Abstract
Purpose
Influenza is one of the most important agents of pandemic outbreak causing substantial morbidity and mortality. Vaccination strategies of influenza must be adapted annually due to constant antigenic changes in various strains. Therefore, the present study was conducted to evaluate protective immunity of the conserved influenza proteins.
Methods
For this purpose, three tandem repeats of M2e (3M2e) and NP were separately expressed in E. coli and were purified using column chromatography. Female Balb/c mice were injected intradermally with a combination of the purified 3M2e and NP alone or formulated with Alum (AlOH3) adjuvant in three doses. The mice were challenged by intranasal administration of H1N1 (A/PR/8/34) 2 weeks after the last vaccination.
Results
The results demonstrated that recombinant NP and M2e proteins are immunogenic and could efficiently elicit immune responses in mice compared to non-immunized mice. The combination of 3M2e and NP supplemented with Alum stimulated both NP and M2e-specific antibodies, which were higher than those stimulated by each single antigen plus Alum. In addition, the secretion of IFN-γ and IL-4 as well as the induction of lymphocyte proliferation in mice received the mixture of these proteins with Alum was considerably higher than other groups. Moreover, the highest survival rate (86%) with the least body weight change was observed in the mice immunized with 3M2e and NP supplemented with Alum followed by the mice received NP supplemented with Alum (71%).
Conclusion
Accordingly, this regimen can be considered as an attractive candidate for global vaccination against influenza.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In today’s corporate climate, acute respiratory predicaments in patients are rudimentarily triggered by a well-known disease called as the influenza, responsible for infection of 10–20% of global population, whose annual epidemics lead to 3–5 million cases of detrimental disease and 250,000–500,000 deaths for which vaccination is the best solution to prevent infection of the influenza virus (Rappazzo et al. 2016).
The ability for mutation and genetic recombination frequency in these viruses, particularly in the influenza A virus causes antigenic changes or new emerging subtypes (Farahmand et al. 2019). Prevalent formulation of the licensed influenza vaccines in human population comprises split viral particles replicated in embryonated eggs or eukaryotic cells. They are based on surface glycoproteins of hemagglutinin (HA) and neuraminidase (NA) (Fiers et al. 2009). Considering this fact, since these two proteins are constantly exposed to mutations, traditional influenza vaccines cannot be effective against the current seasonal influenza epidemics (Slepushkin et al. 1995). Therefore, researchers have focused on using highly conserved regions of viral proteins as effective universal influenza vaccines (UIVs) that can be rapidly expressed and purified by molecular biology techniques (Rappazzo et al. 2016). The M2 extracellular domain (M2e) has been discovered as the basis of UIVs as its sequence is highly conserved in all human isolates since 1933. M2e antigen has a small size and vulnerable immunogenicity, so it does not seem to be a sufficient recombinant influenza vaccine. A promising solution to this problem is using tandem repeats of M2e peptide and adjuvants (Mardanova et al. 2015).
Another conserved antigen that can constitute UIVs is the nucleoprotein (NP) (Bernelin-Cottet et al. 2016). This protein creates protection against influenza A virus (IAV) as a protein vaccine (MacLeod et al. 2013.) Cellular immunity is propagated by protected sequence of NP, which is one of the most significant and ideal targets of host CD8+ T cells (Thomas et al. 2006).
So far, some research groups have focused on the use of M2e and NP proteins. In one study, De Filette et al. showed that the UIV M2e-HBc completely protected mice from a lethal infection when combined with safe and effective CTA1-DD adjuvant (De Filette et al. 2006a,b). In addition, Guo et al. studied on immunization of recombinant nucleoprotein (rNP) of the influenza virus and reported that rNP with cholera toxin B as an adjuvant provides complete protection in mice against the influenza virus (Guo et al. 2010). Moreover, Gao et al. demonstrated that 3M2e-NP-HBc (consisting of three tandem copies of M2e (3M2e), NP epitopes, and hepatitis B virus core) in combination with an oil-in-water SP01 adjuvant caused protection against a lethal challenge induced by the Beijing501 or China097 virus (Gao et al. 2013).
