Abstract
One of the major goals of invasion biology is predicting the effects of invaders on native species, which is often accomplished by linearly scaling-up per-capita effects with invader abundance. However, the relationship between invader density and their ecological impact is poorly understood, and it is likely that the effects of invasive species scale non-linearly with increasing invader density. The Pacific red lionfish (Pterois volitans) is an invasive predator that has reached high abundances throughout the tropical and sub-tropical western Atlantic, Caribbean, and Gulf of Mexico. A single lionfish can have extremely large effects on native reef-fish communities, yet the relationship between lionfish density and their ecological effects is not well known. I manipulated juvenile lionfish densities on small patch reefs in the Bahamas and monitored the subsequent effects on native coral-reef fish abundance, biomass, richness, evenness, composite diversity, and community structure over 7 weeks. Native fish abundance and biomass decreased non-linearly with increasing lionfish density, with the largest drops in abundance and biomass at low lionfish densities and their effects leveling off at high densities. Lionfish density also significantly affected richness and community structure, but these effects could not be clearly classified as either linear or non-linear. Evenness and composite diversity were not significantly affected by lionfish density. Given that the effects of lionfish on native fish abundance and biomass level-off at high lionfish densities, it appears important to remove all lionfish from small patch reefs to have the biggest influence on conserving the native community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive predators can cause severe declines in the abundance and diversity of native species that far exceed the effects of native predators (Vitousek et al. 1997; Salo et al. 2007; Davis 2009). Therefore, understanding how different introduced predators affect specific populations and communities of native species is an important goal in invasion biology, basic ecology, and conservation biology (Parker et al. 1999). To predict an invader’s impact, whether be it on individuals, populations, communities, and/or ecosystems, most studies determine its per-capita effect and then scale-up this effect linearly based on the invader’s density (Parker et al. 1999). However, the assumption that per-capita effects scale linearly with invader density has not been tested extensively and does not account for potential non-linearities in the effects of invasive species at different densities (Thiele et al. 2009).
Intraspecific interactions can cause the per-capita effect of invasive predators to scale non-linearly with increasing invader density. When there is a non-linear relationship between invasive predator density and its impact (on native prey abundance, richness, evenness, diversity, or community structure), then linearly scaling-up its per-capita effect would either overestimate or underestimate the impacts of the invader at high densities. Overestimation would occur if the overall impacts of an invader level-off at high invader densities (e.g. Byrnes and Witman 2003; Gherardi and Acquistapace 2007; Griffen and Byers 2008; Matsuzaki et al. 2009). Potential mechanisms causing this overestimation include depletion of food and/or competition that limits an invasive predator’s consumption rate at high densities. Alternatively, underestimation of invader impact would occur when an invasive species has disproportionately large effects at high compared to low invader densities (e.g. Williams et al. 2002; Keller and Lake 2007). This underestimation may occur if an introduced predator has a higher per-capita consumption rate when foraging in groups due to strategies that include cooperative hunting.
Given that increasing densities of invasive predators may alter native communities in several different ways, more experimental studies examining impacts of a broader range of densities are needed to accurately predict the effects of specific invaders. However, few studies have quantified whether different densities of invaders have linear or non-linear effects on native populations and ecosystems (Yokomizo et al. 2009; Kulhanek et al. 2011; Ricciardi et al. 2013). For example, in a recent meta-analysis, Thomsen et al. (2011) found 900 papers that studied the impacts of invaders on marine and freshwater ecosystems, yet only 39 of these studies manipulated invader abundance. Even those studies that accounted for invader density rarely tested more than three different abundances (Thomsen et al. 2011). Such information on the relationship between invader abundance and its ecological effects is particularly important in controlling invasive species, as understanding the shape of an invader’s impact curve can help managers prioritize resources and maximize the effectiveness of management plans (Byers et al. 2002; Yokomizo et al. 2009).
The Pacific red lionfish (Pterois volitans) is an invasive predator that is a top global conservation issue (Sutherland et al. 2010), and therefore is being extensively managed (Morris 2012). Lionfish are the first non-native marine fish to become invasive throughout the greater tropical and subtropical western Atlantic region (Schofield 2009). Originally recorded off the coast of Florida in the 1980s, lionfish populations have been spreading rapidly since the early 2000s (Schofield 2010). Accompanying their rapid range expansion are exponential population growth rates (Albins and Hixon 2013), with lionfish in their invaded range reaching densities of approximately 400 fish/ha, compared to just 26.3 fish/ha in their native range (Green and Côté 2008; Kulbicki et al. 2012). The current method of controlling lionfish populations is through manual removal, which is a labor-intensive process and is unlikely to fully eradicate them (Barbour et al. 2011). Due to the high effort associated with lionfish removal but the limited resources available, directing efforts to most effectively mitigate the effects of lionfish is essential (Morris 2012; Green et al. 2014).
