, Mailleux AC, Servigne P, Petanovic R (2010) Mites and ticks (Acari). BIORISK Biodivers Ecosyst Risk Assess 4:149–192
Navajas M, Moraes GJ de, Auger P, Migeon A (2013) Review of the invasion of Tetranychus evansi: biology, colonization pathways, potential expansion and prospects for biological control. Exp Appl Acarol 59:43–65. doi:10.1007/s10493-012-9590-5
Nedved O, Lavy D, Verhoef HA (1998) Modelling the time-temperature relationship in cold injury and effect of high-temperature interruptions on survival in a chill-sensitive collembolan. Funct Ecol 12:816–824. doi:10.1046/j.1365-2435.1998.00250.x
Ohashi K, Kotsubo Y, Takafuji A (2003) Distribution and overwintering ecology of Tetranychus takafujii (Acari: Tetranychidae), a species found from Kinki district, Japan. J Acarol Soc Jpn 12:107–113
Parisod C, Trippi C, Gallad N (2005) Genetic variability and founder effect in the pitcher plant Sarracenia purpurea (Sarraceniaceae) in populations introduced into Switzerland: from inbreeding to invasion. Ann Bot 95:277–286. doi:10.1093/aob/mci023
Park I, DeWalt SJ, Siemann E, Rogers WE (2012) Differences in cold hardiness between introduced populations of an invasive tree. Biol Invasions 14:2029–2038. doi:10.1007/s10530-012-0209-x
Parker IM, Rodriguez J, Loik ME (2003) An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Conserv Biol 17:59–72. doi:10.1046/j.1523-1739.2003.02019.x
Paschoal AD (1971) O complexo Tetranychus telarius no Brasil (Acarina : Tetranychidae). Revista de Agricultura 46:3–8
R Core Team (2013) R: A language and environment for statistical computing. 3.0 edn. R Foundation for Statistical Computing, Vienna, Austria
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981–993. doi:10.1111/j.1461-0248.2006.00950.x
Roques A (2012) Biological invasion. Integr Zool 7:227. doi:10.1111/j.1749-4877.2012.00311.x
Roy HE, Roy DB, Roques A (2011) Inventory of terrestrial alien arthropod predators and parasites established in Europe. Biocontrol 56:477–504. doi:10.1007/s10526-011-9355-9
Sexton JP, McKay JK, Sala A (2002) Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol Appl 12:1652–1660. doi:10.2307/3099929
Sutherst RW (2003) Prediction of species geographical ranges. J Biogeogr 30:805–816
Sutherst RW, Maywald GF (1985) A computerised system for matching climates in ecology. Agric Ecosyst Environ 13:281–289
Sutherst RW, Maywald GF, Bottomley W, Bourne A (2004) Climex V2, user guide. CSIRO Publishing, Collingwood
Thuiller W, Lavorel S, Sykes MT, Araujo MB (2006) Using niche-based modelling to assess the impact of climate change on tree functional diversity in Europe. Divers Distrib 12:49–60
Valiente AG, Juanes F, Nuñez P, Garcia-Vazquez E (2010) Brown trout (Salmo trutta) invasiveness: plasticity in life-history is more important than genetic variability. Biol Invasions 12:451–462. doi:10.1007/s10530-009-9450-3
Venette RC et al (2010) Pest risk maps for invasive alien species: A roadmap for improvement. Bioscience 60:349–362. doi:10.1525/bio.2010.60.5.5
Vila M et al (2010) How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front Ecol Environ 8:135–144. doi:10.1890/080083
Williams DW, Liebhold AM (2002) Climate change and the outbreak ranges of two North American bark beetles. Agric For Entomol 4:87–99
Acknowledgments
Funding was provided by the French Agence Nationale de la Recherche (ANR 2010 BLAN 1715 02). This project operated as cofounding for the GI-046 grant from Genome Canada and the Ontario Genomics Institute and the GL2-01-035 grant from the Ontario Research Fund–Global Leadership in Genomics and Life Sciences. RAH acknowledges the support of the United States Department of Agriculture through the Colorado Experiment Station, Fulbright-France, as well as NSF RCN 0541673.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Migeon, A., Auger, P., Hufbauer, R. et al. Genetic traits leading to invasion: plasticity in cold hardiness explains current distribution of an invasive agricultural pest, Tetranychus evansi (Acari: Tetranychidae). Biol Invasions 17, 2275–2285 (2015). https://doi.org/10.1007/s10530-015-0873-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-0873-8