One of the adjuvants used in human and animal vaccines, involving aluminum hydroxide adjuvant is briefly called as the Alum (Eisenbarth et al. 2008). This adjuvant can increase antigen absorption at the site of injection and enhance immunogenicity of an antigen (Mbow et al. 2010; Morefield et al. 2005). Furthermore, Alum is an inexpensive and safe adjuvant. Predominantly, Alum adjuvant is responsible for spurring T Helper 2 (Th2) cytokines and B cells producing Th2-related antibodies, immunoglobulin G1 (IgG1), and immunoglobulin E (IgE) and contributes to boosting humoral immunity (Awate et al. 2013; Jordan et al. 2004; Reed et al. 2009). Additionally, Alum adjuvant raises secretion of interleukin 1 beta (IL-1β), CC-chemokine ligand 2 (CCL2; monocyte chemoattractant protein 1 (MCP1)), CC-chemokine ligand 11 (CCL11) (exotoxin), histamine, and interleukin 5 (IL-5) (Kool et al. 2008).
Therefore, in the present study, NP and 3M2e were expressed in Escherichia coli and were purified using Ni-TED columns. Immunogenic potency and protection efficacy of mixture of these two proteins supplemented with or without Alum adjuvant were evaluated as a universal subunit vaccine against influenza infection in Balb/c mice.
Materials and methods
Materials
Protino™ Ni-TED-IDA 1000 kit and DNA extraction kit were supplied by Macherey Nagel TM Company (Germany) and Bioneer Company (South Korea), respectively. Mouse-adapted human influenza A virus (H1N1, PR/8/34), two E. coli strains [TOP10 and BL21 (DE3)], and 6–8‐week‐old female BALB/c mice were supplied from the Pasteur Institute of Iran, Tehran, Iran. Acrylamide and sodium dodecyl sulphate (SDS) were prepared from Merck Company (Germany). Isopropyl β-d-1-thiogalactopyranoside (IPTG), dialysis membrane bag, tetramethylbenzidine (TMB), MTT salt (3-(4,5-dimethyl tetrazolyl-2) 2,5 diphenyl), and horseradish peroxidase (HRP) conjugated anti-mouse immunoglobulin G (IgG) were supplied from Sigma-Aldrich Company (USA). Enzyme-linked immunosorbent Assay (ELISA) plate was obtained by Greiner Company. Alum adjuvant was prepared from Alhydrogel®2% (Brenntag Biosector, Denmark) (CAS Number:21645-51-2).
Production of recombinant proteins (M2e and NP) in prokaryotic system
NP and 3M2e genes were previously cloned and expressed (Shokouhi et al. 2018; Yousefi-Najafabadi et al. 2013). Confirmed pET28a-3M2e and pET28a-NP plasmids were multiplied by transformation into competent E. coli BL21 (DE3) using a heat shock method. After an overnight incubation of the plates at 37 °C, positive clones were cultured in Luria–Bertani (LB) broth to an OD600 of 0.6, and the bacteria were agitated by adding 1 mM IPTG solution and were incubated for more than 3 h. After harvesting the bacteria by centrifugation, for cell disruption and extraction of recombinant proteins, bacterial pellets were lysed in the lysis-equilibration-wash (LEW) buffer, were incubated at 4 °C for 30 min and then, were sonicated. This process was repeated two more times so that, all the proteins were extracted. Afterwards, for purification of target proteins, Protino™ Ni-TED affinity chromatography technique was used. The purified proteins were evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Low-weight substances, such as urea and salt were removed by a dialysis membrane bag. Finally, NP and 3M2e concentrations were determined by Bradford method.