Invasive lionfish have the potential to have severe impacts on already stressed coral-reef systems (Albins and Hixon 2013). Of particular concern is the fact that lionfish are voracious predators of a variety of coral-reef fishes, including commercially and ecologically important species (Albins and Hixon 2008; Morris and Akins 2009; Muñoz et al. 2011; Cure et al. 2012; Côté et al. 2013). Lionfish are unlike other predators in both their appearance and hunting behavior (Allen and Eschmeyer 1973; Albins and Lyons 2012; Green et al. 2011; Cure et al. 2012), and consequently consume native prey fish at extremely high rates (Albins and Hixon 2008; Côté and Maljivoic 2010; Green et al. 2011). A single lionfish on a 1–4 m2 patch reef can reduce the abundance of small [≤50 mm total length (TL)] native fishes by 80–90 % in as few as 5 weeks (Albins and Hixon 2008; Albins 2013). The effect of invasive lionfish on native fish abundance is over 2.5 times that of an ecologically similar native grouper (Cephalopholis fulva), and is accompanied by a reduction in species richness on patch reefs with lionfish compared to ones with only that native predator (Albins 2013). On a larger temporal and spatial scale, increases in lionfish abundance have coincided with declines of native fishes on large natural reefs over several years (Green et al. 2012).
Despite our growing knowledge of the lionfish invasion, we still do not know how the effects of lionfish on native fish communities vary over a range of lionfish densities. Thus far, field studies of the effects of lionfish on native fishes have primarily been presence/absence experiments (e.g. Albins and Hixon 2008; Albins 2013) or observational studies (e.g. Green et al. 2012). The only study to date to evaluate the response of native fish to various lionfish numbers modeled and tested the effect of removal effort on native prey production on small patch reefs (Green et al. 2014). While this study demonstrated that suppression of lionfish below modeled biomass thresholds can prevent declines in native fish biomass (Green et al. 2014), it focused on site-specific reductions in lionfish biomass, not density per se, and did not evaluate the linearity of lionfish effects.
It is likely that the effects of lionfish will not simply scale-up linearly with increasing density. There is evidence for intraspecific competition among lionfish, as they experience slower growth rates at higher densities (Benkwitt 2013), which would lead to disproportionately smaller effects on native prey populations at higher densities. However, cooperative hunting by some species of lionfish has been documented in their native range (Kendall 1990; Lönnstedt et al. 2014) and anecdotally observed in their invaded range (personal observation). If invasive lionfish hunt cooperatively only at higher densities, and cooperative hunters are more successful than solitary hunters, then they may have accelerating impacts on native fishes as their densities increase.
In this study, I address how increasing invasive lionfish densities affect native coral-reef fishes. Specifically, I conducted a manipulative field experiment in which I adjusted lionfish densities and quantified their effects on native fish abundance and biomass, and species richness, evenness, composite diversity, and composition. I show that some metrics scale non-linearly with increasing lionfish density, with the effects of lionfish leveling off at high densities.
Methods
Field experiment
I conducted this 7-week study from June to August 2011 near Lee Stocking Island, Bahamas, using a matrix of artificial patch reefs (23°45′19.69′′, −76°8′46.50′′) (Online Resource 1). These reefs were constructed in 1991–1992 and are located on a shallow (<4 m deep) sand and seagrass flat (Carr and Hixon 1997). Each reef measures approximately 1 m3 and is separated from its nearest neighbor by 200 m and from the nearest continuous reef by at least 1 km (Hixon and Carr 1997). Over the two decades since construction, the reefs have become essentially natural features, supporting benthic communities of sponges, corals, and seaweeds that completely cover all reefs, as well as over 70 species of fish (Carr and Hixon 1995, 1997; Hixon and Carr 1997). These reefs have been used successfully in a variety of other manipulative experiments (e.g. Carr and Hixon 1997; Hixon and Carr 1997), including studies on invasive lionfish (Albins and Hixon 2008; Pusack 2013; Benkwitt 2013).
To examine the effects of lionfish density on prey-sized native fishes, I adjusted densities of juvenile lionfish on 10 of the artificial reefs, ranging from 0 to 12 lionfish/m2 (Online Resource 2). The highest natural lionfish density observed on these reefs was 8 lionfish/m2, thus the experimental range extended to 150 % the natural maximum. Four reefs were used as 0-lionfish control reefs and there was one reef per remaining lionfish treatment (1, 2, 4, 7, 10, and 12 lionfish/m2). This experimental design was chosen to maximize the range of experimental densities and was preferred over having fewer treatments with more replicates given that regression designs have been called to be used more in ecological experiments, in part because they have more power than ANOVA designs (Cottingham et al. 2005). In addition, this experimental design enables one to determine the relationship between invader abundance and its impact, including important parameters such as the shape, intercept, and slope, rather than merely determining whether or not a relationship exists (Thomsen et al. 2011). Replicates were used for the control reefs to obtain an accurate estimate of recruitment and variability among reefs with no lionfish present. Previous studies using these same reefs have found that there is less variability in native prey abundance on reefs with lionfish present compared to control reefs, likely due to the large negative effect of even a single lionfish on native fish recruits (Albins and Hixon 2008).
