Abstract
The immunogenicity and protective properties of the designed recombinant fusion peptide of 3M2e and truncated nucleoprotein (trNP), originating from Influenza A virus, were investigated in the BALB/c mice model in comparison with the Mix protein (3M2e + trNP). The results were evaluated by antibody response, cytokine production, lymphocyte proliferation assay, and mortality rate after challenge with homologous (H1N1) and heterologous (H3N2) influenza viruses in BALB/c mice. The animals that received the chimer protein with or without adjuvant had more specific antibody responses and elicited memory CD4 T cells, and cytokines of Th1 and Th2 cells compared to the Mix protein. Moreover, the Mix protein, like the recombinant chimer protein, provided equal and effective protection against both homologous and heterologous challenges in mice. Nevertheless, the chimer protein demonstrated superior immune protection compared to the Mix protein. The percentage of survived animals in the adjuvanted protein group (78.4%) was less than the non-adjuvanted one (85.7%). However, the Mix protein plus Alum could induce protective immunity in only 57.1% and 42.8% of homologous and heterologous virus-challenged mice, respectively. Regarding the sufficient immunogenicity and protectivity of the chimer protein construct against influenza viruses, the findings of the study suggest that the chimer protein without a requirement of adjuvant can be used as an adequate vaccine formulation to protect against a broad spectrum of influenza viruses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza viruses are highly contagious respiratory pathogens that are mainly transmitted through respiratory droplets by coughing or sneezing of the infected people. Seasonal influenza epidemics cause about 3–5 million cases of severe illness and about 290,000 to 650,000 deaths each year [1]. The antigenic evolution through the point mutations in the genes of influenza viruses (antigenic drift) and genetic reassortments between human and avian viruses (antigenic shift) have led to annual epidemics and global pandemics [2]. Vaccination is the most effective strategy for preventing the spread of influenza disease. However, seasonal vaccination does not afford protection against pandemic strains of novel subtypes due to antigenic drift and needs to be reformulated annually [3, 4]. Therefore, a universal influenza vaccine could induce cross-reactive antibodies and cross-reactive CD8+ T cell responses against conserved proteins of Influenza viruses [5, 6]. According to the data published by the Centre for Disease Prevention and Control (CDC), influenza vaccination reduces the risk of influenza illness by between 40 and 60% among the overall population during seasons when most circulating influenza viruses are well matched to the influenza vaccine [7].
Ecto-domain of matrix protein 2 (M2e), stem subunit of the hemagglutinin glycoprotein (HA2), and nucleoprotein are the most conserved proteins of the influenza A viruses [8, 9]. Matrix protein 2 (M2) is a homotetrameric proton channel responsible for the release of the viral genome during viral entry. M2 protein has 97 amino acids which consist of three domains [10]. The extracellular domain of M2 (M2e) is composed of 23 amino acid residues which are highly conserved at its 9 N-terminal amino acids among all influenza A subtypes and it is considered an attractive target to produce the global vaccine [11]. Due to the presence of M2e on the surfaces of infected host cells, M2e-specific antibodies are readily bound to these target cells. Thus, effector Cells are capable of antibody crosslinking via FcRs to carry out antibody-dependent cellular cytotoxicity (ADCC) [12,13,14]. However, M2e is poorly immunogenic and presents at a low copy number on the surface of the virion. Many vaccinal strategies have been applied to improve M2e immunogenicity. For that purpose, different approaches were explored to construct tandem copies of the M2e peptide and/or use potent adjuvants [15]. Via a tandem repeat of M2e sequences, more comprehensive protection was achieved against influenza lethal challenge. Zhang et al. showed that 3 copies of the M2e gene provided the best protection to chickens against virus challenge [16]. They also demonstrated that virus-challenged mice that received 3 copies of M2e protein lost weight more slowly and recovered faster than the mice in the other groups [16]. Dabaghian and his colleagues used 4 copies of M2e fused to HSP70c protein and showed that it was remarkably immunogenic and effective in protecting mice against weight loss and severe clinical signs; it could also improve the survival rates after lethal influenza viral challenge [17]. Hence, we developed a vaccine construct with three copies of the M2e gene.
Nucleoprotein (NP) is an internal antigen that is highly conserved and therefore has become an appropriate candidate for an efficient influenza vaccine. Besides its structural role in organizing the RNP complex, NP is involved in viral transcription and replication and affects the host specificity and virulence of viruses. In addition, NP can interact with a variety of viral and cellular macromolecules. Viral nucleoprotein could generate subtype cross-reactive cytotoxic T lymphocyte (CTL) which plays an important role in controlling influenza virus infection [18, 19]. As early as 1987, Wraith et al. purified the NP protein of influenza virus X31 (H3N2) and immunized mice by i.p. injection [20]. They found that NP immunization resulted in significant protection (75%) of mice from a lethal challenge with influenza virus A/PR/8/34(H1N1), which may be attributed to CTL with cross-protective activity [20]. MacLeod et al. found that NP delivered with a universally used and safe adjuvant, which is composed of insoluble aluminum salts, provides protection against viruses that either express the same or an altered version of nucleoprotein [21].
