Abstract
Objectives
To select a microbial consortium from intertidal sludge and evaluate its ability to convert crude glycerol from biodisel to high value-added products such as 1,3-propanediol (1,3-PDO) and lactic acid (LA).
Results
A microbial consortium named CJD-S was selected from intertidal sludge and exhibited excellent performance for the conversion of crude glycerol to 1,3-PDO and LA. The composition of CJD-S was determined to be 85.99% Enterobacteriaceae and 13.75% Enterococcaceae by 16S rRNA gene amplicon high-throughput sequencing. In fed-batch fermentation with crude glycerol under nonsterile conditions, the highest concentrations of 1,3-PDO and LA were 41.47 g/L and 45.86 g/L, respectively.
Conclusions
The selected microbial consortium, CJD-S, effectively converted crude glycerol to 1,3-PDO and LA under nonsterile conditions and can contribute to the sustainable development of the biodiesel industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An excess of crude glycerol, a byproduct from biodiesel (10% w/w), has been produced with the rapid development of the global biodiesel industry (Zhou et al. 2018). Crude glycerol from biodiesel often contains various impurities that limite its utilization, such as residual methanol, fatty acids, catalysts and heavy metals. At the same time, the cost of purifying crude glycerol makes it unviable. The disposal of crude glycerol greatly burdens both enterprises and the environment. Hence, the direct conversion of crude glycerol into value-added products is the key to solving these problems, and the sustainable development of the biodiesel industry (Monteiro et al. 2018). Microbial fermentation with crude glycerol as a carbon source to produce biochemicals, such as 1,3-propanediol, 1,2-propanediol, lactic acid, and ethanol, has received increasing attentation (Pradima et al. 2017).
1,3-Propanediol (1,3-PDO) can be widely used in medicines, paints, cosmetics, adhesives, antifreezes and lubricants. As a monomer of polymers (polyesters, polyethers, and polyurethanes) with excellent properties, there is a growing demand for 1,3-PDO (Mitrea et al. 2017). Lactic acid (LA) has extensive application in the food, cosmetic and medical industries. It can also be used as an important monomer to synthesize biodegradable polymers such as polylactic acid (PLA) (Eiteman and Ramalingam, 2015). The production of 1,3-PDO and LA from crude glycerol by microbial fermentation has received increasing attention due to environmental protection and sustainable social development. Glycerol is metabolized by microorganisms via reductive and oxidative pathways during the production of 1,3-PDO and organic acids such as LA. The coproduction of 1,3-PDO and LA is acceptable because the conversion of crude glycerol can be increased and the compounds are easily separated from each other (Xin et al. 2017; Song et al. 2013).
The traditional microbial fermentation of 1,3-PDO was based on pure cultures by organisms such as Klebsiella and Clostridium (Chatzifragkou et al. 2011; Metsoviti et al. 2012). However, there were some disadvantages in pure cultures, such as requirements for strict anaerobic or aseptic environments, difficulties in the purification caused by byproducts, and low tolerances to the impurities in crude glycerol (Xiu and Zeng 2008; Yang et al. 2016). Compared to pure cultures, fermentation with a microbial consortium, which possesses properties such as the utilization of complicated substrates, no sterility requirement, and the use of regulatory metabolites, has been extensively studied and proven to be viable in recent years (Dietz and Zeng, 2014; Jiang et al. 2017a, b; Zhou et al. 2017, 2018; Wang et al. 2019). Microbial consortia are complex systems, and further research on their fermentation performance will be beneficial to their application in industry.
In this study, a microbial consortium efficiently producing 1,3-PDO was selected from intertidal sludge, and its bacterial composition was analyzed. Then, the conversion of crude glycerol from biodiesel to 1,3-PDO and LA under unsterile conditions by the selected microbial consortium was evaluated in batch and fed-batch fermentations.
Methods
Inoculum and media
The inoculum was intertidal sludge obtained from seashore, Liaoning, China. The seed and fermentation media for 1,3-PDO and LA production were similar to those previously described (Jiang et al. 2017a). Crude glycerol was characterized as described by Zhou et al. (2017).