Because initial fish surveys revealed no significant differences in native fish communities among reefs (see “Statistical analyses” below), I assigned lionfish density treatments to reefs using constrained randomization to ensure that similar densities were not clustered spatially. Using SCUBA and handnets, I collected lionfish ranging in initial size from 40 to 71 mm TL from nearby reefs. There was no difference in initial body size among lionfish treatments (linear regression t = 0.839, p = 0.41; Online Resource 2). Each lionfish was given a unique elastomer tag (Northwest Marine Technology Inc., Shaw Island, WA, USA; Frederick 1997) to differentiate between lionfish at the start of the experiment and any new immigrants over the course of the study and to monitor demographic rates as part of another study (Benkwitt 2013). Treatments were started on all reefs within a 2-week period. To maintain treatments, I monitored lionfish density during weekly visits and removed any new lionfish recruits (total of 15 throughout experiment) and immigrants (total of 5 throughout experiment). In addition, I removed resident native piscivores and standardized the number of Nassau grouper (Epinephelus striatus) and territorial damselfishes (Stegastes spp.) weekly to mitigate any confounding effects of these strong interactors on fish recruitment (Almany 2003).
Of the lionfish initially placed on the reefs, only 6 out of 40 disappeared. To account for the small changes in lionfish density throughout the experiment, I averaged the weekly lionfish densities on each reef over the course of the experiment and used these average densities in all analyses.
Following the establishment of lionfish density treatments, a pair of divers using SCUBA censused the entire fish community on each reef weekly for 7 weeks, recording the species, abundance, and body size (TL estimated to the nearest centimeter) of all fish present both on the reefs and within a 1-m radius around the reefs. Divers slowly approached the reefs and first counted all planktivorous and active species hovering above the reefs from a distance of approximately 3 m. From a distance of 1 m, the divers slowly circled the reefs and counted all other species, using dive lights to count cryptic species in holes (Hixon and Beets 1989, 1993). Because the study was conducted during the summer recruitment period, the null expectation was that the abundance, biomass, and richness of small fishes would increase during the experiment.
Statistical analyses
I focused my analyses on native fishes <50 mm TL, which were small enough to be vulnerable to lionfish predation throughout the experiment. Lionfish can consume prey up to about 2/3 of their body size, but the average prey size is approximately 20–25 mm, even for lionfish larger than the ones used in this study (Albins and Hixon 2008; Morris and Akins 2009).
I calculated the proportional change in abundance, biomass, species richness, species evenness, and composite species diversity on each reef over time. For example, proportional change in abundance was calculated as:
where N ti is the proportional change in native fish abundance at week t for reef i, n tij is the abundance of species j on reef i at week t, n1ij is the abundance of species j on reef i at week 1, and s is the total number of species present on the reef. Therefore, on each reef N ti is >1 when there was an increase in abundance, =1 when there was no change in abundance, and <1 when there was a decrease in abundance relative to the initial census. For all analyses, I used the proportional change in each metric (abundance, biomass, species richness, species evenness, or composite species diversity) at the final censuses (t = 7) compared to the initial censuses (t = 1).
The biomass (B) of each native fish was calculated using the formula:
where a and b are species-specific constants obtained from the primary literature by Albins (2011), and L is the length of the individual fish. Proportional change in biomass was then calculated for each reef over time as for abundance above.
Species richness was the total number of species present on each reef. I used Pielou’s J as the species evenness index (Pielou 1966) and the Shannon–Wiener index (H’) for composite species diversity, which combines richness and evenness (Shannon and Weaver 1949). These indices were chosen to facilitate comparisons between this study and the only other published study on the effects of lionfish on native species evenness and diversity (Albins 2013). I also used Simpson’s index (1-D) (Simpson 1949) to compare a composite species diversity index that is less sensitive to variation in species richness with the Shannon–Wiener index, which is more sensitive (Magurran 2004).
For each metric, I compared the fit of a null intercept-only, linear, and non-linear (exponential or negative exponential) model with lionfish density as the explanatory variable. For analyses of species evenness and composite diversity, I excluded the 12-lionfish treatment because there was only one individual native fish remaining at the end of the experiment. The model with the lowest Akaike information criterion corrected for small sample size (AICc; Burnham and Anderson 2002) was selected as the best model. If the difference in AICc between the model with the lowest AICc and another model (∆AICc) was <2, then there was no evidence to select one model over the other (Burnham and Anderson 2002). Using the best-fit model, I then calculated the estimated change in each metric for each additional lionfish on a reef. Based on a linear model, this estimate was equal to the slope parameter. For an exponential model, the estimated percent change was equal to e slope. Due to the seemingly large influence of control reefs on the shapes of the curves for native fish abundance and biomass, I also repeated the analyses for these responses with the control reefs excluded. Visual analysis of plots and formal tests showed no departures from normality or homoscedascity in the best fit models.
To determine the effect of lionfish density on the final native fish community structure (species composition and relative abundances), I conducted an ordination of reefs in species space using non-metric multidimensional scaling (NMDS, Kruskal 1964; Mather 1976; McCune and Grace 2002). I log(x + 1) transformed species abundance data to help reduce the influence of the most abundant species. After this transformation, there were no extreme outliers and I did not perform any other transformations or modifications. I used Bray–Curtis distance and a random starting configuration, performing 100 runs with real data. I also included a Monte–Carlo (randomization) test to determine whether the extracted axes were stronger than expected by chance (McCune and Grace 2002). Because I was interested in the effect of lionfish density on community structure, all ordinations were rotated to maximize the positive correlation between lionfish density and axis 1, and I overlaid lionfish density on the NMDS plot. I then extracted the site scores and compared the fits of intercept-only, linear, and non-linear models using lionfish density as the explanatory variable and NMDS axis 1 score as the response variable as described above. There was no difference in native fish communities among treatments at the start of the experiment, as for all metrics (abundance, biomass, richness, evenness, composite diversity, and community structure, in terms of species composition and relative abundances as ordinated by NMDS) the null intercept-only model was a better fit than any other model (all ∆AICc > 3.73).
