Abstract
Assessing the implications of species invasion for native communities requires determining whether effects of invaders are novel, or are redundant with effects of species that are already present. Using a pair of field experiments conducted over two successive years, we examined factors that influence community impacts of a recent predatory crab invader (Hemigrapsus sanguineus) and a previously established invasive crab (Carcinus maenas) on New England coasts. We demonstrate that effects of these species differ temporally with changes in the ambient prey community, and are influenced by density differences between the two species and by different strengths and types of indirect effects that each elicits. Our study highlights the importance of including bottom-up processes (i.e., prey recruitment) when examining the redundancy of consumers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species can alter species composition or richness in invaded areas by causing extinction of native species (Clavero and Garcia-Berthou 2005), replacing previously established non-native species (Lohrer and Whitlatch 2002a), or increasing local species diversity (Sax 2002; Sax and Gaines 2003). These changes may in turn have important consequences for ecosystem function (Parker et al. 1999; Hooper et al. 2005). Whether a new invader alters ecosystem function depends largely on the novelty of its effects within the invaded community (Crooks 2002). If its effects are redundant with those of species already present (sensu Lawton and Brown 1993), then impacts of the introduction on the wider native community may be small. Ultimately, the level of understanding needed for many conservation goals is to assess the redundancy of invading species at the community level (Byers et al. 2002).
Effects of invasive populations are often determined by scaling up from per capita measurements made using single individuals, or several individuals of the same size, sex, etc. (e.g., Rossong et al. 2006; Schooler et al. 2006). This can be problematic when per capita effects scale nonlinearly due to intraspecific interactions (e.g., Byrnes and Witman 2003), or when per capita effects are age, size, or sex specific within a demographically heterogeneous population (e.g., Bergmann and Motta 2005). Further, with the notable exception of plant invaders and a limited number of studies on animal invaders (e.g., zebra mussels: Karatayev et al. 2002; fire ants: Sanders et al. 2003), impacts of invasive species on entire communities, rather than on just one or two focal native species, have rarely been quantified (Parker et al. 1999).
Study system
Two invasive predatory crabs, the European green crab Carcinus maenas and the Asian shore crab Hemigrapsus sanguineus, can strongly affect native communities on the east coast of North America. C. maenas was introduced to the western Atlantic in the mid 1800’s and now ranges from Nova Scotia to Maryland (de Rivera et al. 2005), where it affects the native community both through direct consumption (Glude 1955, Richards et al. 1999; Lohrer and Whitlatch 2002b; Whitlow et al. 2003) and through altering behavior and morphology of native species (Hadlock 1980; Seeley 1986; Trussell et al. 2003; Freeman and Byers 2006). The more recently introduced crab, H. sanguineus, was first noted in New Jersey in 1988, spread quickly, and now ranges from central Maine to North Carolina (McDermott 1998). Populations of this new invader are often very dense, and a recent survey of 30 sites throughout New England found that mean densities of H. sanguineus were approximately six times higher than current and historic C. maenas densities (Griffen and Delaney 2007). Recent studies imply that H. sanguineus may have broad impacts on the native community (Tyrrell and Harris 1999; Ledesma and O’Connor 2001; Bourdeau and O’Connor 2003; Brousseau and Baglivo 2005), as well as large species-specific effects on bivalve prey (Lohrer and Whitlatch 2002b).
While C. maenas is found in a broader range of habitats, both species are found abundantly in rocky intertidal areas. Negative interactions between these species are common (Jensen et al. 2002; Griffen 2006; Griffen and Byers 2006b), and the spread of dense H. sanguineus populations has apparently caused the disappearance of C. maenas from most rocky intertidal habitats in Long Island Sound (Lohrer and Whitlatch 2002a; Kraemer et al. 2007). H. sanguineus populations in the Gulf of Maine are also on the rise and a similar species replacement may be in progress in these northern regions (Griffen and Delaney 2007).
The observed and predicted replacements of C. maenas by H. sanguineus underscore the need to determine the relative impacts of these species on intertidal communities. Previous studies have compared the impacts of these species by examining diets using gut contents (Lohrer et al. 2000a) and food preferences (Tyrrell and Harris 1999). Multiple studies have experimentally compared consumption rates on individual prey taxa (Lohrer and Whitlatch 2002b; DeGraaf and Tyrrell 2004; Griffen and Byers 2006a) or have examined broader predatory impacts using small numbers of crabs of the same size and sex over short time frames (Tyrrell et al. 2006).
However, several factors not accounted for in previous studies may influence the population impacts of these species on the invaded community. First, negative interactions between these species can alter their impacts in areas where they still coexist (Jensen et al. 2002; Griffen 2006; Griffen and Byers 2006b; Griffen and Byers 2006a; Griffen et al. 2008). Second, the densities of each species vary widely, with maximum H. sanguineus densities that are many times higher than C. maenas densities (Lohrer and Whitlatch 2002a; Griffen and Delaney 2007). Further, density-dependent intraspecific interference is stronger for C. maenas than for H. sanguineus (Griffen and Delaney 2007). Impacts of C. maenas may therefore increase more weakly with increasing crab density. Third, the impacts of these omnivores on the native prey community may be influenced by temporal variation in environmental conditions and prey availability (Elner 1980). Thus the redundancy of the two species may not be constant, but may vary as the prey community fluctuates. And fourth, indirect effects can occur as both carnivorous and herbivorous snails alter foraging in the presence of the invasive crab predators (Trussell et al. 2002; Trussell et al. 2003). In this study, we used field experiments to examine how each of these factors influences the overall impacts of C. maenas and H. sanguineus on the invaded community.