Culture conditions
Approximately 2 g of the intertidal sludge was inoculated into a 250 mL sealed flask with 100 mL of seed medium at 37 °C, and shaken at 200 rpm for 24 h. Then, 1 mL of the culture broth was incubated in another flask with the same seed medium and conditions for 24 h, and the subculturing was repeated one more time.
After the subculture, 1 mL of the culture broth was grown in a 250-mL sealed flask with 100 mL of seed medium at 37 °C, and shaken at 200 rpm for 12 h. Then, seed cells were prepared for batch and fed-batch fermentations.
Batch and fed-batch fermentations were performed in a 5 L bioreactor (Baoxing Biotech, Shanghai, China) with a working volume of 2 L and an inoculum volume of 10% (v/v). The pH was controlled at 7.0 by automatic addition of 5 M NaOH. The temperature was maintained at 37 °C with an agitation rate of 250 rpm. Nitrogen gas at 0.1 vvm was pumped into the bioreactor to produce an anaerobic environment during fermentations. In fed-batch fermentation, the initial concentration of crude glycerol was 40 g/L, and 20 g/L glycerol was maintained by adding crude glycerol during cultivation.
Analysis of microbial consortium composition
The bacterial composition of the selected microbial consortium was analyzed by 16S rRNA gene amplicon high-throughput sequencing according to Zhou et al. (2017). The sequencing raw data of the microbial consortium have been submitted to the sequence read archive database of NCBI under accession number SRR12496099.
Analytical procedures
Cell growth was measured at 650 nm and converted to dry cell weight (DCW) as previously described (Mu et al. 2006). Glycerol was assayed as described by Wang et al. (2001). The concentrations of 1,3-PDO, LA, ethanol, 2,3-butandiol, acetate, formate, and succinate were determined by HPLC (Jiang et al. 2017a). The results were the averages of two experiments under the same conditions. The carbon recovery was calculated after fermentation according to Wang et al. (2011).
Results and discussion
Selection and composition analysis of the microbial consortium
After three subcultures, a microbial consortium named CJD-S was obtained, and the diversity was analyzed by 16S rRNA gene amplicon high-throughput sequencing. The microbial consortium CJD-S, obtained from the enrichment of intertidal sludge, was mainly composed of Enterobacteriaceae and Enterococcaceae, which accounted for 86.25% and 13.75% of the total family abundance, respectively. The bacterial genera used in the production of 1,3-PDO in nature, such as Klebsilla, Enterobacter and Citrobacter, belong to Enterobacteriaceae (Sun et al. 2018). Enterococcus faecalis, from Enterococcaceae, is commonly used for LA production (Murakami et al. 2016). Therefore, 1,3-PDO and LA were expected to be the main products of the microbial consortium CJD-S when it transformed glycerol.
Effect of seawater on batch fermentation of the microbial consortium CJD-S
Batch fermentations with 40 g/L initial crude glycerol were performed to investigate the effect of seawater on metabolites produced by the microbial consortium CJD-S under nonsterile conditions with no nitrogen. The microaerobic environment was created by not pumping nitrogen into the bioreactor during fermentation. As shown in Table 1, 1,3-PDO was the main product in the batch fermentations by microbial consortium CJD-S, and there was obvious influence on the consumption of glycerol, and the duration and products of batch fermentations with seawater. Compared to the control values, the concentration, yield and productivity of 1,3-PDO were all decreased. Because the duration of the fermentation process was prolonged by seawater, the productivity of 1,3-PDO was reduced significantly. The production levels of products other than acetate, including lactate, ethanol and formate, were higher than those of the control. When the ionic concentration was reduced by a lack of KH2PO4, MgCl2 or CaCl2 addition, the production of lactate, ethanol and formate was somewhat decreased. The inhibitory effect on glycerol assimilation was attributed to high osmotic pressure caused by salts in seawater. The effects of seawater on the microbial community in microbial consortium CJD-S might contribute to the changes in the production of metabolites during batch fermentation. Considering that the lower productivity of bath fermentation with seawater was not conducive to industrial production, tap water was selected for subsequent experiments. These experiments showed that the high diversity of the microbial consortium CJD-S enabled it to adapt to complex environments without a sterility requirement, which was the same result as in the previous report by Dietz and Zeng (2014).