All statistical analyses were conducted in R version 3.0 (R Development Core Team 2013), with associated packages AICcmodavg (Mazerolle 2013), nlstools (Baty and Delignette-Muller 2013), and vegan (Oksanen et al. 2013).
Results
Native fish abundance and biomass
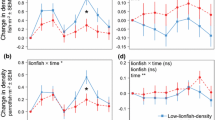
Over the course of the 7-week experiment, the abundance of prey-sized native fishes nearly tripled on the lionfish-free control reefs, increased by approximately 18 % on the reef with one lionfish, and decreased on all reefs with two or more lionfish (Fig. 1a). On the reef with the highest lionfish density, the abundance of native fishes decreased by 97 %, with only a single recruit goldspot goby (Gnatholepis thompsoni) remaining at the end of the experiment. By the final census, the abundance of small fishes decreased non-linearly with increasing lionfish density on each reef (Fig. 1b). The decline followed a negative exponential curve, with an estimated 49.2 % reduction in the proportional abundance of prey-sized fishes for each additional lionfish on a reef (95 % CI 27.8–86.9; Table 1).
Proportional change in abundance of prey-sized native fishes (0–50 mm TL) on patch reefs with manipulated lionfish densities (0–12 lionfish/m2). a Weekly over the 7-week experiment and b at the final census only. Curve shows negative exponential model (best fit model based on ∆AICc). Points for 0-lionfish control treatment represent mean ± SEM. Sample size (# of reefs) equals 4 for the 0-lionfish treatment and 1 for all other treatments
When lionfish-free control reefs were excluded from the analysis, there was no evidence to choose among the intercept-only, linear, and non-linear models as the best fit (∆AICc < 1.31). However, in both the linear and negative exponential models there was a significant relationship between lionfish density and native fish abundance. Based on a linear model, for each additional lionfish on a reef the proportional abundance of native fish decreased by 0.08 (95 % CI 0.06–0.10, t = −3.773, p = 0.02). Based on an exponential model, for each additional lionfish on a reef there was an estimated 85.2 % reduction in proportional abundance (95 % CI 80.2–90.6, t = 2.611, p = 0.059).
The species that had the largest increase in abundance on lionfish-free control reefs and the largest decrease in abundance on all lionfish reefs was the bridled goby (Coryphopterus glaucofreanum) (Online Resource 3). On control reefs, 18 out of 31 species increased in abundance between the beginning and the end of the experiment, whereas on the reef with the highest lionfish density the abundance of all native species decreased.
Similarly, biomass of small fishes tripled on reefs with no lionfish, yet decreased on all of the reefs with lionfish present by between 3 % and nearly 100 % (Fig. 2a). By the end of the experiment, biomass of small fishes also followed a negative exponential decline with increasing lionfish density (Fig. 2b; Table 1). The relationship between lionfish density and native fish biomass was primarily driven by the difference between control reefs and reefs with lionfish present. When control reefs were removed, there was no effect of lionfish density on native fish biomass and the intercept-only model provided the best fit (∆AICc > 6.65).
Proportional change in biomass of prey-sized native fishes (0–50 mm TL) on patch reefs with manipulated lionfish densities (0–12 lionfish/m2). a Weekly over the 7-week experiment and b at the final census only. Curve shows negative exponential model (best fit model based on ∆AICc). Points for 0-lionfish control treatment represent mean ± SEM. Sample size (# of reefs) equals 4 for the 0-lionfish treatment and 1 for all other treatments
Native fish richness, evenness, and composite diversity
While lionfish-free control reefs gained an average of 3.5 native fish species during the summer recruitment season, all of the lionfish reefs experienced no gain or a loss in species richness. Like abundance and biomass, species richness declined with increasing lionfish density (Fig. 3a), and both the linear and negative exponential models fit better than the null intercept-only model (Table 1). However, there was little evidence to distinguish between the linear and non-linear models (∆AICc = 0.208; Table 1). Based on the linear model, for each additional lionfish on a reef species richness decreased proportionally by 0.10 (95 % CI 0.07–0.13; Table 1). Based on the non-linear model, for each additional lionfish on a reef there was an estimated 86.9 % reduction in proportional species richness (95 % CI 81.1–93.2; Table 1).
Proportional change in a species richness, b species evenness, c Shannon–Wiener diversity, and d Simpson’s diversity of prey-sized native fishes (0–50 mm TL) on patch reefs with manipulated lionfish densities (0–12 lionfish/m2) at the end of the 7-week experiment. Curves show best fit models based on ∆AICc: a linear and negative exponential, b–d no other curve fit better than a null intercept-only model. Points for 0-lionfish control treatment represent mean ± SEM. Sample size (# of reefs) equals 4 for the 0-lionfish treatment and 1 for all other treatments
There was no relationship between species evenness (J) or species diversity (H’ and 1-D) and lionfish density (Fig. 3b–d). For evenness and both diversity indices, the null intercept-only model fit better than both the linear and non-linear models (∆AICc > 2.09; Table 1).