Methods
Experimental design
We conducted two field enclosure experiments in 2005 and 2006 to examine the factors influencing community impacts of invasive predatory crabs on rocky New England shores. The semi-exposed study site at Odiorne Point, New Hampshire, U.S.A., is dominated by cobble and boulders. Fully enclosed cages (0.6 × 0.45 × 0.3 m) constructed of lobster wire and lined with 0.5 cm plastic mesh were placed along a 50 m stretch of beach at approximately 0.3 m above mean low water where C. maenas and H. sanguineus are found in high abundances. Cages were deployed each year in the beginning of April. Five to eight small boulders were placed inside of each cage in order to standardize the total abundance of naturally occurring flora and fauna. This standardization process was greatly facilitated by the low species richness found in New England rocky intertidal sites (Menge 1976). Additionally, we allowed communities contained inside cages to equilibrate for six weeks without crab predators (until mid-May) and then randomly assigned treatments to cages to avoid biasing our results.
Dominant prey species that were followed in this study included three species of red algae (Chondrus crispus, Mastocarpus stellatus, and Polysiphonia lanosa), two groups of brown algae (Fucus sp. and Ascophyllum nodosum), the barnacle Semibalanus balanoides, the mussel Mytilus edulis, the carnivorous whelk Nucella lapillus, and two herbivorous snails Littorina littorea and Littorina obtusata. Small, highly mobile prey that could pass through cage mesh such as amphipods and isopods were not explicitly examined due to the difficulty of accurately quantifying these species. However, these are readily consumed by both crab species (Griffen and Byers 2006b; Griffen and Byers 2006a), and likely provided an additional food source for crabs in our experiments.
Experiments during each year tested different factors (Table 1). In 2005 we examined how interactions between the crab species influenced their overall impacts on the prey community. Four different treatments were each replicated eight times: 10 C. maenas, 10 H. sanguineus, 5 C. maenas + 5 H. sanguineus, and a no crab control. These densities are consistent with those quantified at our field site and at other sites where both species are common (Griffen et al. 2008).
In 2006 we examined the influence of conspecific predator density on community impacts of C. maenas and H. sanguineus. Treatments including 10, 20, and 40 individuals of each species alone (n = 4), and a no crab control (n = 3) (Table 1). The lowest density (37 m−2) was chosen to represent C. maenas’ carrying capacity (Lohrer and Whitlatch 2002b) and the highest density (148 m−2) was chosen to roughly approximate H. sanguineus’ carrying capacity (Kraemer et al. 2007), thus allowing an explicit comparison of the population level effects of these two species. This four-fold difference is less than the six-fold differences in mean densities throughout their invaded ranges (Griffen and Delaney 2007), and thus provides a conservative comparison of density-scaling in the effects of these two species.
We did not initially intend to examine how variation in prey abundance across years influenced crab impacts. However, large differences in recruitment of several important prey species occurred between 2005 and 2006 and were important in interpreting our results. We therefore examined both temporal variation due to changes in prey availability and the influences of indirect effects using data from both the 2005 and 2006 experiments.
Our goals were to compare the relative effects of these two crabs by comparing the community in their presence/absence and at different relative densities. Further, our aim was to increase tractability and to isolate impacts of each crab species independent of effects by other predators (fish, birds, etc.). Comparing the communities in fully enclosed cage treatments with crabs against those without crabs accomplished each of these goals. As such, results of these experiments are intended to represent the relative, not absolute impacts of these species.
All crabs were collected by hand on site at Odiorne Point. We used a 50:50 sex ratio and a range of sizes of both species in each treatment, mimicking population demographics at our field site. Specifically, we used a 7:2:1 ratio of small:medium:large crabs, where small, medium, and large C. maenas were 12–18, 20–25, and 40–55 mm carapace width (CW), respectively, and H. sanguineus were 12–15, 20–25, and 29–34 mm CW, respectively.
At monthly intervals the contents of each cage were monitored and missing crabs were replaced. This monthly interval was chosen as a compromise between maintaining experimental crab densities and minimizing disturbance to the experiments. Crabs could not pass through cage mesh, and missing crabs thus resulted from cannibalism and intraguild predation rather than escape (this was verified by the presence of carapace fragments found inside of enclosures). While treatments with 20 and 40 C. maenas exceed natural densities of this species, this facilitated comparison between species without confounding density differences. Maintaining constantly high densities likely elevated the influence of aggression between crabs (which we quantified – see cannibalism), but was necessary to examine the long term impacts of these species at the high densities that have been reported (e.g., Lohrer and Whitlatch 2002b).