In this research, a chimer protein containing three tandem repeats of M2e fused to a truncated nucleoprotein (trNP1 − 158aa) was expressed in a prokaryotic system. The immunogenicity of chimer protein (3M2e-trNP) alone or in combination with Alum adjuvant was assessed in BALB/c mice in a challenge with homologous (H1N1) and heterologous (H3N2) influenza viruses. In addition, the protective efficacy of chimer protein was compared to the combination of 3M2e and trNP supplemented with Alum which was named Mix protein.
Materials and Methods
Materials (Viruses, Cells, and Proteins)
Mouse-adapted human influenza A viruses PR8 (H1N1) and X47 (H3N2), E. coli strains of Top10fʹ and BL21 (DE3) as well as female BALB/c mice aged 6–8 weeks old were supplied by the Pasteur Institute of Iran, Tehran, Iran. The proliferation of antigen-specific T lymphocytes was evaluated using Roswell Park Memorial Institute Medium (RPMI)-1640 cell culture medium and Fetal Bovine Serum (FBS), and penicillin/streptomycin (Pen/Strep) were purchased from Gibco Company (USA). The expression of Chimer Protein in the prokaryotic system was conducted using Isopropyl b-D-1Thiogalactopyranoside (IPTG) (Sigma-Aldrich, USA). The lymphocyte proliferation assay was performed using (3-(4, 5-dimethyl tetrazolyl-2) 2,5 diphenyl) tetrazolium bromide (MTT) obtained from Sigma-Aldrich Company (USA). The antibody titers to influenza vaccine were determined using Tetramethylbenzidine (TMB), Horseradish Peroxidase (HRP) conjugated anti-mouse Immunoglobulin G (IgG) and HRP-conjugated rabbit antigoat IgG, and 96-well plates were obtained from Sigma-Aldrich Company (USA). The SDS-PAGE method was performed utilizing Acrylamide and SDS, which were purchased from Merck Company (Germany). The target protein was purified using ProtinoTM Ni-TED-IDA 1000 kit supplied by Macherey NagelTM Company (Germany) and the DNA extraction kit was supplied by Bioneer Company (South Korea). All materials were of analytical grade. Alum adjuvant was prepared from Alhydrogel2% (Brenntag Biosector, Denmark).
Construction of Recombinant Vector
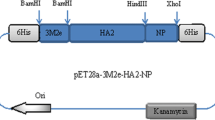
Total RNA of the influenza A/PR/8/34 (H1N1) was extracted; the virus cDNA was synthesized and a truncated form of nucleoprotein (trNP1 − 158aa) was amplified by PCR using specific forward (5´-GGATCCATGGCGTCTCAAGGCAC-3) and reverse (5´-CTCGAGTCCGGTGCGAACAAGAG-3´) primers which incorporate a Bamh1 and Xho1 restriction site into the 5’ and 3’-end of PCR product, respectively. The amplified gene was cloned into an expression vector (pET28a), confirmed by restriction enzyme analysis and sequencing (pET28a/trNP) [22, 23]. Synthesized three tandem repeat sequences of M2e (3M2e) cloned by BamHI sites into pET28a vector (pET28a/3M2e) were previously constructed in the Department of Influenza and Respiratory Viruses, Pasteur Institute of Iran [24]. In the next step, the 3M2e synthetic fragment was subcloned upstream of the trNP gene in BamHI enzymatic site in pET28a/trNP vector [23]. The correct orientation of the 3M2e gene in the recombinant expression vector was verified by colony PCR using specific primers, and restriction analysis by single and double enzyme digestions, and then sequencing. The confirmed construct was named pET28a/3M2e-trNP. Fig. S1 indicates the nucleotide sequences and translated amino acids of three recombinant vectors namely (A) pET28a/3M2e, (B) pET28a/trNP, and (C) pET28a/3M2e-trNP.
An overview of the study design was shown in Fig. S2.
Expression and Purification of Recombinant Proteins
The recombinant chimer constructs, pET28a/3M2e-trNP, pET28a/trNP, and pET28a/3M2e were transformed into the E. coli BL21 (DE3) strain chemically competent cells. A single colony of transformed cells was cultured in 5 ml of LB broth containing Kanamycin (50 µg/ml) and incubated for 2–3 h at 37 °C in a shaker incubator, induced with IPTG at the final concentration of 0.5 mM and incubated in the shaker incubator once again (37 °C, 195 RPM). The protein expression was evaluated at various time intervals by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by staining with Coomassie blue R250. For Western blotting, the expressed target protein separated by SDS-PAGE was transferred to a nitrocellulose membrane using a semi-dry transfer system [25]. Then, the immunoblotting was carried out using horseradish peroxidase (HRP) conjugated anti-His antibody (Qiagen, Germany). Finally, the antibody-reactive bands were revealed by chromogenic detection by adding a diaminobenzidine (DAB) substrate.