Immunization of Balb/c mice
Eighty female mature Balb/c mice were housed in the animal facility at the Pasteur Institute of Iran, Tehran, Iran; and they were randomly assigned into eight groups of ten animals. Two groups of animals were injected by recombinant proteins (3M2e and NP) alone or supplemented with Alum. Other two groups were injected by the mixture of 3M2e and NP alone or plus Alum. Two negative control groups received Alum adjuvant or phosphate-buffered saline (PBS). Mice were injected subcutaneously in 2-week interval in three doses. The recombinant proteins’ dose used in all the study groups was equal to 15 μg per injection in a total volume of 100 μl (Table 1). All these procedures were performed based on the protocols approved by the Ethics Committee of the Pasteur Institute of Iran (IR.PII.REC.1395.82).
Antibody responses in mice
Fourteen days after the last immunization, sera samples were collected and assayed for total specific anti-M2e and anti-NP IgG using ELISA technique. In brief, M2e synthetic peptide (GeneScripte: RP20206) and NP synthetic peptide (Biomatik: RPC20340) with a final concentration of 10–4 and 10–2 mg/ml were coated, respectively, were placed in ELISA plate and were incubated overnight at 4 °C. Mice sera were prepared at 1:1000 dilutions. Total specific anti-M2e and anti-NP IgG were evaluated by a number of reagents composed of HRP conjugated anti-mouse IgG (Sigma), or secondary antibody; the TMB substrate, and stop solution (H2SO4). Absorbance was measured by ELISA reader at 450 nm (Saleh et al. 2020).
Cell proliferation assay
For determining T-lymphocyte functions, the spleens of three mice from each group were removed and the cells were prepared as described previously (Shokouhi et al. 2018). Briefly, spleen cells were cultured in 96-well plates (2 × 104 cells/well) in triplicate, were incubated at 37 °C in 5% CO2 atmosphere for 1 h and were agitated by stimuli consisting of M2e peptide (accession number: ACF41880), NP (accession number: LC120392), PBS, and phytohemagglutinin (PHA). Amount of each stimulus was equal to 2 μg per well. After 48 h, 30 μl of 5 mg/ml MTT salt was added to each well and was incubated at 37 °C for 4 h in darkness. When, MTT was reduced to formazan, 100 μl of dimethyl sulfoxide (DMSO) was added to dissolve formazan crystals. The OD450 was measured using the microplate reader. Finally, stimulation index (SI) was obtained for each mouse using the following formula.
Cytokine assay
Cytokine profile was investigated in the immunized mice. Briefly, 48 h after isolation of splenocytes’ culture and stimulation, interferon-γ (IFN-γ) and interleukin 4 (IL-4) were detected using commercial ELISA kit (R&D). The optical density was measured at 45 nm.
Influenza challenge
Fifteen days after the last vaccination, the immunized mice were challenged by intranasal administration of one LD90 (lethal dose of the H1N1 (A/PR/8/34) to 90% of mice) and were kept under safety cabinet. Survival rate and weight loss were monitored daily for 2 weeks. Weight percentage of each mouse was assessed by comparing daily weight with the pre-challenge weight.
Data analysis
Data were analyzed using the Excel software and Graph Pad Prism 6.0 software. We have used ANOVA with Tukey’s honestly significant difference (HSD) post hoc test which indicates the groups which are significantly different from each other while correcting alpha for multiple comparisons. Additionally, significant differences between body weight curves were evaluated via Kaplan–Meier survival analysis.
Results
Expression, extraction, and purification of recombinant proteins (3M2e and NP)
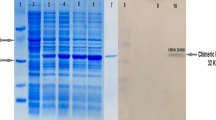
Expression of 2 peptides, 3M2e and NP with molecular mass corresponding to 14 and 57 KD, respectively was analyzed by SDS-PAGE (Fig. 1). Recombinant proteins reached their maximum level of expression 4 h after IPTG induction. Also, extraction and purification steps of recombinant proteins are shown in Fig. 1.