Native fish community structure
The non-metric multidimensional scaling ordination of reef fish communities at the final censuses converged on a stable 2-D solution (Fig. 4a; final stress = 5.44, linear r2 = 0.989, Monte–Carlo p = 0.001). Lionfish density was strongly positively correlated with axis 1 (r = 0.92, randomization p = 0.003). Based on a regression of axis 1 scores versus lionfish density, community structure was clearly affected by lionfish density, but there was little evidence to distinguish between a linear and non-linear model (∆AICc = 1.61; Table 1; Fig. 4b). Of the 27 native coral reef fish species present during the final surveys, only the cleaner goby (Elacatinus genie) was positively correlated with axis 1, but this was an extremely weak correlation (r = 0.11, Online Resource 4). Eight species were strongly negatively correlated with axis 1 (r < −0.50), with the beaugregory damselfish (Stegastes leucostictus) (r = −0.79), bridled goby (Coryphopterus glaucofraenum) (r = −0.77), and rosy blenny (Malacoctenus macropus) (r = −0.76) having the strongest negative relationships.
a Non-metric multidimensional scaling (NMDS) ordination of reefs in species space at the end of the 7-week experiment. Each point represents a reef with a minimum convex hull polygon around 0-lionfish control reefs. Labels correspond to the density of lionfish (#/m2) on each reef. The solid line represents the correlation (angle) and strength (length) of the effect of lionfish density. The distances between points approximate the dissimilarities among the communities at those reefs in terms of species composition and relative abundance. b Effect of manipulated lionfish density on prey-sized native fish community structure, represented by NMDS axis 1 score extracted from ordination in (a). Curves show linear and negative exponential models (best fit models based on ∆AICc). Points for 0-lionfish control treatment represent mean ± SEM. Sample size (# of reefs) equals 4 for the 0-lionfish treatment and 1 for all other treatments
Discussion
Native fish abundance and biomass
Increasing lionfish density negatively affected the abundance and biomass of prey-sized native reef fishes over the course of the experiment. The largest declines in native fish abundance and biomass occurred between the zero and one lionfish treatments. For biomass in particular, there was no additional effect of adding more than one lionfish to the reefs. Therefore, if one were to predict the impact of lionfish on native fish abundance and biomass by linearly scaling-up their per-capita effect (Parker et al. 1999), one would severely overestimate the effects of large numbers of lionfish in this study system.
A likely explanation for such a leveling off of impacts at higher lionfish densities is intraspecific competition among lionfish. The juvenile lionfish in this study experienced slower growth rates in both length and mass with increasing density (Benkwitt 2013), which combined with the fact that there was a non-linear decrease in native fish abundance and biomass, suggests that lionfish were competing for food at higher densities. While native fish biomass declined on reefs with lionfish, lionfish biomass increased throughout the study, which is indicative of a potential ‘worst-case scenario’ whereby the majority of native fish biomass is converted to lionfish biomass (Albins and Hixon 2013). A similar pattern has been observed on larger reefs, as increasing lionfish biomass co-occurred with declining prey biomass around New Providence, Bahamas (Green et al. 2012).
The species that had the largest difference in abundance between the lionfish-free control and the lionfish reefs was the bridled goby (Coryphopterus glaucofraenum), followed by the beaugregory damselfish (Stegastes leucostictus), and the rosy blenny (Malacoctenus macropus). This result is consistent with those of Albins and Hixon (2008) and Albins (2013) who also documented that bridled goby and beaugregory damselfish experienced the largest declines in abundance between lionfish-free reefs and reefs with one lionfish present. Throughout the Bahamas, gobies, damselfishes, and blennies comprise a large part of the prey species found in lionfish stomach contents (Albins and Hixon 2008; Morris and Akins 2009; Côté et al. 2013). However, at the highest experimental lionfish density, populations of all native species were severely depleted, with only a single native fish recruit present on the reef at the end of the experiment.
In addition to predation by lionfish, cues emitted by lionfish could have contributed to the reduction in native fish abundance and biomass on reefs with lionfish present. For example, Vail and McCormick (2011) found a 24–43 % reduction in recruitment of damselfish to patch reefs with predator odor compared to control reefs in the Pacific. Thus far, there is no evidence that fish recognize chemical cues from lionfish in either their native or invaded ranges (Lönnstedt and McCormick 2013; Marsh-Hunkin et al. 2013), which suggests that the majority of lionfish effect in this study was due to direct predation and not differential recruitment. However, further research should address whether chemical or other cues from invasive lionfish affect native fish recruitment, especially at extremely high lionfish densities.
Native fish richness, evenness, and composite diversity
Although native fish abundance and biomass declined non-linearly with increasing lionfish density, the patterns of species richness, evenness, and composite diversity were less consistent. Species richness decreased with more lionfish on a reef, with extirpations of many native prey species occurring as lionfish density increased. By contrast, there was no relationship between evenness and composite diversity across lionfish treatments after excluding the highest lionfish treatment (where there was only one individual native fish remaining). Evenness and composite diversity did not change significantly because at higher lionfish densities there were fewer individuals of the most abundant species present (such as the bridled goby and the beaugregory damselfish) and also fewer total species due to extirpations. Albins (2013) found a similar pattern whereby there was a decrease in species richness, an increase in species evenness, and a comparable change in composite species diversity on reefs with one lionfish present compared to lionfish-free control reefs.