Effect of nitrogen on batch fermentation of the microbial consortium CJD-S
Batch fermentations with 40 g/L initial crude glycerol were conducted to evaluate the effect of nitrogen on metabolites from microbial consortium CJD-S under nonsterile conditions. According to Table 2, compared to the values obtained without nitrogen, the concentration, yield and productivity of 1,3-PDO significantly increased from 11.67 g/L, 0.33 mol/mol and 1.30 g/(L·h) to 15.70 g/L, 0.43 mol/mol and 1.96 g/(L·h), respectively, at the end of batch fermentation with 0.1 vvm nitrogen. At the same time, the concentrations of other products also increased, and the carbon recovery increased from 0.75 to 0.99.
Therefore, an anaerobic environment that was conducive to the growth of facultative anaerobes in the microbial consortium CJD-S was beneficial to the production of 1,3-PDO and other metabolites from glycerol. When no nitrogen was pumped, the dissolved oxygen in the medium facilitated the growth of aerobic bacteria in the microbial consortium at the beginning of fermentation, which competed for substrate utilization and reduced the conversion rate of glycerol. The effect of nitrogen on batch fermentations was different from that reported by Wang et al. (2019). The reason may be that the main family of the microbial consortium LS30 selected by Wang et al. (2019), was Enterobacteriaceae, which accounted for 97.22% of the total after 30 serial transfers under anaerobic conditions and maintained steady fermentation performance under anaerobic and aerobic conditions. In contrast, the bacterial composition of consortium CJD-S was more similar to that of the natural consortium, and the microbial diversity was also more varied, so that the growth of aerobic bacteria was inhibited by nitrogen and more glycerol was converted to value-added products by facultative anaerobes, such as 1,3-PDO and LA. In addition, the continuous sparging with N2 during fermentation also has a significant influence on the spectrum of final products produced by a single strain, such as found for the production of 1,3-PDO (Metsoviti et al. 2012). Therefore, the effect of nitrogen on final products produced by microbial consortium CJD-S can be regarded as the result of the comprehensive influence on all microorganisms in the microbial community. Due to the more satisfactory of batch fermentation results (15.70 g/L 1,3-PDO, 6.42 g/L LA and carbon recovery of 0.99), nonsterile and anaerobic culture conditions were performed for further experiments.
Fed-batch fermentation of the microbial consortium CJD-S with crude glycerol
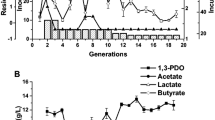
Fed-batch fermentation was performed to evaluate the ability of the microbial consortium CJD-S to convert crude glycerol to 1,3-PDO and other products without the inhibition by a high initial glycerol concentration or toxic impurities. The fermentation results are shown in Fig. 1. The dominant products of fed-batch fermentation of microbial consortium CJD-S with crude glycerol were 1,3-PDO (41.47 g/L) and LA (45.86 g/L), followed by ethanol (17.98 g/L), acetate (11.26 g/L) and succinate (4.96 g/L). Formate was undetectable in the late stage of fermentation. The yields of 1,3-PDO and LA were 0.34 mol/mol and 0.32 mol/mol, respectively, at the end of the fed-batch fermentation by the microbial consortium CJD-S, and 147.65 g/L glycerol was consumed.
Although the production of 1,3-PDO was not as high as that reported by Dietz and Zeng (2014), Zhou et al. (2017) and Jiang et al. (2017a), these were the best results reported for 1,3-PDO and LA coproduction by a microbial consortium from crude glycerol under unsterile conditions (Table 3). Specifically, Chatzifragkou et al. (2011) produced 67.9 g/L 1,3-PDO and 1.9 g/L LA by Clostridium butyricum VPI 1718 in fed-batch fermentation with crude glycerol under nonsterile conditions. Metsoviti et al. (2012) produced 50.1 g/L 1,3-PDO and 16.8 g/L LA by Klebsiella oxytoca FMCC-197 during fed-batch fermentation. Metsoviti et al. (2013) also produced 66.3 g/L 1,3-PDO and 33.7 g/L LA by the Citrobacter freundii strain [FMCC-B 294 (VK-19)] during a nonsterilized fed-batch process. In our research, the microbial consortium CJD-S exhibited good robustness and efficient 1,3-PDO and LA production from crude glycerol, which enabled it to simplify the fermentation process and reduce the production costs in industrial production. The different fermentation performances with microbial consortia are mainly caused by the differences in microbial communities among the microbial consortia selected.