To purify the chimer protein, the bacterial pellet was lysed in LEW buffer (300 mM NaCl, 50 mM NaH2PO4, pH 8.0) containing 5 mM Imidazole, and then the cells were lysed by sonication with 20 pulses at 20-sec intervals for seven times on the ice. Recombinant proteins were eluted using Ni-TED affinity columns according to the manufacturer’s instructions. Briefly, after loading of the lysates on the Ni-TED column and washing steps, the proteins were eluted using imidazole buffer containing 8 M urea. The proteins were desalted by dialysis against Phosphate Buffered Saline (PBS, pH 7.2) at 4 °C overnight to remove the urea. The high purity of the recombinant protein was verified by SDS-PAGE.
Immunization Schedule
Six to eight-week-old female BALB/c mice were divided randomly into 6 groups and immunized subcutaneously, three times at two weeks intervals with 15 µg of chimer protein either alone or supplemented with adjuvant of Alum, and Mix protein plus Alum (Equal volumes of NP and 3M2e proteins were mixed and injected with a total concentration of 15 µg). The control group received phosphate-buffered saline (PBS), and groups of Mice were immunized with inactivated influenza viruses, either A/H1N1/PR8 or A/X-47(H3N2), as positive controls (Table 1). Two weeks after the last immunization, the mice were bled through the orbital sinus, and the sera were kept at − 20 °C for ELISA analysis. All animal experiments were carried out in accordance with the Ethics Committee of the Pasteur Institute of Iran (IR.PII.REC.1394.39).
Lethal Homologous and Heterologous Challenge
Three weeks after the last immunization, the mice of each group were challenged intranasally with 10 LD50 titers of homologous (A/H1N1/PR8) or heterologous (A/H3N2/X47) viruses. Animals were monitored daily for mortality rate and body weight changes for two weeks. Mice that lost body weight by more than 25% were humanely euthanized.
Measurement of the Specific anti-M2e Antibodies
The potency of the chimer protein and Mix protein in the production of specific antibodies was evaluated in the mouse model using a modified enzyme-linked immunosorbent assay (ELISA). The mice were inoculated with Mix protein plus Alum and chimer proteins with or without adjuvant. The mice were bled and specific antibody responses against the M2e protein were evaluated using an ELISA test 2 weeks after the first, second, and third immunizations. Antibody titer increased significantly after the third injection compared to the first and second immunizations (data not shown). Blood samples were collected from several mice before vaccination, and these samples were used as negative control serum. Briefly, 96-well plates were coated overnight at 4 °C using 100 µl of 10− 4 mg/ml of the M2e synthetic peptide (GenScript: RP20206). After washing the plates with PBS buffer containing 0.05% Tween-20 (PBST), they were blocked with 5% BSA in PBST per well for 1 h at 37 °C. Serum samples of the mice were diluted in PBS (1:1000). The concentrations of coated antigen and serum dilution were obtained by a checkerboard titration assay. To evaluate specific IgG, an Anti-mouse antibody conjugated with horseradish peroxidase (HRP) was added into the wells and incubated for one hour. Finally, plates were washed and developed for 20 min with the substrate 3, 3′, 5, 5′-tetramethylbenzidine [26]. The reaction was ceased by the addition of 1 N sulfuric acid. Optical density was measured using a microplate reader at 450 nm of wavelength.
Lymphocyte Proliferation Assay
The MTT assay was carried out to evaluate lymphocyte proliferation. Two weeks after the last (third) immunization, the spleens of three mice per group were removed to evaluate the cellular immune response [27]. Briefly, splenocytes were isolated by sieving through a 40-µm cell strainer, depleting red blood cells with NH4Cl lysis solution, and washing twice with RPMI 1640 medium. The suspension of isolated spleen cells was cultured in RPMI-1640 medium supplemented with 10% of FBS, 1% of 4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid (HEPES), 1% of pen/strep, and 2 mM L-glutamine to reach a final density of 2 × 105 cells/ml. The splenocytes were cultured in 96-well plates and incubated at 37 °C in 5% CO2 for 4 h, induced in the presence of 2 µg/ml M2e synthetic peptide, and 3 µg/ml NP-specific antigen or left without stimulation and incubated at 37 °C for 48 h. Lymphoproliferation assay was performed using MTT assay in which mitochondrial activity of living cells turns MTT to purple formazan. The results were indicated as stimulation index (SI). The SI was calculated using the following formula: SI= (Cs-Cu)/Cu, where Cs is the OD value of stimulated cells, and Cu is the OD value of the unstimulated cells. All the tests were performed in triplicate for each mouse.
Cytokine ELISA
A cytokine assay was performed to investigate the ability of chimer protein and Mix protein to elicit specific cellular immune responses. Spleen cells were stimulated for 24 h with the same antigens and concentrations used in proliferative assays (NP and M2e). Cell-free supernatants were collected and analyzed for the incidence of IFN-γ and IL-4 using Sandwich-Based ELISA kits (R&D Systems, Minneapolis, USA) following the manufacturer’s instructions. All tests were performed in duplicate for each mouse and read at 450 nm on an automatic ELISA plate reader. The concentrations were measured with reference to a standard curve.