SDS-PAGE results related to expression and purification of the recombinant proteins in (DE3) BL21 E. coli. Lane 1: protein marker; Lanes 2 & 3: expression of 3M2e before induction and 4 h after IPTG induction, Lanes 5 & 6: expression of NP before induction and 4 h after IPTG induction, Lanes 4 & 7: purification of 3M2e and NP, respectively
Measurement of specific antibodies
For measuring IgG antibodies, blood samples of the vaccinated mice were taken 14 days after the last immunization and were tested by the ELISA technique. The results obtained from measuring anti-M2e IgG demonstrated that antibody induction was significantly higher in all the vaccinated groups than the control groups. Mice immunized with the mixture of 3M2e and NP with Alum adjuvant indicated the highest level of specific anti-M2e antibodies (Fig. 2a).
Analysis of IgG antibody responses in the blood samples of the vaccinated mice against M2e protein (A) and NP peptide (B) using ELISA test. Value of each serum was evaluated at OD 450 nm. Antibody induction in all the immunized groups showed significant results compared to control groups (p value ≤ .01–.0001)
The results of anti-NP IgG evaluation also showed that the group vaccinated by the mixture of 3M2e and NP formulated with Alum had the increased anti-NP antibody titer considerably more than the groups immunized by NP, NP plus Alum, and the combination of 3M2e and NP (Fig. 2b).
Lymphocyte proliferation assay
For determining T lymphocyte activation, three mice from each group were sacrificed; the spleen cells were cultured, and were stimulated by 3M2e and NP for measuring specific cellular responses. PBS was also used for induction as negative control. The results of lymphocyte proliferation assay are shown in Fig. 3a and b. All the vaccinated groups could induce significantly higher level of lymphocyte proliferation than the control groups when they were stimulated by antigen peptide. Among the vaccinated groups, the group immunized with the combination of 3M2e and NP plus Alum had the highest SI.
Measurement of lymphocyte proliferation in response to in-vitro specific stimulation by M2e (A) and NP (B) peptides. Proliferation of splenocytes was measured via the MTT method one week after the final vaccination. P-values less than 0.001 (***) and 0.0001 (****) indicate high significant differences among the specified groups
Cytokine assay
The results of cytokine assay indicated that 3M2e and NP stimulated cell immunity responses and raised production of IFN-γ and IL-4 in the immunized mice compared to the unvaccinated control groups.
The group that received the mixture of these two proteins showed a significantly higher difference in production of IFN-γ and IL-4 compared to the groups that received the protein alone. Furthermore, the combination of 3M2e and NP plus Alum caused secretion of higher concentrations of IFN-γ and IL-4 than the other groups (Fig. 4). In this group, IFN-γ concentration of NP-stimulated splenocytes (mean = 279.14) was detected more than that of 3M2e-stimulated splenocytes (mean = 150.89).
Detection of IFN-γ after stimulation of splenocytes’ culture with M2e peptide (a) and NP protein (b) and concentration of IL-4 in splenocytes’ culture stimulated with M2e peptide (c) and NP protein (d). Cytokine concentration was measured using ELISA test one week after the last vaccination. P-values less than 0.05 (*), 0.01 (**), 0.001 (***), and 0.0001 (****) demonstrate significant differences among the specified groups
Lethal challenge
Two weeks after the last immunization, all animal groups were challenged with one LD90 of the A/PR8/H1N1 virus and were monitored for 15 days to measure mortality and morbidity rate. The highest survival rate (86%) was observed in the mice immunized with 3M2e and NP supplemented with Alum followed by the mice received NP supplemented with Alum (71%), while all the animals in negative control groups died at the end of 11 days (Fig. 5a). The least protection (29%) was seen in the mice injected with 3M2e alone. Since, number of the survived mice in 3M2e-vaccinated group was not adequate for statistical analysis; this group was not considered for calculation of morbidity rate. As shown in Fig. 5b, body weight was reduced quickly in both vaccinated and control groups after viral inoculation and it was recovered 7 days post-challenge. Among the vaccinated groups, change in body weight was slower in the mice vaccinated by the combination of 3M2e and NP plus Alum than the other groups and remaining animals in this group achieved their primary body weight at the end of experiment.