In conclusion, the microbial consortium CJD-S, consisting of 86.25% Enterobacteriaceae and 13.75% Enterococcaceae, was selected from intertidal sludge to convert crude glycerol to high-value products such as 1,3-PDO and LA under unsterile conditions, and its fermentation performance was evaluated. In fed-batch fermentation, the highest concentration of 41.47 g/L 1,3-PDO and 45.86 g/L LA were the highest concentrations achieved from crude glycerol. As the microbial community is constantly changing under the influence of internal and external environmental factors and has a decisive role in the conversion of glycerol to high-value products, regulation strageties will be investigated to synthesize products by a microbial consortium in accordance with people’s expectations in the future. This work provides an alternative method for the production of value-added chemicals from crude glycerol by microbial consortia.
Data availability
The datasets generated during and analysed during the current study are available in the NCBI Sequence Read Archive (SRA) repository, https://dataview.ncbi.nlm.nih.gov/?search=SUB7972898&archive=sra.
Abbreviations
- 1,3-PDO:
-
1,3-Propanediol
- LA:
-
Lactic acid
References
Chatzifragkou A, Papanikolaou S, Dietz D, Doulgeraki AI, Nychas GJE, Zeng AP (2011) Production of 1,3-propanediol by Clostridium butyricum growing on biodiesel-derived crude glycerol through a non-sterilized fermentation process. Appl Microbiol Biotechnol 91:101–112
Dietz D, Zeng AP (2014) Effificient production of 1,3-propanediol from fermentation of crude glycerol with mixed cultures in a simple medium. Bioprocess Biosyst Eng 37:225–233
Eiteman MA, Ramalingam S (2015) Microbial production of lactic acid. Biotechnol Lett 37:955–972
Gallardo R, Faria C, Rodrigues LR, Pereira MA, Alves MM (2014) Anaerobic granular sludge as a biocatalyst for 1,3-propanediol production from glycerol in continuous bioreactors. Bioresour Technol 155(4):28–33
Jiang LL, Liu HF, Mu Y, Sun YQ, Xiu ZL (2017a) High tolerance to glycerol and high production of 1,3-propanediol in batch fermentations by microbial consortium from intertidal sludge. Eng Life Sci 17:635–644
Jiang LL, Zhou JJ, Quan CS, Xiu ZL (2017b) Advances in industrial microbiome based on microbial consortium for biorefifinery. Bioresour Bioprocess 4:11
Metsoviti M, Paraskevaidi K, Koutinas A, Zeng AP, Papanikolaou S (2012) Production of 1,3-propanediol, 2,3-butanediol and ethanol by a newly isolated Klebsiella oxytoca strain growing on biodiesel-derived glycerol based media. Process Biochem 47:1872–1882
Metsoviti M, Zeng AP, Koutinas AA, Papanikolaoua S (2013) Enhanced 1,3-propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel-derived waste glycerol through sterile and non-sterile bioprocesses. J Biotechnol 163:408–418
Mitrea L, Trif M, Catoi AF, Vodnar DC (2017) Utilization of biodiesel derived-glycerol for 1,3-PD and citric acid production. Microb Cell Fact 16:190
Monteiro MR, Kugelmeier CL, Pinheiro RS, Batalha MO, César ADS (2018) Glycerol from biodiesel production: technological paths for sustainability. Renew Sust Energy Rev 88:109–122
Mu Y, Teng H, Zhang DJ, Wang W, Xiu ZL (2006) Microbial production of 1,3-propanediol by Klebsiella pneumoniae using crude glycerol from biodiesel preparations. Biotechnol Lett 28:1755–1759
Murakami N, Oba M, Iwamoto M, Tashiro Y, Noguchi T, Bonkohara K, Abdel-Rahman MA, Zendo T, Shimoda M, Sakai K, Sonomoto K (2016) l-Lactic acid production from glycerol coupled with acetic acid metabolism by Enterococcus faecalis without carbon loss. J Biosci Bioeng 121(1):89–95