Statistical Analysis
Statistical analyses were performed using Microsoft Excel and GraphPad Prism 6.0 software. The Kaplan–Meier curve was used for the analysis of the survival rate. Pearson’s chi-squared test was performed. Results were indicated as mean ± SD. * (P < 0.05) and **** (P < 0.0001) indicate statistically significant and highly significant differences, respectively between the designated groups as determined by one-way ANOVA.
Results
Biological Information Analysis
The physicochemical properties of chimer protein (3M2e-trNP) obtained using ProtParam and ProtScale software were as follows: the average molecular weight of 28 kDa, approximate half-life of more than 10 h in E. coli, the stability index of about 32.91, estimated isoelectric pH of 6.3, aliphatic index of 74.65, and GRAVY of about − 0.494. The negative GRAVY value indicates the nonpolar characteristic of the protein.
The allergenicity and toxicity of (3M2e-trNP) chimer protein were evaluated using the Structural Database of Allergenic Proteins (SDAP) and the results showed that the protein is not allergenic or toxic. Moreover, it is predicted that the chimer protein is soluble in E. coli. B-cell epitopes of chimer protein were analyzed using web-based B-cell epitope prediction tools and T-cell epitopes were screened using ProPred software (Imtech server) and IEDB for predicting MHC Class-I and II alleles. The results showed numerous shared peptide regions with high-scored immunogenic epitopes over multiple mouse and human alleles from the chimer protein.
Preparation of Chimer Protein
The results of SDS-PAGE and Western blotting confirmed the expression of recombinant chimer protein with a molecular weight of about 32 kDa. After comparing the results, the best expression occurred after 4 h of induction with IPTG. A large scale of recombinant proteins was produced and the Protino Ni-TED Nickel column was employed to purify the recombinant chimer protein containing His-tag (Fig. 1).
Analysis of expression and purification of the recombinant chimer protein in E. coli by gel electrophoresis (SDS-PAGE) and western blotting: Lane (1) marker protein; Lane (2) before induction; Lanes (3, 4, 5, 6) 1, 2, 3, 4 h after induction by IPTG, respectively; Lane (7) purified chimer protein; Lane (8) marker protein (rainbow); Lanes (9,10) immunostaining of recombinant protein before and after induction, respectively
Humoral Immune Responses
Results of the last immunization indicated that antibody production in all immunized groups was considerably higher compared to the control group (PBS). Results showed that chimer-receiving groups with or without adjuvant elicited a significantly higher antibody response than the Mix group plus Alum and control group (P ˂ 0.0001). Moreover, Animals that received chimer proteins alone showed a higher specific IgG titer compared to animals receiving chimer proteins adjuvanted with Alum. Although the differences observed between these two groups were not highly significant (P ˂ 0.05) (Fig. 2).
Measurement of IgG antibodies in sera of immunized mice using human type M2e peptide coated ELISA plates: Values for individual serum were measured at 450 nm of OD and were indicated as mean ± SD obtained from the experiment. According to ANOVA results, the differences between all treatment and control groups were statistically highly significant (****P ˂ 0.0001). *P ˂0.05, ****P ˂ 0.0001
Lymphocyte Proliferation Assay
To evaluate specific cellular immunity against the chimer protein, the stimulation indices in different groups were stimulated with (A) NP-specific antigen or (B) M2e antigen (Fig. 3).
Results regarding lymphocyte proliferation after in vitro stimulation with: (A) NP; (B) M2e. Splenocyte proliferation levels of immunized mice (three per group) were determined using MTT assay 14 days after the last immunization. The values were indicated as mean ± SD obtained from the 2 independent experiments. According to ANOVA results, the differences between all treatment and control groups were statistically highly significant (****P ˂ 0.0001). ****P ˂ 0.0001
The results showed that lymphocytes were effectively and almost similarly stimulated and proliferated in response to NP or M2e antigens. Mice in the group immunized with chimer protein with or without adjuvant showed a significantly higher stimulation index, compared to the Mix group plus Alum, on day fourteen after the last immunization (P ˂ 0.0001). Mice immunized with the chimer protein supplemented with Alum had almost similar responses to the mice that received the chimer protein alone; the differences observed between these two groups were not statistically significant. As expected, negligible cell proliferation was detected in mice that received PBS (Fig. 3A, B).
Cytokine Assay
The results of the cytokine assay showed that NP and M2e almost similarly increased IFN- and IL-4 production in all immunized mice as shown in Fig. 4 (A, B) and (C, D), respectively. The concentration of IFN-γ in groups that received chimer protein alone or supplemented with Alum was significantly higher than Mix protein plus alum and control groups (P ˂ 0.0001). In addition, animals that received chimer proteins alone showed higher INF- production compared to animals receiving chimer proteins adjuvanted with Alum. Although, the differences observed between these two groups were not highly significant (P ˂ 0.05) (Fig. 4A, C).