During the 2006 experiment we also mimicked the availability of allocthanous drift algae as a potential food source by placing 20 g of Chondrus crispus (the most abundant species of drift algae at our field site) in each cage at monthly intervals. At monthly maintenance periods, remaining C. crispus from the previous addition was subsequently removed, weighed, and replaced with fresh algae. We included drift algae because it may reduce impacts on the intertidal community by providing an alternative food for crabs. However, because it is allocthanous rather than a resident of the intertidal community, we analyzed its response separately (two-way ANOVA on loss of algal mass with crab species and crab density as fixed factors).
The experiment was terminated each year in mid-October. The experimental duration (May-October) thus encompassed the portion of the year when active foraging by these species is greatest (Elner 1980). We collected the contents of each cage, including all flora, fauna, and shell fragments. In the laboratory, the number of each species of animal was assessed (live and dead). Herbivorous and carnivorous snails were enumerated in large and small categories, with the distinction between sizes set by the ability to pass through the 0.5 cm mesh used on experimental cages. Algae were separated by species and the wet weight determined. We also assessed predation by the whelk Nucella lapillus on mussels (using characteristic drill holes in mussel shells) and barnacles (using empty barnacle tests) in order to quantify indirect effects of crab presence. The abundance of live barnacles, empty barnacle tests, mussel recruits (which settled in July-August of each year and were distinguished from initial mussels by their small size, <1 mm), and fucoid algae recruits inside each cage were determined by counting the number within 156 cm2 quadrats placed on each of five separate boulders (at the site of highest barnacle density on each boulder).
Statistical analyses of overall impacts on the prey community
Our primary goal was to examine factors that influence the effects of C. maenas and H. sanguineus on the native community. We therefore analyzed the data from each year using MANOVAs combined with planned linear contrasts (detailed below) to examine the impacts of different predator treatments across all prey types (using red algae, brown algae, mussels [log transformed], barnacles, L. littorea, L. obtusata, and N. lapillus as response variables). When these whole-community analyses indicated a significant difference in the impacts of the two crab species, we then used post hoc comparisons to examine the impacts of specific predator treatments on each prey type individually.
In 2005 we used a one-way MANOVA with predator treatment as a fixed factor (four levels: C. maenas only, H. sanguineus only, both species together, no-crab control) to compare the impacts of the two species, both when they foraged alone and when they foraged together. This was followed by three planned linear contrasts: C. maenas vs. H. sanguineus, C. maenas vs. both species together, and H. sanguineus vs. both species together. Because these contrasts indicated that community impacts differed across treatments, we used post hoc ANOVAs and Tukey’s tests to compare the difference in each prey type individually across the three predator treatments.
In 2006 we used a two-way MANOVA with predator species (two levels) and predator density (four levels) as fixed factors. This was followed by post hoc individual two-way ANOVAs (with the same factors) for each prey type. The treatments used also allowed us to examine the overall impacts of C. maenas and H. sanguineus while accounting for natural differences in their equilibrium population sizes. We therefore followed each ANOVA with planned linear contrasts to compare the effects of 10 C. maenas and 40 H. sanguineus on each prey type.
We examined how the impacts of C. maenas and H. sanguineus varied across years using data from both years when 10 individuals of either species foraged alone. We used a two-way MANOVA with predator species and year (each with two levels) as fixed factors. This was followed by planned linear contrasts to compare redundancy in the overall impacts of the two species within each year.
We also compared the importance of cannibalism for each species at different densities using ANOVA on mean percent monthly mortality of crabs in 2006, with species and density as fixed factors. In addition, we compared the importance of cannibalism between years using data from both years when 10 individuals of either species foraged alone. We used a two-way ANOVA with predator species and year as fixed factors.
Statistical analyses of indirect effects
Our study system and design allowed us to examine the contribution of the indirect effects of these predators to their overall impacts on the community within our experiments. Several indirect effects potentially occur within our system, although the number of important pathways is limited by the relatively low species richness of the Gulf of Maine intertidal. We focus here on indirect effects that have previously been documented for C. maenas or that are likely to be important given our experimental community and the diet preferences of these crabs. Understanding the relative strengths of indirect effects of C. maenas and H. sanguineus may help to mechanistically explain differences in the overall community-level impacts of these species.
First, crabs can influences food consumption by carnivorous snails (Trussell et al. 2002; Trussell et al. 2003). Using data from both experimental years, we examined the influence of the two crab species on mussel and barnacle consumption by the carnivorous whelk N. lapillus by quantifying drill holes in mussel shells and empty barnacle tests. (In contrast, crab predation on these species results in chipped mussel shells and removal of the entire barnacle from rock surfaces.) We made comparisons between the three treatments that were conducted in both years: 10 C. maenas, 10 H. sanguineus, and the no-crab controls. We first compared the influence of crabs on small and large N. lapillus using separate ANOVAs with crab treatment (3 levels) and year (two levels) as fixed factors. We then compared the number of drilled mussels and empty barnacle tests (both log transformed) using separate ANCOVAs with predator treatment and year as the main factors and the total number of whelks in each cage as a covariate. Significant interaction terms of main factor effects were followed by Tukey’s test to examine specific differences between predator treatments across the two years.