Discussion
Regarding the use of subunit vaccines, it should be mentioned that they have insufficient immunogenicity of most protein antigens when rendered as pure substances. For eliminating this restriction, these low immunogen proteins should be linked to immune-enhancing substances or appropriate adjuvants (Rafati et al. 2007). Alum has been most commonly used as a safe adjuvant for over 70 years. It acts as an antigen depot in the administration site and releases antigen slowly in a particular form to the antigen-presenting cells (APCs), thereby, stability and immunogenicity of the antigen are enhanced. Alum has been shown to stimulate innate immune system and likely promoting secretion of T Helper 2 (Th2) cytokines and B cells resulting in robust antibody production (Lee et al. 2014). Vaccination strategies of influenza must be adapted annually, due to high variability of hemagglutinin and neuraminidase (Huleatt et al. 2008). A UIV consisting of virus conserved antigen induces broader spectrum protection against multiple strains. Among these conserved antigens, an 18–24 amino acid region of the M2 protein and NP are candidate target antigens of the current studies on influenza vaccination (Kaiser 2006; Pica and Palese 2013; Tompkins et al. 2007). It has been shown that M2e raised adequate antibody titers in blood of the mice after vaccination (Zheng et al. 2014); anti-M2e antibodies blocked the virus replication in lungs of the mice and protected them effectively against IAV infection (Ebrahimi and Tebianian 2011). It is conceived that protection against influenza infection is somewhat related to specific cytotoxic T lymphocyte (CTL) responses against NP (Li et al. 2013) and anti-NP antibodies could not play a critical role in virus neutralization. However, Carragher et al. demonstrated that anti-NP induced high titers of antibodies, which could interact with NP in early stage of infection likely reducing virus replication and morbidity (Carragher et al. 2008). Since, new subunit vaccines only consist of disease antigenic proteins, and they have insufficient auxiliary stimuli to provoke the immune system; researchers have focused on improving immunogenicity of subunit vaccine. Huleatt et al. reported that four copies of M2e in fused form to the toll-like receptor 5(TLR5) ligand flagellin boosted potency of specific immune response to antigens and protected mice after challenging with influenza virus (Huleatt et al. 2008). Other reports have indicated that repeated copies of M2e induced significantly stronger antibody responses when fused to the protein carriers (De Filette, et al. 2006a,b; Zhao et al. 2010a,b). In our previous study, the 3M2e was conjugated with Leishmania major heat shock protein (HSP70) as an adjuvant and improved the ability of 3M2e to elicit an effective immune response in mice (Shokouhi et al. 2018). Another study has shown that NP is capable of protecting mice against challenge with IAV when encoded by heterologous viral carriers (Li et al. 2013). Yang et al. showed that fusion of full-length NP and M1 proteins and HSP60 induced high levels of specific IgG antibodies, Th1/Th2-associated immune responses, and completely protected mice from lethal challenge with influenza H7N9 virus (Yang et al. 2014). Also, protection against a heterologous IAV using an NP–M2e fusion protein supplemented with Al(OH)3 adjuvant in a mouse model has been also reported (Wang et al. 2012). In this study, a UIV was produced based on NP and M2e proteins and was expressed in prokaryotic system. For assessing immunogenicity of these conserved proteins, measuring the specific antibody responses, lymphocyte proliferation test, and cytokine assay were done and finally, mice were challenged with LD90 of the H1N1. The results of ELISA tests in this study indicated that IgG antibody responses were elevated in all the vaccinated groups. A vaccine regimen containing 3M2e and NP formulated with Alum induced the highest anti-M2e and anti-NP antibodies. In fact, Co-administration of two different antigens elicited stronger antigen-specific IgG responses in comparison with the single-antigen vaccines that might due to synergistic effects. Similarly, in a recent study, a dual-antigen influenza vaccine with interior NP/exterior M2e or with interior M2e/exterior NP concurrently induced humoral immune responses—that is—M2e and NP-specific antibodies for which synergistic effects were observed (Wei et al. 2020).