Detection of IFN-γ after stimulation of splenocytes culture from immunized mice (three per group) were obtained 14 days after the last immunization and were stimulated with: (A) NP; (C) M2e and concentration of (IL-4) in splenocytes culture was stimulated with: (B) NP; (D) M2e. The levels of Cytokines in splenocytes culture supernatants were measured by ELISA. The values were indicated as mean ± SD obtained from the 2 independent experiments. According to ANOVA results, the differences between all treatment and control groups were statistically highly significant (****P ˂ 0.0001). *P ˂0.05, ****P ˂ 0.0001
The results also indicated that the secretion of IL-4 in mice that received chimer protein alone was significantly higher than those which received chimer protein supplemented with Alum and Mix protein plus Alum (P˂ 0.0001). Moreover, highly significant levels of IL-4 were detected in mice immunized with chimer protein supplemented with Alum compared to the Mix protein plus Alum (P ˂ 0.0001) (Fig. 4B, D).
Viral Challenge; Morbidity and Mortality rate
To confirm the potency of protective immunity, mice were challenged with two mouse-adapted subtypes of the influenza A virus, PR8 and X47. The 50% lethal dose (LD50) for each virus was calculated using the method of Reed and Muench.
As shown in Fig. 5A and B, the chimer-vaccinated groups supplemented with or without adjuvant, A/H1N1/PR8 or A/X-47(H3N2), exhibited a significantly higher survival rate in comparison with the Mix group plus Alum or PBS (P < 0.0001). The Mix protein, similar to the recombinant chimer protein, protected mice equally and effectively against both homologous and heterologous challenges. In this connection, 14 days after the challenge, the chimer group with or without adjuvant had 78.4% and 85.7% survival, respectively. However, the Mix protein plus Alum could protect only 57.1% and 42.8% of homologous and heterologous virus-challenged mice, respectively. All animals in the control groups, which received PR8 or X-47 survived at the end of the experiment and all mice in the non-vaccinated control group died 9–12 days after the challenge date.
Protection of mice groups against homologous and heterologous lethal challenges: (A) Homologous challenge: survival% post-infection with acute mouse-adapted H1N1 influenza A virus in immunized mice groups with different antigens. (B) Heterologous challenge: survival% in days post-infection with acute mouse-adapted H3N2 influenza A virus in immunized mice groups with different antigens. (C) Homologous challenge: body weight changes post-infection with acute mouse-adapted H1N1 influenza A virus in immunized mice groups with different antigens. (D) Heterologous challenge body weight changes post-infection with acute mouse-adapted H3N2 influenza A virus in immunized mice groups with different antigens. Error bars show standard deviations. According to ANOVA results and Kaplan-Meier curves, the differences between all treatment and control groups were statistically highly significant (****P ˂ 0.0001). ****P ˂ 0.0001
The mice were monitored daily for weight loss for 2 weeks after the challenge with PR8 and X47 viruses. As shown in Fig. 5C and D, mouse groups injected with chimer protein alone or adjuvanted with alum, A/H1N1/PR8 or A/X-47(H3N2), experienced only a small and transient weight loss after challenge with homologous and heterologous viruses compared to groups injected with Mix protein plus Alum or PBS; the differences observed between these groups were statistically significant (P < 0.0001). Other observed differences between groups were not significant. Moreover, in the challenge against heterologous viruses, weight decrease in All groups was almost similar to the homologous challenge, and the differences were not statistically significant. Mice injected with PBS died 9–12 days after the challenge date.
Discussion
In this study, a recombinant chimer protein (3M2e-trNP) was produced in a prokaryote system and was purified successfully. The immunogenicity of the chimer protein was assessed in the BALB/c mice model compared to Mix protein (3M2e + NP). The fusion of truncated nucleoprotein (trNP1-158aa) as an internal antigen with conserved epitopes, and three tandem repeats of M2e as a surface antigen with immunogenic epitopes, resulted in a greater increase in both humoral (antibody-mediated) and cellular (T-cell-mediated) responses than the Mix protein. However, the reason behind the chimer protein’s superior immune response compared to the Mix protein remained uncertain. The presence of antigens on the surface of pathogens can stimulate the production of specific antibodies, which can provide protection against infection. Due to the presence of M2e on the surfaces of infected host cells, M2e can give rise to humoral immunity (antibodies), which protect against virus infection. Also, Protection by M2e-based influenza vaccines is provided mainly by M2e-specific IgG antibodies [28, 29]. Whereas, Nucleoprotein (NP) is an internal antigen and could generate subtype cross-reactive cytotoxic T lymphocyte (CTL) which plays an important role in controlling influenza virus infection [30]. It was found that the chimer receiving group alone or adjuvanted with Alum is effective in inducing significantly higher Ab responses against M2e compared to Mix group plus Alum.
In addition to inducing strong humoral immune responses, chimer proteins were reported to elicit robust specific T-cell responses in stimulation with M2e and NP antigens.