Second, crab effects on barnacle density may have important indirect effects. The presence of barnacles can enhance mussel recruitment by providing complex surface areas for attachment of settling individuals (Navarrete and Castilla 1990) and can enhance establishment of fucoid algae through inhibiting snail herbivory on new recruits (Lubchenco 1983). We therefore examined how crabs influenced the facilitation of mussel settlement and fucoid establishment by barnacles. Specifically, we examined mussel recruitment (log transformed) during our 2005 experiment using ANCOVA with predator treatment as a main factor and barnacle density as a covariate. For comparison, we also verified the influence of barnacle density on mussel recruitment on ambient rocks surrounding our experimental cages using regression. Similarly we examined the importance of barnacle density for establishment of new fucoids (log transformed) in 2005 using ANCOVA with predator treatment as a main factor and barnacle density as a covariate.
Results
Species interactions
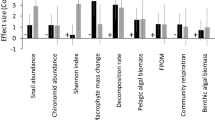
Community impacts in the 2005 experiment varied across predator treatments, with the two predators having different effects in the absence of the other species (MANOVA, Table 2A, Fig. 1). Combined effects of the two species foraging together were similar to those produced by C. maenas foraging alone, but were different from the effects of H. sanguineus foraging alone (planned linear contrasts, Table 2B, Fig. 1). All predator treatments differed in their impacts on specific prey. For mussels and herbivorous snails, impacts of H. sanguineus were weaker than those of C. maenas or both predators together. In contrast, when both predators foraged together there was a trend towards weaker impacts on barnacles (survival increased by ≈40%) and brown algae (biomass increased by ≈30%, though the increase was not significant) than when either species foraged alone (ANOVAs and Tukey’s tests, Table 2C, Fig. 1).
Survival of different prey types in 2005 (left side of vertical bar, mean ± SE, n = 8) and 2006 (right side of vertical bar, mean ± SE, n = 4). Horizontal dashed line represents mean value in no-crab control cages each year (±SE shaded area, n = 8 in 2005 and n = 3 in 2006). Values above line indicate that survival increased in presence of crabs, values below the line indicate that crabs had negative impact. Mussels include both experimentally added and naturally settling individuals. Red algae does not include 2006 additions of drift
Effects of predator population density
The impacts of both species in the 2006 experiment differed with predator density, though the effect of predator density on prey was weaker than expected (MANOVA, Table 3A, Fig. 1). When individual prey types were examined, greater impacts at higher predator densities were only found in the most abundant (red and brown algae) and most preferred prey (mussels and to a lesser extent barnacles) (ANOVAs, Table 3B, Fig. 1). Consumption of ‘drift’ red algae also increased with predator density (ANOVA, F 2,23 = 4.99, P = 0.017), but was not different between the two crab species (ANOVA, F 2,23 = 1.49, P = 0.24). In addition, while impacts of the two predators on most prey types were similar, C. maenas had a greater positive impact on brown algae at lower densities (ANOVA, Table 3B, Fig. 1).
When approximate equilibrium densities of the two species were compared (10 C. maenas vs. 40 H. sanguineus), H. sanguineus had a 30–50% larger impact across the entire prey community (Fig. 1). However, a significant difference was detected only for brown algae (linear contrasts, Table 3B). Due to low replication and high variability within treatments, our analyses had low power to detect a difference between the two species (mean power across all prey types = 0.29),
Influences of prey variability on crab effects
Prey communities differed between years in the absence of predators, largely due to greater barnacle recruitment (before the experiment started) and mussel recruitment (during the experiment) in 2005 (Fig. 1). Impacts of both crabs on barnacles, mussels, and snails were greatest in 2005 when these prey were more abundant (Fig. 1). Overall community impacts of 10 C. maenas were stronger than impacts of 10 H. sanguineus in 2005, but not in 2006 (planned linear contrast, Table 4B). However, this increase in redundancy between the crab species in 2006 was not consistent across all prey types, with the crabs becoming more similar in their effects on mussels and herbivorous snails in 2006, but less so for some taxa such as brown algae.
Cannibalism
Cannibalism was an important factor causing high levels of mortality among the small size class of crabs for both species in our experiments, and became stronger for both species as predator densities increased (ANOVA, density F 2,23 = 5.92, P = 0.02, species × density F 2,23 = 0.05, P = 0.83). Across all densities, cannibalism was 13% stronger for C. maenas than H. sanguineus in 2006, though this difference was not significant (ANOVA, species F 1,23 = 1.25, P = 0.28). Further comparison only at similar densities of 10 crabs per cage across years indicated that cannibalism among C. maenas remained consistently high over both years at approximately 40% mortality each month, while cannibalism among H. sanguineus increased from 11% per month in 2005 to 33% per month in 2006 when other animal prey were less available (ANOVA, species F 1,24 = 12.45, P = 0.002, species × year F 1,24 = 4.44, P = 0.047).