Although, M2 is scarcely present on influenza virus envelope, it is expressed on the influenza-infected cell surface abundantly and is accessible to antibodies (Lamb et al. 1985). In our study, 3M2e promoted more antibody induction than internal NP protein and NP could enhance cellular immune responses as a major internal virion protein that can be recognized by host CTLs (Thomas et al. 2006). Considering this fact, results of this study revealed that vaccination with the combination of NP and 3M2e plus Alum led to the highest induction of lymphocyte proliferation, IFN-γ, and IL-4. Th2 cytokines (IL-4, IL-5, interleukin 6 (IL-6), and interleukin 10 (IL-10)) are associated with antibody production, whereas Th1 cytokines (IFN-γ and interleukin 2 (IL-2)) are related to both B- and T-cell activities (Bernstein et al. 1998). Thus, as observed in this study, the combination of 3M2e and NP supplemented with Alum induced superior humoral and cellular immune responses and was able to provide protection against infection by reducing weight loss and increasing survival rate. Although, the mechanism by which this cocktail vaccine could improve immunogenicity and protection of the subunit protein is not clear, it can be said that this phenomenon is mostly related to stimulation of T cells by NP. However, further cytokines involving in immune responses including IL-2, interleukin 12 (IL-12), interleukin 17 (IL-17), and tumor necrosis factor alpha (TNF-α) should be investigated and the immunized animals should be challenged against other mouse-adapted IAVs in order to be more confident about immunogenicity and effectiveness of these proteins.
Data availability
All data are available in case of need.
Abbreviations
- 3M2e:
-
Three tandem repeats of M2e
- M2e:
-
M2 extracellular domain
- HA:
-
Hemagglutinin
- NA:
-
Neuraminidase
- UIV:
-
Universal influenza vaccines
- NP:
-
Nucleoprotein
- IAV:
-
Influenza A virus
- rNP:
-
Recombinant nucleoprotein
- Th2:
-
T Helper 2
- IgG1:
-
Immunoglobulin G1
- IgE:
-
Immunoglobulin E
- SDS:
-
Acrylamide and sodium dodecyl sulphate
- IPTG:
-
Isopropyl β-d-1-thiogalactopyranoside
- TMB:
-
Tetramethylbenzidine
- 3-(4,5-dimethyl tetrazolyl-2) 2,5 diphenyl:
-
MTT salt
- HRP:
-
Horseradish peroxidase
- ELISA:
-
Enzyme-linked immunosorbent assay
- LEW:
-
Lysis-equilibration-wash
- PBS:
-
Phosphate buffer saline
- DMSO:
-
Dimethyl sulfoxide
- SI:
-
Stimulate index
- IFN-γ:
-
Interferon-γ
- IL-4:
-
Interleukin 4
- LD90 :
-
Lethal Dose 90
- APCs:
-
Antigen-presenting cells
- HSP70:
-
Heat shock proteins
- CTL:
-
Cytotoxic T lymphocyte
- LB Broth:
-
Luria-Bertani Broth
References
Awate S, Babiuk L, Mutwiri G (2013) Mechanisms of action of adjuvants. Front Immunol 4:114
Bernelin-Cottet C, Deloizy C, Stanek O, Barc C, Bouguyon E, Urien C, Da Costa B (2016) A universal influenza vaccine can lead to disease exacerbation or viral control depending on delivery strategies. Front Immunol 7:641
Bernstein ED, Gardner EM, Abrutyn E, Gross P, Murasko DM (1998) Cytokine production after influenza vaccination in a healthy elderly population. Vaccine 16:1722–1731
Carragher D, Kaminski D, Moquin A, Hartson L, Randall T (2008) A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol Res 181:4168–4176
De Filette M, Fiers W, Martens W, Birkett A, Ramne A, Löwenadler B, Saelens X (2006a) Improved design and intranasal delivery of an M2e-based human influenza A vaccine. Vaccine 24:6597–6601
De Filette M, Ramne A, Birkett A, Lycke N, Löwenadler B, Jou W, Fiers W (2006b) The universal influenza vaccine M2e-HBc administered intranasally in combination with the adjuvant CTA1-DD provides complete protection. Vaccine 24:544–551
Ebrahimi S, Tebianian M (2011) Influenza A viruses: why focusing on M2e-based universal vaccines. Virus Genes 42:1–8