Native fish community structure
Lionfish density was important in structuring small native fish communities on small patch reefs in this study. By the end of the experiment, reefs clearly separated along axis 1 of the NMDS based on lionfish density, with the lionfish-free control reefs clustering on the negative side and the higher lionfish treatment reefs separated by increasing lionfish density along the positive side of axis 1. As with species richness, however, the effect of lionfish density on community structure could not be clearly classified as either linear or non-linear. Native species that were most negatively correlated with axis 1 in the ordinations were the same species (beaugregory damselfish, bridled goby, and rosy blenny) that experienced the largest drops in abundance between lionfish-free and lionfish-present reefs. Only one native species, the cleaner goby (Elacatinus genie), was positively correlated with axis 1, but this was a very weak correlation. Although lionfish occasionally consume cleaner gobies in the laboratory (Tuttle personal communication), there is thus far no evidence that invasive lionfish consume cleaner gobies in the wild. Given that cleaner gobies provide a service on reefs by removing ectoparasites from fishes (Côté and Soares 2011) it is worthwhile to note that they may not be consumed readily by lionfish, even at high densities of this invasive predator.
Implications and conclusions
Due to the small reef size and limited duration of this field experiment, caution must be used when applying conclusions from this study to managing the lionfish invasion in general. Still, these findings may have some implications for the management of invasive lionfish, at least in small patch reef systems. The largest decrease in native fish abundance and biomass occurred between the zero and one lionfish treatments, with additional lionfish causing smaller subsequent declines. This result implies that removal efforts in small patch reef habitats should be thorough and aim to remove all lionfish to have the biggest conservation impact on native fish abundance and biomass. This result differs slightly from model predictions and tests of lionfish removal effort necessary to minimize their impacts on native fish biomass, which found that patch reefs with high and moderate removal effort have comparable increases in native fish biomass when prey production is accounted for (Green et al. 2014). These discrepancies between studies are likely due to several factors, including the differences in size and type of reefs used and prey production on the reefs. While this study used small (1 m3) artificial reefs, Green et al. (2014) used natural patch reefs of 100–150 m2. As a result of the large differences in patch size, natural recruitment was likely much lower to the reefs in this study. Consequently, the effects of lionfish in this study, particularly at high densities, may have been limited by recruitment of prey fish to the reefs. The differences between these studies further demonstrate that there will likely not be one universal rule to aid in the management in lionfish. Instead, the effectiveness of removal strategies will be context-dependent based on factors including reef size, habitat type, and natural prey production in the area.
Removal strategies will also vary based on the management goals that the removals are trying to accomplish. Even at this small scale, increasing lionfish density did not affect all metrics in the same manner. For example, species richness declined with increasing lionfish density, but the decline curve was not necessarily non-linear while evenness and diversity did not change significantly with increasing lionfish density. This difference in pattern among metrics has been found for a variety of other invaders (e.g. Williams et al. 2002; Gherardi and Acquistapace 2007; Matsuzaki et al. 2009). Therefore, management targets may depend on whether the removals are primarily trying to conserve native fish abundance, biomass, or diversity. In this system, it appears that removing all lionfish is important for conserving native fish abundance and biomass on small patch reefs. However, if conserving native species richness is the goal of lionfish removals, then removing any lionfish may be of equal help regardless of how many other lionfish are present.
In addition to having some implications for managing the lionfish invasion, these results also apply to invasive species in general. A major goal of invasion biology is measuring the impact of invasive species, and thus far this has often been accomplished by linearly scaling-up per-capita effects (Parker et al. 1999). In the past few years, however, there have been several calls to incorporate potential non-linearities in invader impacts with increasing invader abundance when measuring and predicting the effects of invasive species (Thiele et al. 2009; Thomsen et al. 2011; Ricciardi et al. 2013). Still, most other studies of invasive species effects do not account for invader abundance, and those that do usually compare only a few invader densities, making it difficult to create a unified framework for predicting the effects of different types of invaders (Thomsen et al. 2011; Ricciardi et al. 2013). The results from this study comparing the effect of a broad range of densities of invasive lionfish on several different native community metrics demonstrate that accounting for non-linearities in invader impacts is important in managing the ecological effects of biological invasions.