Indirect effects
Carcinus maenas had a greater positive indirect effect on barnacles and mussels than H. sanguineus by reducing N. lapillus predation, though only small N. lapillus seemed to be influenced by either crab. Small N. lapillus were more abundant overall in 2005 than 2006 and were reduced in C. maenas cages through emigration and/or consumption relative to control cages, with intermediate numbers in H. sanguineus cages (ANOVA, year: F1,31 = 7.63, P = 0.01; treatment: F2,31 = 3.71, P = 0.04, only C. maenas and control cages differed statistically from each other, Fig. 2a). Large N. lapillus were slightly more abundant in 2006 than in 2005, but were not influenced by the presence of either crab species (ANOVA, year: F1,31 = 3.17, P = 0.08; treatment: F2,31 = 0.94, P = 0.40, 2A). Barnacle consumption by N. lapillus was independent of the number of N. lapillus present (ANCOVA, covariate, F1,28 = 1.72, P = 0.20) and decreased in response to both predator species in 2005, though more so with C. maenas, but was low across all treatments in 2006 (ANCOVA predator treatment × year, F2,28 = 3.24, P = 0.05, followed by Tukey’s test, Fig. 2b). While mussel consumption by N. lapillus increased with N. lapillus abundance (ANCOVA, covariate, F1,28 = 4.91, P = 0.04), it decreased in 2005 in the presence of C. maenas, but was not influenced by either crab in 2006 (ANCOVA predator treatment × year, F2,28 = 3.31, P = 0.05, Fig. 2c).
(a) Final number of large and small N. lapillus in cages with 10 C. maenas, 10 H. sanguineus, or no crabs in both years. Large and small snails were distinguished by their ability to pass through 0.5 cm cage mesh. (b) Final density of empty barnacle tests on rocks inside cages. (c) Final number of mussel shells with drill holes. Bars are means ± SE (n = 8 in 2005, n = 4 in 2006 predator treatments, and n = 3 in 2006 control treatment). Letters above bars give results of Tukey’s tests
While both crab species decreased barnacle consumption by N. lapillus, both also heavily consumed barnacles themselves and thus had an indirect negative impact on mussels and fucoids that settle on and around barnacle tests. Specifically, the number of mussel recruits inside experimental cages in 2005 was not influenced by the presence of either crab species directly (main effect of ANCOVA, F 3,27 = 0.88, P = 0.46), but both crabs reduced the presence of barnacles and thus influenced mussel recruitment by reducing the density of prime mussel settlement sites (covariate [barnacle density] in ANCOVA, F 1,27 = 8.93, P = 0.006). Effects of barnacle density on mussel recruitment inside cages were even stronger when pooling data over both years (regression, F 1,56 = 54.58, P < 0.0001, R2 = 0.49), highlighting the importance of this relationship. A similar positive correlation between barnacle density and mussel recruitment was also observed on ambient rocks surrounding our experimental cages in 2005 (regression, F 1,37 = 121.07, P < 0.0001, R2 = 0.77). The number of fucoid recruits increased with barnacle density (covariate in ANCOVA, F 1,27 = 4.76, P = 0.04), and was further influenced by crab predators (main effect of ANCOVA, F 3,27 = 3.68, P = 0.02). Relative to controls, the mean number of fucoid recruits decreased (through reduced settlement, direct consumption, or indirectly through removal of barnacles) by 80% in C. maenas cages, and by >99% in cages with H. sanguineus in 2005. In contrast, fucoid recruitment in 2006 was largely absent in all cages.
Discussion
Our study found that the effects of the C. maenas and H. sanguineus on the intertidal community were very different in 2005, but were similar in 2006. This temporal variation in species redundancy was apparently driven by the large differences between the two years in recruitment of important/favored prey species. In contrast, interactions between the two species, the density of each species, and the indirect effects caused by each species only minimally affected their community impact.
Interactions between the species
Contrary to previous reports of large interference effects of H. sanguineus on C. maenas foraging (Jensen et al. 2002; Griffen 2006; Griffen et al. 2008), we found that interference between the two species only weakly reduced mortality of barnacles and brown algae, and did not alter impacts on the rest of the prey community. Strong interference effects that reduce mussel mortality (Griffen 2006; Griffen and Williamson 2008) were likely absent here because experiments were sufficiently long that mussel depletion could occur even at reduced predation rates (Fig. 1). Thus, while interference with H. sanguineus does reduce mussel consumption rates by C. maenas (Jensen et al. 2002; Griffen et al. 2008), this reduction may not provide considerable predation relief for mussels over long time periods in areas where crabs are abundant.
Predator density
The influence of crab density on community impacts of these predators was lower than anticipated. This may be attributed to multiple factors. First, we examined the influence of predator density in 2006 when lower availability of favored prey (barnacles, mussels, juvenile snails) led to weaker overall impacts of these predators (Fig. 1; Table 3). The strength of predator effects are limited by the amount that prey can be depressed from predator-free conditions. Thus when prey recruitment is strong, prey levels have further to fall and predators can potentially have stronger effects. Our results therefore indicate that bottom up forces control the strength of top down processes in this system.