Eisenbarth S, Colegio O, O’Connor W, Sutterwala F, Flavell RA (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453:1122–1126
Farahmand B, Taheri N, Shokouhi H, Soleimanjahi H, Fotouhi F (2019) Chimeric protein consisting of 3M2e and HSP as a universal influenza vaccine candidate: from in silico analysis to preliminary evaluation. Virus Genes 55:22–32
Fiers W, De Filette M, El Bakkouri K, Schepens B, Roose K, Schotsaert M, Saelens X (2009) M2e-based universal influenza A vaccine. Vaccine 27:6280–6283
Gao X, Wang W, Zhang S, Duan Y, Xing L, Wang X (2013) Enhanced influenza VLP vaccines comprising matrix-2 ectodomain and nucleoprotein epitopes protects mice from lethal challenge. Antivir Res 98:4–11
Guo L, Zheng M, Ding Y, Yang Z, Wang H, Chen Z (2010) Protection against multiple influenza A virus subtypes by intranasal administration of recombinant nucleoprotein. Arch Virol 155:1765–1775
Huleatt J, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, Umlauf S (2008) Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine 26:201–214
Jordan M, Mills D, Kappler J, Marrack P, Cambier J (2004) Promotion of B cell immune responses via an alum-induced myeloid cell population. Science 304:1808–1810
Kaiser J (2006) A one-size-fits-all flu vaccine. Sience|AAAS 380–382
Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, Van Nimwegen M, Tschopp J (2008) Cutting edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol Res 181:3755–3759
Lamb R, Zebedee S, Richardson C (1985) Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell 40:627–633
Lee Y, Kim K, Ko E, Lee Y, Kim M, Kwon Y, Kang S (2014) New vaccines against influenza virus. Clin Exp Vaccine Res 3:12–28
Li Z, Gabbard J, Mooney A, Gao X, Chen Z, Place R, He B (2013) Single-dose vaccination of a recombinant parainfluenza virus 5 expressing NP from H5N1 virus provides broad immunity against influenza A viruses. J Virol 87:5985–5993
MacLeod M, David A, Jin N, Noges L, Wang J, Kappler J, Marrack P (2013) Influenza nucleoprotein delivered with aluminium salts protects mice from an influenza A virus that expresses an altered nucleoprotein sequence. PLoS ONE 8:e61775
Mardanova E, Kotlyarov R, Kuprianov V, Stepanova L, Tsybalova L, Lomonossoff G, Ravin N (2015) High immunogenicity of plant-produced influenza based on the M2e peptide fused to flagellin. Biotechnol Lett 15:25
Mbow M, De Gregorio E, Valiante N, Rappuoli R (2010) New adjuvants for human vaccines. Curr Opin Immunol 22:411–416
Morefield G, Sokolovska A, Jiang D, HogenEsch H, Robinson J, Hem S (2005) Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 23:1588–1595
Pica N, Palese P (2013) Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med 64:189–202
Rafati S, Gholami E, Hassani N, Ghaemimanesh F, Taslimi Y, Taheri T, Soong L (2007) Leishmania major heat shock protein 70 (HSP70) is not protective in murine models of cutaneous leishmaniasis and stimulates strong humoral responses in cutaneous and visceral leishmaniasis patients. Vaccine 25:4159–4169
Rappazzo C, Watkins H, Guarino C, Chau A, Lopez JL, DeLisa M, Putnam D (2016) Recombinant M2e outer membrane vesicle vaccines protect against lethal influenza A challenge in BALB/c mice. Vaccine 34:1252–1258
Reed S, Bertholet S, Coler R, Friede M (2009) New horizons in adjuvants for vaccine development. Trends Immunol 30:23–32
Saleh M, Nowroozi J, Farahmand B, Fotouhi F (2020) An approach to the influenza chimeric subunit vaccine (3M2e-HA2-NP) provides efficient protection against lethal virus challenge. Biotechnol Lett. https://doi.org/10.1007/s10529-020-02822-3