References
Albins MA (2011) Effects of the invasive Pacific red lionfish Pterois volitans on native Atlantic coral-reef fish communities. Dissertation, Oregon State University
Albins MA (2013) Effects of invasive Pacific red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish communities. Biol Invasions 15:29–43
Albins MA, Hixon MA (2008) Invasive Indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reef fishes. Mar Ecol Prog Ser 367:233–238
Albins MA, Hixon MA (2013) Worst case scenario: potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ Biol Fish 96:1151–1157
Albins M, Lyons P (2012) Invasive red lionfish Pterois volitans blow directed jets of water at prey fish. Mar Ecol Prog Ser 448:1–5
Allen GR, Eschmeyer WN (1973) Turkeyfishes at Eniwetok. Pac Discov 26:3–11
Almany GR (2003) Priority effects in coral reef fish communities. Ecology 84:1920–1935
Barbour AB, Allen MS, Frazer TK, Sherman KD (2011) Evaluating the potential efficacy of invasive lionfish (Pterois volitans) removals. PLoS ONE 6:e19666
Baty F, Delignette-Muller ML (2013) nlstools: tools for nonlinear regression diagnostics. R package version 0.0-15
Benkwitt CE (2013) Density-dependent growth in invasive lionfish (Pterois volitans). PLoS ONE 8:e66995
Burnham KP, Anderson DR (2002) Model selection and multimodel interference: a practical information–theoretic approach. Springer, New York
Byers JE, Reichard S, Randall JM et al (2002) Directing research to reduce the impacts of nonindigenous species. Conserv Biol 16:630–640
Byrnes J, Witman JD (2003) Impact assessment of an invasive flatworm, Convoluta convoluta, in the Southern Gulf of Maine. J Exp Mar Biol Ecol 293:173–191
Carr MH, Hixon MA (1995) Predation effects on early post-settlement survivorship of coral-reef fishes. Mar Ecol Prog Ser 124:31–42
Carr MH, Hixon MA (1997) Artificial reefs: the importance of comparisons with natural reefs. Fisheries 22:28–33
Côté IM, Malijovic A (2010) Predation rates of Indo-Pacific lionfish on Bahamian coral reefs. Mar Ecol Prog Ser 404:219–225
Côté IM, Soares MC (2011) Gobies as cleaners. In: Patzner RA, Van Tassell JL, Kovacic M, Kapoor BG (eds) The biology of gobies. Science Publishers, St. Helier, pp 531–558
Côté IM, Green SJ, Morris JA, Akins JL, Steinke D (2013) Diet richness of invasive Indo-Pacific lionfish revealed by DNA barcoding. Mar Ecol Prog Ser 472:249–256
Cottingham KL, Lennon JT, Brown BL (2005) Knowing when to draw the line: designing more informative ecological experiments. Front Ecol Environ 3:145–152
Cure K, Benkwitt CE, Kindinger TL, Pickering EA, Pusack TJ, McIlwain JL, Hixon MA (2012) Comparative behavior of red lionfish Pterois volitans on native Pacific versus invaded Atlantic coral reefs. Mar Ecol Prog Ser 467:19–181
Davis MA (2009) Invasion biology. Oxford University Press, Oxford
Frederick JL (1997) Evaluation of fluorescent elastomer injection as a method for marking small fish. Bull Mar Sci 61:399–408
Gherardi F, Acquistapace P (2007) Invasive crayfish in Europe: the impact of Procambarus clarkii on the littoral community of a Mediterranean lake. Freshw Biol 52:1249–1259
Green SJ, Côté IM (2008) Record densities of Indo-Pacific lionfish on Bahamian coral reefs. Coral Reefs 28:107
Green SJ, Akins JL, Côté IM (2011) Daily patterns of foraging behaviour and prey consumption in the Indo-Pacific lionfish Pterois volitans on Bahamian coral reefs. Mar Ecol Prog Ser 433:156–167
Green SJ, Akins JL, Maljković A, Côté IM (2012) Invasive lionfish drive Atlantic coral reef fish declines. PLoS ONE 7:e32596
Green SJ, Dulvy NK, Brooks ALM, Akins JL, Cooper AB, Miller S, Côté IM (2014) Linking removal targets to the ecological effects of invaders: a predictive model and field test. Ecol Appl 24:1311–1322
Griffen BD, Byers JE (2008) Community impacts of two invasive crabs: the interactive roles of density, prey recruitment, and indirect effects. Biol Invasions 11:927–940
Hixon MA, Beets JP (1989) Shelter characteristics and Caribbean fish assemblages: experiments with artificial reefs. Bull Mar Sci 44:666–680
Hixon MA, Beets JP (1993) Predation, prey refuges, and the structure of coral-reef fish assemblages. Ecol Monogr 63:77–101
Hixon MA, Carr MH (1997) Synergistic predation, density dependence, and population regulation in marine fish. Science 277:946–949
Keller RP, Lake PS (2007) Potential impacts of a recent and rapidly spreading coloniser of Australian freshwaters: oriental weatherloach (Misgurnus anguillicaudatus). Ecol Freshw Fish 16:124–132
Kendall JJ (1990) Further evidence of cooperative foraging by the turkeyfish Pterois miles in the Gulf of Aqaba, Red Sea, with comments on safety and first aid. Proc Am Acad Underw Sci Diving Symp 10:209–223
Kruskal JB (1964) Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29:1–27
Kulbicki M, Beets J, Chabanet P et al (2012) Distributions of Indo-Pacific lionfishes Pterois spp. in their native ranges: implications for the Atlantic invasion. Mar Ecol Prog Ser 446:189–205
Kulhanek SA, Ricciardi A, Leung B (2011) Is invasion history a useful tool for predicting the impacts of the world’s worst aquatic invasive species? Ecol Appl 21:189–202