In a lymphocyte proliferation assay, results indicated that the mice immunized with chimer protein alone or adjuvanted with Alum led to a significantly stronger lymphocyte proliferation than Mix group plus Alum. Regarding cytokine responses, the influenza vaccine caused a marked increase in IL-2 and IFN-γ production. Since these cytokines act as stimulators of cellular and humoral activity and are pivotal for the antiviral defense of the organism. High levels of IFN-γ reduce viral replication, activate cytokine production by T cells, and enhance cytotoxic T lymphocyte-killing activity, but these levels are not sustained [31]. On the other hand, IL-4 levels may inhibit naive CD4 + cells from proceeding to Th1 maturation or block IFN-γ gene transcription, consequences of influenza virus vaccination. Therefore, an effective Th1 response is not sustained, and the adaptive immune system probably evaluates the balancing between the Th1 and Th2 response followed by the development of the Th2 immune adaptive response [31, 32]. Cytokine profiles of splenocytes from the group of immunized mice with the chimer protein alone or adjuvanted with Alum showed a significantly stronger response of INF-γ compared to Mix group plus Alum. Also, we found that IL-4 levels significantly increased in the lymphocytes isolated from the splenocytes of the mice which were immunostimulated with chimer protein alone compared to the other groups. Furthermore, the chimer-receiving group compared to the chimer-Alum-receiving group could induce better results in response to IgG evaluation and cytokine assay, but the statistical differences between these two groups were not highly significant.
Our study aimed to evaluate the protective efficacy of chimer and Mix proteins against homologous and heterologous influenza A viruses in mice. Both proteins contained 3M2e and trNP antigens, which are highly conserved among various influenza virus strains, allowing for good cross-protection against challenges with 10 LD50 titers of either virus type. Specifically, the presence of both proteins, either as a chimer or cocktail (Mix protein), provided at least 78.4% and 42.8% protection, respectively, against different strains of the influenza virus. However, the chimer protein demonstrated superior immune protection compared to the Mix protein, and the reason for this difference remained unclear. Additionally, fusing the two gene fragments into a single protein allowed for more efficient expression and purification, leading to significant savings in time and production expenses. The group receiving the chimer protein, with or without the Alum adjuvant, demonstrated a significantly higher survival rate and less weight loss compared to both the Mix protein plus Alum group and the control group. Moreover, during the challenge with heterologous viruses, the weight loss observed in all groups was nearly equivalent to that observed during the homologous challenge.
We showed that the recombinant chimer protein without the requirement of Alum adjuvant can induce humoral and cellular immune responses, which can protect against both homologous (H1N1) and heterologous (H3N2) lethal virus challenges in mice. Although adjuvants are crucial in improving immune responses to purified protein-based antigens with low immunogenicity, there are instances where additional immune system activation may not necessarily provide better responses against the influenza virus in animals [33].
Firstly, the immune response to a vaccine can depend on the specific formulation of the vaccine, such as the type and amount of antigen or the method of delivery. The effectiveness of an adjuvant can depend on how it interacts with the vaccine components to stimulate the immune system [34].
Secondly, Alum can cause local and systemic side effects, such as injection site pain, redness, swelling, fever, and fatigue. These side effects can affect patient adherence to vaccination and limit Alum’s use in certain populations, such as pregnant women and individuals with allergies [35].
Lastly, the immune response to a vaccine is complex and may involve multiple immune system components [36]. We hypothesize that adjuvants were able to stimulate different aspects of the immune response, which were not investigated in this study. For instance, Alum-induced innate immune responses consist of an influx of neutrophils, eosinophils, NK cells, CD11b + monocytes, and dendritic cells (DCs) to the site of injection were found to play an essential role in the immune response against influenza infection [37, 38]. Moreover, other factors limited the study, including the insufficient number of animals in each group to draw statistically and medically significant conclusions about the efficacy of the chimer protein without an adjuvant.
Overall, the choice to use an adjuvant in a vaccine formulation depends on multiple factors, including the vaccine antigen, the desired immune response, safety considerations, and the efficacy of the adjuvant itself. In some cases, a vaccine without an adjuvant may be a more effective and safer option [39, 40]. Consequently, using an adjuvant, like alum, with chimer protein would be an advantage for an influenza virus vaccine, but chimer protein containing influenza virus trNP and 3M2e were found to be effective in inducing protective immune responses in the absence of the adjuvant.
Conclusion
The construct of the chimer protein (3M2e-trNP) is characterized as a potential universal vaccine candidate due to the conserved nature regions of the human influenza A virus, and its efficacy was evaluated against influenza infection in an animal model in comparison to Mix protein (3M2e + NP). This research showed that immunization with chimer protein with or without adjuvant induced significantly higher humoral and cellular immune responses in comparison to Mix protein plus Alum. Moreover, the chimer protein and the Mix protein, both containing 3M2e and trNP antigens from the H1N1 subtype virus, provided effective cross-protection against H1N1 and H3N2 challenges in mice. However, the chimer protein demonstrated superior immune protection compared to the Mix protein. Therefore, the chimer protein, constructed without any adjuvant, could be a potential universal vaccine candidate, but its immunogenicity needs to be studied in animal models along with other influenza virus-conserved proteins. While additional activation of the immune system is critical for protecting animals against the influenza virus, it may not always be advantageous.