Shokouhi H, Farahmand B, Ghaemi A, Mazaheri V, Fotouhi F (2018) Vaccination with three tandem repeats of M2 extracellular domain fused to Leismania major HSP70 protects mice against influenza A virus challenge. Virus Res 251:40–46
Slepushkin VA, Katz JM, Black RA, Gamble WC, Rota PA, Cox NJ (1995) Protection of mice against influenza A virus challenge by vaccination with baculovirus-expressed M2 protein. Vaccine 13:1399–1402
Thomas P, Keating R, Hulse-Post D, Doherty P (2006) Cell-mediated protection in influenza infection. Emerg Infect Dis 12:48
Tompkins S, Zhao Z, Lo C, Misplon J, Liu T, Epstein S (2007) Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis 13:426
Wang W, Huang B, Jiang T, Wang X, Qi X, Gao Y, Ruan L (2012) Robust immunity and heterologous protection against influenza in mice elicited by a novel recombinant NP–M2e fusion protein expressed in E. coli. PLoS ONE 7:e52488
Wei J, Li Z, Yang Y, Ma X, An W, Ma G, Zhang S (2020) A biomimetic VLP influenza vaccine with interior NP/exterior M2e antigens constructed through a temperature shift-based encapsulation strategy. Vaccine 38:5987–5996
Yang P, Wang W, Gu H, Li Z, Zhang K, Wang Z, Wang X (2014) Protection against influenza H7N9 virus challenge with a recombinant NP–M1–HSP60 protein vaccine construct in BALB/c mice. Antivir Res 111:1–7
Yousefi-Najafabadi Z, Behzadian F, Fotouhi-Chahooki F, Farahmand B (2013) Prokaryotic expression of influenza A virus nucleoprotein fused to mycobacterial heat shock protein70. Iran J Virol 7:7–14
Zhao G et al (2010a) Induction of protection against divergent H5N1 influenza viruses using a recombinant fusion protein linking influenza M2e to Onchocerca volvulus activation associated protein-1 (ASP-1) adjuvant. Vaccine 28:7233–7240
Zhao G, Lin Y, Du L, Guan J, Sun S, Sui H, Zheng B (2010b) An M2e-based multiple antigenic peptide vaccine protects mice from lethal challenge with divergent H5N1 influenza viruses. Virol J 7:1–8
Zheng M, Luo J, Chen Z (2014) Development of universal influenza vaccines based on influenza virus M and NP genes. Infection 42:251–262
Acknowledgements
This study was performed in the Department of Influenza and Other Respiratory Viruses, Pasteur Institute of Iran and was also financially supported by the Pasteur Institute of Iran (Grant Number: 759). Hereby, the authors would like to show their gratitude to all the staff working in Pasteur Institute of Iran for their valuable assistance and cooperation.
Funding
This work was supported by the Pasteur Institute of Iran (Grant Number 759).
Author information
Authors and Affiliations
Contributions
(MS): Study concept and design; Development of the protocol; Acquisition, analysis and interpretation of data; Draft of the manuscript, wrote and prepared the manuscript; and Critical revision of the manuscript for important intellectual content. (JN): Study concept and design; Supervision; Administrative; Technical and material support. (BF): Study concept and design; Supervision; Administrative; Technical, and material support. (FF): Study concept and design; Development of the original idea and the protocol; Administrative; Technical, and material support; Supervision; and Interpretation of data.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interests.
Ethical approval
All the animal experiments were approved and performed based on the guidelines of the Ethical Committee of the Pasteur Institute of Iran, Tehran, Iran (IR.PII.REC.1395.82).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Forqani, M., Hosseini, S.M., Farahmand, B. et al. Combination of conserved recombinant proteins (NP & 3M2e) formulated with Alum protected BALB/c mice against influenza A/PR8/H1N1 virus challenge. Biotechnol Lett 43, 2137–2147 (2021). https://doi.org/10.1007/s10529-021-03174-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03174-2