Pradima J, Rajeswari Kulkarni M, Archna (2017) Review on enzymatic synthesis of value added products of glycerol, a by-product derived from biodiesel production. Resour-Efficient Technol 3: 394-405
Song Z, Sun Y, Wei B, Xiu Z (2013) Two-step salting-out extraction of 1,3-propanediol and lactic acid from the fermentation broth of Klebsiella pneumoniae on biodieselderived crude glycerol. Eng Life Sci 13:487–495
Sun YQ, Shen JT, Yan L, Zhou JJ, Jiang LL, Chen Y, Yuan JL, Feng EM, Xiu ZL (2018) Advances in bioconversion of glycerol to 1,3-propanediol: prospects and challenges. Process Biochem 71:134–146
Wang JF, Xiu ZL, Fan SD (2001) Determination of glycerin concentration during the fermentation of glycerin to 1,3-propanediol. Ind Microbiol 31:33–35
Wang YH, Teng H, Xiu ZL (2011) Effect of aeration strategy on the metabolic flux of Klebsiella pneumoniae producing 1,3-propanediol in continuous cultures at different glycerol concentrations. J Ind Microbiol Biotechnol 38:705–715
Wang XL, Zhou JJ, Sun YQ, Xiu ZL (2019) Bioconversion of raw glycerol from waste cooking-oil-based biodiesel production to 1,3-propanediol and lactate by a microbial consortium. Front Bioeng Biotechnol 7:14
Xin B, Tao F, Wang Y, Liu HY, Ma CQ, Xu P (2017) Coordination of metabolic pathways: enhanced carbon conservation in 1,3-propanediol production by coupling with optically pure lactate biosynthesis. Metab Eng 41:102–114
Xiu ZL, Zeng AP (2008) Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl Microbiol Biotechnol 78:917–926
Yang XG, Kim DS, Choic HS, Kim CK, Thapa LP, Park C, Kim SW (2016) Repeated batch production of 1,3-propanediol from biodiesel derived waste glycerol by Klebsiella pneumoniae. Chem Eng J 314:660–669
Zhou JJ, Shen JT, Jiang LL, Sun YQ, Mu Y, Xiu ZL (2017) Selection and characterization of an anaerobic microbial consortium with high adaptation to crude glycerol for 1,3-propanediol production. Appl Microbiol Biotechnol 101:5985–5996
Zhou JJ, Shen JT, Wang XL, Sun YQ, Xiu ZL (2018) Stability and oscillatory behavior of microbial consortium in continuous conversion of crude glycerol to 1,3-propanediol. Appl Microbiol Biotechnol 102:8291–8305
Acknowledgements
The authors acknowledge the Key Lab of Biomass Energy and Materials Open Foundation, Jiangsu Province (No. JSBEM202019), the Liaoning Natural Science Foundation Program, China (No. 2019BS238), the Liaoning Key Laboratory of Additive Synthesis and Separation Foundation Program, China (No. 112381), and the Program for Excellent Talents of Science and Technology in Yingkou Institute of Technology.
Funding
This study was supported by the Key Lab of Biomass Energy and Materials Open Foundation, Jiangsu Province (No. JSBEM202019), the Liaoning Natural Science Foundation Program, China (No. 2019BS238), the Liaoning Key Laboratory of Additive Synthesis and Separation Foundation Program, China (No. 112381), and the Program for Excellent Talents of Science and Technology in Yingkou Institute of Technology.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LLJ, FYL and WY. The first draft of the manuscript was written by LLJ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, LL., Liu, FY., Yang, W. et al. Production of 1,3-propanediol and lactic acid from crude glycerol by a microbial consortium from intertidal sludge. Biotechnol Lett 43, 711–717 (2021). https://doi.org/10.1007/s10529-020-03063-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-03063-0