Second, crab density may have been less important than anticipated because of cannibalism. During our monthly maintenance of the experiment, we noted that cannibalism generally increased at higher crab densities, consistent with previous findings for these species (Moksnes 2004). This reduced the time-averaged density differences between our treatments and weakened the influence of predator density, in addition to providing crabs with an alternative food source that may have reduced consumption of ‘normal’ food sources.
Finally, nonlethal predator interference also increases with predator density and likely reduced per capita effects. Predator interference has a greater moderating effect on predation by C. maenas than by H. sanguineus (Griffen and Delaney 2007). This may explain why higher individual consumption rates for C. maenas compared to H. sanguineus that have previously been reported (Lohrer and Whitlatch 2002b; Griffen 2006; Griffen and Byers 2006a) did not translate into higher impacts of C. maenas in our experiments as multiple individuals all foraged together (Fig. 1).
Though effects of increasing predator density were smaller and more variable than expected, incorporating natural differences in population densities between these two species resulted in a 30–50% larger reduction of all prey types by 40 H. sanguineus than by 10 C. maenas. This trend was not statistically significant because of low power in our experiments, but may still be ecologically important, particularly as impacts accumulate over successive years.
Indirect effects
Carcinus maenas and H. sanguineus caused different indirect effects. Carcinus maenas greatly reduced predation by N. lapillus on barnacles and mussels, consistent with previous reports (Trussell et al. 2003; Trussell et al. 2006). Hemigrapsus sanguineus also reduced N. lapillus predation on barnacles, though its effect was weaker; and H. sanguineus did not reduce N. lapillus predation on mussels (Fig. 2). Indirect effects of crabs on barnacles were trait-mediated (nonsignificant covariate [N. lapillus density] in ANCOVA), but were only apparent in 2005 when the majority of N. lapillus were small, and thus more vulnerable to crab predation.
While both crabs had positive indirect effects on barnacle survival, these were overshadowed by strong direct negative effects of direct barnacle consumption. This was especially true of H. sanguineus at high densities (Fig. 1). Low barnacle densities in turn reduce mussel recruitment by limiting settlement sites (Navarrete and Castilla 1990). Heavy consumption of barnacles by dense H. sanguineus populations has also been reported in other parts of H. sanguineus’ invaded range (Lohrer et al. 2000b). The indirect negative effect of barnacle removal on mussel recruitment could therefore be partially responsible for the large decreases in juvenile mussels in intertidal regions where H. sanguineus has become very abundant, effects that have previously been attributed solely to direct mussel consumption by H. sanguineus (Lohrer and Whitlatch 2002b).
Increased abundance of brown algae in 2006 crab treatments compared to no-crab control treatments (Fig. 1) may represent positive indirect effects of crabs on algae by reducing snail abundance and/or foraging, as has been previously documented (Trussell et al. 2002; Trussell et al. 2003).
Temporal variation in prey recruitment
Community impacts of the two invasive crab predators differed between years (Fig. 1). Carcinus maenas generally had stronger impacts in 2005, while impacts of the two species were more similar in 2006 (Fig. 1, Table 4). These differences in impacts and in redundancy between years may have been influenced by multiple factors, but were likely influenced strongly by differences between years in prey availability. Large temporal and spatial variation in prey recruitment may therefore be a large factor altering the redundancy of these species. Our study thus verifies the hypothesized variation in functional redundancy with environmental variability proposed by Wellnitz and Poff (2001). This context dependency in species effects is likely a widespread, yet underinvestigated, aspect of natural communities (Agrawal et al. 2007). We summarize how both the direct and indirect impacts of these species on the invaded community differed across years in Fig. 3.
Relative effects of 10 C. maenas and 10 H. sanguineus in 2005 and 2006. Circle size gives survival relative to controls in each year (gray circle). Solid lines show direct trophic interactions (all have negative effects on prey). Dashed lines were inferred from experiments in both years and illustrate both density and trait mediated indirect effects. Arrows end at the species affected and show the sign of the interaction. The pathways of indirect interactions are demonstrated by the community member that the arrows pass through en route to the affected species. Note the difference in circle sizes in middle row of food web in 2005 but not 2006
Natural variation in prey recruitment in this system is high, suggesting that community impacts of C. maenas and H. sanguineus are highly variable across years. However, recruitment levels in 2005 appears to be closer to the norm than those in 2006 (Petraitis 1991; Petraitis and Methratta 2006), implying that these species may generally have strong, different community impacts. Further, results here indicate that when natural densities of these species are considered, that H. sanguineus may have larger population level impacts than C. maenas (i.e., 40 H. sanguineus were consistently, although not significantly, higher than 10 C. maenas).
Results here also suggest that detecting nonredundant impacts of multiple consumers is easier when recruitment, and thus prey levels, are high, because high levels provide greater resolution when measuring differences in consumer impact. Consistent with this argument, redundant impacts of these two predators in 2006 likely represented actual similarities in the impacts of these species rather than low power of our experiments to detect differences. Posterior power analyses indicated that, given the small effect sizes (because of low recruitment) and the observed level of variation in predator impacts, we would have needed more than 40 replicates of each treatment to detect differences in impacts of 10 C. maenas and 10 H. sanguineus for any of the prey species.