References
Thompson MG, Shay DK, Zhou H, Bridges CB, Cheng PY et al (2010) Estimates of deaths associated with seasonal influenza-United States. 1976–2007. MMWR Morb Mortal Wkly Rep 59:1057–1062
Nakajima K (2003) The mechanism of antigenic shift and drift of human influenza virus. Nihon rinsho 61:1897–1903
Du L, Zhou Y, Jiang S (2010) Research and development of universal influenza vaccines. Microbes Infect 12:280–286. https://doi.org/10.1016/j.micinf.2010.01.001
Pica N, Palese P (2013) Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med 64:189–202. https://doi.org/10.1146/annurev-med-120611-145115
Zhang H, Wang L, Compans RW, Wang BZ (2014) Universal influenza vaccines, a dream to be realized soon. Viruses 6:1974–1991. https://doi.org/10.3390/v6051974
Price GE, Soboleski MR, Lo CY, Misplon JA, Pappas C et al (2009) Vaccination focusing immunity on conserved antigens protects mice and ferrets against virulent H1N1 and H5N1 influenza a viruses. Vaccine 27:6512–6521. https://doi.org/10.1016/j.vaccine.2009.08.053
Sheet CF (2014) Influenza—“Vaccine effectiveness: how well does the Flu Vaccine work? Centers for Disease Control and Prevention (CDC)
Li CKF, Rappuoli R, Xu XN (2013) Correlates of protection against influenza infection in humans—on the path to a universal vaccine? Curr Opin Immunol 25:470–476. https://doi.org/10.1016/j.coi.2013.07.005
Rimmelzwaan GF, McElhaney JE (2008) Correlates of protection: novel generations of influenza vaccines. Vaccine 26:D41–D44. https://doi.org/10.1016/j.vaccine.2008.07.043
Kolpe A, Schepens B, Fiers W, Saelens X (2017) M2-based influenza vaccines: recent advances and clinical potential. Expert Rev Vaccines 16:123–136. https://doi.org/10.1080/14760584.2017.1240041
Tompkins SM, Zhao ZS, Lo CY, Misplon JA, Liu T et al (2007) Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N. Emerg. Infect Dis 13:426–435. https://doi.org/10.3201/eid1303.061125
Fiers W, De Filette M, El Bakkouri K, Schepens B, Roose K et al (2009) M2e-based universal influenza a vaccine. Vaccine 27:6280–6283. https://doi.org/10.1016/j.vaccine.2009.07.007
Ebrahimi SM, Tebianian M (2011) Influenza a viruses: why focusing on M2e-based universal vaccines. Virus Genes 42:1–8
Wu F, Huang JH, Yuan XY, Huang WS, Chen YH (2007) Characterization of immunity induced by M2e of influenza virus. Vaccine 25:8868–8873. https://doi.org/10.1016/j.vaccine.2007.09.056
Stepanova LA, Mardanova ES, Shuklina MA, Blokhina EA, Kotlyarov RY et al (2018) Flagellin-fused protein targeting M2e and HA2 induces potent humoral and T-cell responses and protects mice against various influenza viruses a subtypes. J Biomed Sci 25:1–15
Zhang X, Liu M, Liu C, Du J, Shi W et al (2011) Vaccination with different M2e epitope densities confers partial protection against H5N1 influenza a virus challenge in chickens. Intervirology 54:290–299. https://doi.org/10.1159/000319440
Dabaghian M, Latify AM, Tebianian M, Nili H, Ranjbar ART et al (2014) Vaccination with recombinant 4× M2e. HSP70c fusion protein as a universal vaccine candidate enhances both humoral and cell-mediated immune responses and decreases viral shedding against experimental challenge of H9N2 influenza in chickens. Vet Microbiol 174:116–126. https://doi.org/10.1016/j.vetmic.2014.09.009
Grant E, Wu C, Chan KF, Eckle S, Bharadwaj M et al (2013) Nucleoprotein of influenza a virus is a major target of immunodominant CD8 + T-cell responses. Immunol Cell Biol 91:184–194. https://doi.org/10.1038/icb.2012.78
Guo L, Zheng M, Ding Y, Li D, Yang Z et al (2010) Protection against multiple influenza a virus subtypes by intranasal administration of recombinant nucleoprotein. Arch Virol 155:1765–1775
Wraith DC, Vessey AE, Askonas BA (1987) Purified influenza virus nucleoprotein protects mice from lethal infection. J Gen Virol 68:433–440. https://doi.org/10.1099/0022-1317-68-2-433
MacLeod MK, David A, Jin N, Noges L, Wang J et al (2013) Influenza nucleoprotein delivered with aluminum salts protects mice from an influenza A virus that expresses an altered nucleoprotein sequence. PloS one 8: e61775.https://doi.org/10.1371/journal.pone.006177522.20
Nazeri E, Farahmand B, Fotouhi F, Hashemi M, Taheri N et al (2018) In Silico Analysis and Expression of Influenza Virus 3M2e-HA2 Chimer Protein Fused to C-Terminal Domain of Leishmania major HSP70. Jundishapur J. Microbiol 11. https://doi.org/10.5812/jjm.13777
Li QY, Xu MM, Dong H, Zhao JH, Xing JH et al (2020) Lactobacillus plantarum surface-displayed influenza antigens (NP-M2) with FliC flagellin stimulate generally protective immune responses against H9N2 influenza subtypes in chickens. Vet Microbiol 249:108834. https://doi.org/10.1016/j.vetmic.2020.108834