Lönnstedt OM, McCormick MI (2013) Ultimate predators: lionfish have evolved to circumvent prey risk assessment abilities. PLoS ONE 8:e75781
Lönnstedt OM, Ferrari MCO, Chivers DP (2014) Lionfish predators use flared fin displays to initiate cooperative hunting. Biol Lett 10:20140281
Magurran AE (2004) Measuring biological diversity. Blackwell, Malden
Marsh-Hunkin KE, Gochfeld DJ, Slattery M (2013) Antipredator responses to invasive lionfish, Pterois volitans: interspecific differences in cue utilization by two coral reef gobies. Mar Biol 160:1029–1040
Mather PM (1976) Computational methods of multivariate analysis in physical geography. Wiley, London
Matsuzaki SS, Usio N, Takamura N, Washitani I (2009) Contrasting impacts of invasive engineers on freshwater ecosystems: an experiment and meta-analysis. Oecologia 158:673–686
Mazerolle MJ (2013) AICcmodavg: model selection and multimodal inference based on (Q)AIC(c). R package version 1.33. http://CRAN.R-project.org/package=AICcmodavg
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Morris JA (2012) Invasive lionfish: a guide to control and management. Gulf and Caribbean Fisheries Institute Special Publications Series, Marathon
Morris JA, Akins JL (2009) Feeding ecology of invasive lionfish (Pterois volitans) in the Bahamian archipelago. Environ Biol Fishes 86:389–398
Muñoz RC, Currin CA, Whitfield PE (2011) Diet of invasive lionfish on hard bottom reefs of the Southeast USA: insights from stomach contents and stable isotopes. Mar Ecol Prog Ser 432:182–193
Oksanen J, Blanchet FG, Kindt R, et al (2013) vegan: community ecology package. R package version 2.0-9. http://CRAN.R-project.org/package=vegan
Parker IM, Simberloff D, Lonsdale WM et al (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Pielou EC (1966) Shannon’s formula as a measure of specific diversity: its use and misuse. Am Nat 100:463–465
Pusack TJ (2013) Coral reef fishes: insights into larval dispersal and invasion ecology. Dissertation, Oregon State University
Ricciardi A, Hoopes MF, Marchetti MP, Lockwood JL (2013) Progress toward understanding the ecological impacts of non-native species. Ecol Monogr 83:263–282
Salo P, Korpimäki E, Banks PB, Nordström M, Dickman CR (2007) Alien predators are more dangerous than native predators to prey populations. Proc R Soc B Biol Sci 274:1237–1243
Schofield PJ (2009) Geographic extent and chronology of the invasion of non-native lionfish (Pterois volitans [Linnaeus 1758] and P. miles [Bennett 1828]) in the Western North Atlantic and Caribbean Sea. Aquat Invasions 4:473–479
Schofield PJ (2010) Update on geographic spread of invasive lionfishes (Pterois volitans [Linnaeus, 1758] and P. miles [Bennett, 1828]) in the Western North Atlantic Ocean, Caribbean Sea and Gulf of Mexico. Aquat Invasions 5:S117–S122
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Simpson EH (1949) Measurement of diversity. Nature 163:688
Sutherland WJ, Clout M, Côté IM et al (2010) A horizon scan of global conservation issues for 2010. Trends Ecol Evol 25:1–7
R Development Core Team (2013) R: a language and environment for statistical computing. R foundation for statistical computing. http://www.R-project.org/
Thiele J, Kollmann J, Markussen B, Otte A (2009) Impact assessment revisited: improving the theoretical basis for management of invasive alien species. Biol Invasions 12:2025–2035
Thomsen MS, Olden JD, Wernberg T, Griffin JN, Silliman BR (2011) A broad framework to organize and compare ecological invasion impacts. Environ Res 111:899–908
Vail AL, McCormick MI (2011) Metamorphosing reef fishes avoid predator scent when choosing a home. Biol Lett 7:921–924
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of earth’s ecosystems. Science 277:494–499
Williams AE, Moss B, Eaton J (2002) Fish induced macrophyte loss in shallow lakes: top–down and bottom–up processes in mesocosm experiments. Freshw Biol 47:2216–2232
Yokomizo H, Possingham HP, Thomas MB, Buckley YM (2009) Managing the impact of invasive species: the value of knowing the density-impact curve. Ecol Appl 19:376–386
Acknowledgments
This work was supported by National Science Foundation (NSF) Grants (08-51162 and 12-33027) to M.A. Hixon and an NSF Graduate Research Fellowship to C.E. Benkwitt. This paper is a chapter of my doctoral dissertation (Committee: M.A. Hixon [advisor], S.A. Heppell, S.S. Heppell, L. Madsen, and B.A. Menge). B. Beechler, E. Cerny-Chipman, S.L. Close, A.C.D. Davis, E. Gorsich, S.J. Green, K.I. Ingeman, A.C. Iles, T.L. Kindinger, T.J. Pusack, and L.J. Tuttle also provided constructive feedback on parts of this manuscript. I also thank M.A. Albins for assistance with R code, A. Adler for verifying biomass conversions, M. Crell and S. Vojnovich for data entry, and A.C.D. Davis, T.J. Pusack, K.I. Ingeman, T.L. Kindinger, L.J. Tuttle, and the Perry Institute for Marine Science staff and interns for field assistance and support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Benkwitt, C.E. Non-linear effects of invasive lionfish density on native coral-reef fish communities. Biol Invasions 17, 1383–1395 (2015). https://doi.org/10.1007/s10530-014-0801-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-014-0801-3