Conclusions
The persistent accumulation of invasive species within habitats will likely continue to bring species into contact that perform similar and disparate functions. Results here demonstrate that redundancy of invasive species with each other or with native species may still be highly variable and may be influenced by factors not detected when comparing specific effects of isolated individuals. Determining the consequences of species invasion will require more studies that document species impacts within a community context and under varying conditions.
References
Agrawal AA et al (2007) Filling key gaps in population and community ecology. Front Ecol Environ 5:145–152. doi:10.1890/1540-9295(2007)5[145:FKGIPA]2.0.CO;2
Bergmann GT, Motta PJ (2005) Diet and morphology through ontogeny of the nonindigenous Mayan cichlid ‘Cichlasoma (Nandopsis)’ urophthalmus (Gunther 1862) in southern Florida. Environ Biol Fishes 72:205–211. doi:10.1007/s10641-004-1480-1
Bourdeau PE, O’Connor JN (2003) Predation by the nonindigenous Asian shore crab Hemigrapsus sanguineus on macroalgae and molluscs. North East Nat 10:319–334
Brousseau DJ, Baglivo JA (2005) Laboratory investigations of food selection by the Asian shore crab Hemigrapsus sanguineus: algal versus animal preference. J Crust Biol 25:130–134. doi:10.1651/C-2530
Byers JE et al (2002) Directing research to reduce the impacts of nonindigenous species. Conserv Biol 16:630–640. doi:10.1046/j.1523-1739.2002.01057.x
Byrnes J, Witman JD (2003) Impact assessment of an invasive flatworm, Convoluta convoluta, in the Southern Gulf of Maine. J Exp Mar Biol Ecol 293:173–191. doi:10.1016/S0022-0981(03)00166-7
Clavero M, Garcia-Berthou E (2005) Invasive species are a leading cause of animal extinctions. Trends Ecol Evol 20:110. doi:10.1016/j.tree.2005.01.003
Crooks JA (2002) Characerizing ecosystem-level consequences of biological invasion: the role of ecosystem engineers. Oikos 97:153–166. doi:10.1034/j.1600-0706.2002.970201.x
DeGraaf JD, Tyrrell MC (2004) Comparison of the feeding rates of two introduced crab species, Carcinus maenas and Hemigrapsus sanguineus, on blue mussel, Mytilus edulis. Northeastern Nat 11:163–167. doi:10.1656/1092-6194(2004)011[0163:COTFRO]2.0.CO;2
deRivera CE, Ruiz GM, Hines AH, Jivoff P (2005) Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology 86:3364–3376
Elner RW (1980) The influence of temperature, sex, and chela size in the foraging strategy of the shore crab, Carcinus maenas (L.). Mar Behav Physiol 7:15–24
Freeman AS, Byers JE (2006) Divergent induced responses to an invasive predator in marine mussel populations. Science 313:831–833
Glude JB (1955) The effects of temperature and predators on the abundance of the soft-shell clam Mya arenaria in New England. Trans Am Fish Soc 84:13–26
Griffen BD (2006) Detecting emergent effects of multiple predator species. Oecologia 148:702–709
Griffen BD, Byers JE (2006a) Intraguild predation reduces redundancy of predator species in multiple predator assemblage. J Anim Ecol 75:959–966
Griffen BD, Byers JE (2006b) Partitioning mechanisms of predator interference in different habitats. Oecologia 146:608–614
Griffen BD, Delaney DG (2007) Species invasion shifts the strength of predator dependence. Ecology 88:3012–3021
Griffen BD, Williamson T (2008) Influence of predator density on nonindependent effects of multiple predators. Oecologia 155:151–159
Griffen BD, Guy T, Buck J (2008) Inhibition between invasives: a newly introduced predator moderates the impacts of a previously established invasive predator. J Anim Ecol 77:32–40
Hadlock RP (1980) Alarm response of the inter-tidal snail Littorina littorea (L) to predation by the crab Carcinus maenas (L). Biol Bull 159:269–179
Hooper DU et al (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Jensen GC, McDonald PS, Armstrong DA (2002) East meets west: competitive interactions between green crab Carcinus maenas, and native and introduced shore crab Hemigrapsus spp. Mar Ecol Prog Ser 225:251–262
Karatayev AY, Burlakova LE, Padilla DK (2002) The impact of zebra mussels on aquatic communities and their role as ecosystem engineers. In: Leppäkoski E, Olenin S, Gollasch S (eds) Invasive aquatic species of Europe: distributions, impacts and management. Kluwer Scientific Publishers, Germany
Kraemer GP, Sellberg M, Gordon A, Maine J (2007) Eight-year record of Hemigrapsus sanguineus invasion: population dynamics of the invader, resident crabs, and Littorina littorea in western Long Island Sound estuary. North East Nat 14:207–224
Lawton JH, Brown VK (1993) Redundancy in ecosystems. In: Schulze ED, Mooney HA (eds) Biodiversity and ecosystem function, vol 99, pp 255–270
Ledesma ME, O’Connor JN (2001) Habitat and diet of the non-native crab Hemigrapsus sanguineus in southeastern New England. North East Nat 8:63–78