Farahmand B, Taheri N, Shokouhi H, Soleimanjahi H, Fotouhi F (2019) Chimeric protein consisting of 3M2e and HSP as a universal influenza vaccine candidate: from in silico analysis to preliminary evaluation. Virus Genes 55:22–32
Jalili N, Taheri N, Tavakoli R, Fotoohi F, Akbari A et al (2016) Expression and purification of a recombinant chimeric protein (3M2e-HA2) composed of influenza virus hemaglutinin and matrix protein conserved domain for universal subunit vaccine development. J Maz Univ Med Sci 26:12–22
Shokouhi H, Farahmand B, Ghaemi A, Mazaheri V, Fotouhi F (2018) Vaccination with three tandem repeats of M2 extracellular domain fused to Leismania major HSP70 protects mice against influenza a virus challenge. Virus Res 251:40–46. https://doi.org/10.1016/j.virusres.2018.05.003
Saleh M, Nowroozi J, Farahmand B, Fotouhi F (2020) An approach to the influenza chimeric subunit vaccine (3M2e-HA2-NP) provides efficient protection against lethal virus challenge. Biotechnol Lett 42:1147–1159
Saelens X (2019) The role of matrix protein 2 Ectodomain in the development of Universal Influenza Vaccines. J Infect Dis 219:S68–S74. https://doi.org/10.1093/infdis/jiz003
Mezhenskaya D, Isakova-Sivak I, Rudenko L (2019) M2e-based universal influenza vaccines: a historical overview and new approaches to development. J Biomed Sci 26:1–15. https://doi.org/10.1186/s12929-019-0572-3
Yewdell JW, Bennink JR, Smith GL, Moss B (1985) Influenza a virus nucleoprotein is a major target antigen for cross-reactive anti-influenza a virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A 82:1785–1789. https://doi.org/10.1073/pnas.82.6.1785
Bessler H, Djaldetti M (2016) The effect of influenza vaccine on cytokine production by human mononuclear cells. https://doi.org/10.15761/GVI.2000120
Dittmer U, Peterson KE, Messer R, Stromnes IM et al (2001) Role of interleukin-4 (IL-4), IL-12, and gamma interferon in primary and vaccine-primed immune responses to friend retrovirus infection. J Virol J 75:654–660. https://doi.org/10.1128/JVI.75.2.654-660.2001
Bungener L, Geeraedts F, Ter Veer W, Medema J et al (2008) Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine 26:2350–2359. https://doi.org/10.1016/j.vaccine.2008.02.063
Marciani DJ (2003) Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov Today 8:934–943. https://doi.org/10.1016/s1359-6446(03)02864-2
Mohan T, Verma P, Rao DN (2013) Novel adjuvants & delivery vehicles for vaccines development: a road ahead. Indian J Med Res 138:779
Petrovsky N, Aguilar JC (2004) Vaccine adjuvants: current state and future trends. Immunol Cell Biol 82:488–496
Ghimire TR (2015) The mechanisms of action of vaccines containing aluminum adjuvants: an in vitro vs in vivo paradigm. Springerplus 4:1–18
Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M et al (2011) Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29:1812–1823. https://doi.org/10.1016/j.vaccine.2010.12.090
Harandi AM, Medaglini D, Shattock RJ, convened by EUROPRISE WG (2010) Vaccine adjuvants: a priority for vaccine research. Vaccine 28:2363–23636. https://doi.org/10.1016/j.vaccine.2009.12.084
Sarkar I, van Garg R, Drunen Littel-van, den Hurk S (2019) Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev Vaccines 18:505–521. https://doi.org/10.1080/14760584.2019.1604231
Acknowledgements
This study was performed in the Department of Influenza and Other Respiratory Viruses, Pasteur Institute of Iran, Tehran (Grant Number 759) hereby, thanks to all colleagues for their valuable cooperation.
Funding
This study was funded by the Pasteur Institute of Iran, Tehran (grant number 759).
Author information
Authors and Affiliations
Contributions
FF and SMH: Project administration and supervision, FF: Funding acquisition, BF: Bioinformatics analysis, MM: Data analysis and manuscript preparation, MM; MS; HS and AT: Experimental work. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This article contains studies with animals performed by the authors. All animal experiments were approved and performed based on the guidelines of the Ethics Committee of the Pasteur Institute of Iran (IR.PII.REC.1395.82). This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maleki, M., Hosseini, S.M., Farahmand, B. et al. Induction of Homosubtypic and Heterosubtypic Immunity to Influenza Viruses Using a Conserved Internal and External Proteins. Curr Microbiol 80, 212 (2023). https://doi.org/10.1007/s00284-023-03331-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03331-y