Lohrer AM, Whitlatch RB (2002a) Interactions among aliens: apparent replacement of one exotic species by another. Ecology 83:710–732
Lohrer AM, Whitlatch RB (2002b) Relative impacts of two exotic brachyuran species on blue mussel populations in Long Island Sound. Mar Ecol Prog Ser 227:135–144
Lohrer AM, Fukui Y, Wada K, Whitlatch RB (2000a) Structural complexity and vertical zonation of intertidal crabs, with focus on habitat requirements of the invasive Asian shore crab, Hemigrapsus sanguineus (de Haan). J Exp Mar Biol Ecol 244:203–217
Lohrer AM, Whitlatch RB, Wada K, Yasuo F (2000b) Home and away: comparison of resource utilization by a marine species in native and invaded habitats. Biol Invasions 2:41–57
Lubchenco J (1983) Littorina and Fucus: effects of herbivores, substratum heterogeneity and plant escapes during succession. Ecology 64:1116–1123
McDermott JJ (1998) The western Pacific brachyuran (Hemigrapsus sanguineus: Grapsidae), in its new habitat along the Atlantic coast of the United States: geographic distribution and ecology. J Mar Sci 55:289–298
Menge BA (1976) Organization of the New England rocky intertidal community: role fo predation, competition, and environmental heterogeneity. Ecol Monogr 46:355–393
Moksnes P (2004) Self-regulating mechanisms in cannibalistic populations of juvenile shore crabs Carcinus maenas. Ecology 85:1343-1354
Navarrete SA, Castilla JC (1990) Barnacle walls as mediators of intertidal mussel recruitment: effects of patch size on the utilization of space. Mar Ecol Prog Ser 68:113–119
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Petraitis PS (1991) Recruitment of the mussel Mytilus edulis L. on sheltered and exposed shores in Maine, USA. J Exp Mar Biol Ecol 147:65–80
Petraitis PS, Methratta ET (2006) Using patterns of variability to test for multiple community states on rocky intertidal shores. J Exp Mar Biol Ecol 338:222–232
Richards MG, Huxham M, Bryant A (1999) Predation: a causal mechanism for variability in intertidal bivalve populations. J Exp Mar Biol Ecol 241:159–177
Rossong MA, Williams PJ, Comeau M, Mitchell SC, Apaloo J (2006) Agonistic interactions between the invasive green crab, Carcinus maenas (Linnaeus) and juvenile American lobster, Homarus americanus (Milne Edwards). J Exp Mar Biol Ecol 329:281–288
Sanders NJ, Gotelli NJ, Heller NE, Gordon DM (2003) Community disassembly by an invasive species. Proc Natl Acad Sci USA 100:2474–2477
Sax DF (2002) Species invasions exceed extinctions on islands worldwide: a comparative study of plants and birds. Am Nat 160:766–783
Sax DF, Gaines SD (2003) Species diversity: from global decreases to local increases. Trends Ecol Evol 18:561–566
Schooler SS, McEvoy PB, Coombs EM (2006) Negative per capita effects of purple loosestrife and reed canary grass on plant diversity of wetland communities. Divers Distrib 12:351–363
Seeley RH (1986) Intense natural selection caused a rapid morphological transition in a living marine snail. Proc Natl Acad Sci USA 83:6897–6901
Trussell GC, Ewanchuk PJ, Bertness MD (2002) Field evidence of trait-mediated indirect interactions in a rocky intertidal food web. Ecol Lett 5:241–245
Trussell GC, Ewanchuk PJ, Bertness MD (2003) Trait-mediated effects in rocky intertidal food chains: predator risk cues alter prey feeding rates. Ecology 84:629–640
Trussell GC, Ewanchuk PJ, Matassa CM (2006) Habitat effects on the relative importance of trait-and density-mediated indirect interactions. Ecol Lett 9:1245–1252
Tyrrell MC, Harris LG (1999) Potential impact of the introduced Asian shore crab Hemigrapsus sanguineus, in northern New England: diet, feeding preferences, and overlap with the green crab, Carcinus maenas. In: Pederson J (ed) Proceedings of the National Conference on Marine Bioinvasions, pp 208–220
Tyrrell MC, Guarino PA, Harris LG (2006) Predatory impacts of two introduced crab species: inferences from mesocosms. North East Nat 13:375–390
Wellnitz T, Poff L (2001) Functional redundancy in heterogeneous environments: implications for conservation. Ecol Lett 4:177–179
Whitlow WL, Rice NA, Sweeney C (2003) Native species vulnerability to introduced predators: testing an inducible defense and a refuge from predation. Biol Invasions 5:23–31
Acknowledgments
We thank D. Niemaszyk, G. Goldsmith, and A. Malek for help with field experiments. We also thank I. Altman, A. Blakeslee, A. Freeman, J. H. Grabowski, W. J. Lee, L. Page, M. J. Shulman, and anonymous reviewers for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Griffen, B.D., Byers, J.E. Community impacts of two invasive crabs: the interactive roles of density, prey recruitment, and indirect effects. Biol Invasions 11, 927–940 (2009). https://doi.org/10.1007/s10530-008-9305-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-008-9305-3