Abstract
Objective
To construct recombinant Lactococcus lactis (L. lactis) expressing viral protein 1 (VP1) of enterovirus 71 (EV71) and evaluate its immunogenicity to be used as an oral vaccine in BALB/c mice.
Results
Recombinant L. lactis competent in secreting VP1 (~ 30 kDa) into the extracellular environment with the aid of the signal peptide Usp45 was produced. Enzyme-linked immunosorbent assay showed that significant VP1-specific antibody response including the production of both serum IgG and fecal IgA (p < 0.05) was elicited in BALB/c mice upon oral immunization with recombinant L. lactis. Moreover, in contrast to negative control, recombinant L. lactis induced adequate neutralizing antibodies in mouse sera (p < 0.05) as demonstrated in virus neutralization assay, whereas the presence of neutralizing antibodies in fecal samples was obvious but not significant (p > 0.05).
Conclusions
Recombinant L. lactis expressing VP1 of EV71 has the potential to be used as an oral vaccine candidate. The findings may provide some preliminary evidences for further development of effective and needle-free EV71 vaccines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hand, foot, and mouth disease (HFMD) is an infectious disease that poses a serious threat to infants and young children worldwide, especially in the Asia–Pacific region. Various enteroviruses can cause HFMD, but severe complications including neurological symptoms and heart diseases have been mostly associated with enterovirus 71 (EV71) (Ventarola et al. 2015). In the absence of specific treatment against EV71, vaccination is considered as the best choice to control EV71-associated HFMD. To date, three formalin inactivated EV71 vaccines have been approved in China, which greatly decreased HFMD incidences (Yi et al. 2017). However, the use of whole inactivated viruses needs several intramuscular injections and may address some potential safety issues (Mao et al. 2016). Therefore, it is desired to develop novel, safe, and needle-free vaccines against EV71.
Viral proteins 1–3 (VP1–VP3) are exposed on the surface of EV71 virion and mediate virus entry through interacting with cellular receptors (Wang et al. 2012). Particularly, VP1 is able to elicit the production of neutralizing antibodies that can block virus infection (Gao et al. 2012; Ku et al. 2015). Therefore, VP1 has been considered to be an ideal subunit vaccine candidate (Peng et al. 2016; Yuan et al. 2015). In view of the fact that EV71 is mostly transmitted via the fecal–oral route, we asked whether direct presentation of VP1 at the mucosa could elicit immunoprotection against EV71 infection.
Lactococcus lactis (L. lactis), which is a non-pathogenic food grade lactic acid bacterium, has been developed as a promising vaccine delivery vehicle for oral vaccination (Wyszynska et al. 2015). It is well known that L. lactis strains have innate adjuvant properties that can enhance specific immune responses to the administered antigens. Therefore, this study aimed to produce recombinant L. lactis expressing secretory VP1 and investigate the possibility of using this recombinant strain as an oral vaccine against EV71 infection. Immunogenicity of the vaccine was preliminarily evaluated in mice by testing VP1-specific IgG and IgA and neutralizing antibodies. To our knowledge, this is the first study to construct recombinant L. lactis secreting VP1 of EV71 and evaluate its potential as an oral vaccine.
Materials and methods
Bacterial strains and plasmids
Lactococcus lactis MG1363 strains and the shuttle plasmid pMG36e, a type of non-induced expression vector containing a P32 promoter, were employed as expression host and vector, respectively. Escherichia coli (E. coli.) DH5α cells were used for plasmid screening and amplification. For selection of positive pMG36e-carrying strains, erythromycin (EM) was added to the growth medium at 200 μg/ml for E. coli. and 10 μg/ml for L. lactis.
Cells and viruses
Human rhabdomyosarcoma (RD) cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) under humidified environment at 37 °C with 5% CO2. The prototype strain EV71/BrCr (ATCC, VR-1775) was cultured and propagated in RD cells. The viral titer was calculated by the Reed and Muench method using RD cells based on cytopathic effect (CPE) developing and expressed as the 50% tissue culture infectious dose (TCID50).
Plasmid construction and transformation
VP1 gene of EV71/BrCr (GenBank Accession No. AB777928.1) with the signal peptide sequence of Usp45 (SPusp45) (GenBank Accession No. EU382094.1) at its amino-terminus was codon-optimized for expression in L. lactis and commercially synthesized by Sangon Biotech company (Shanghai, China). Fusion gene of VP1 and SPusp45 was then cloned into the multiple cloning site of pMG36e under the control of the P32 promoter by digestion with Cla I and Sal I and ligation with T4 DNA ligase (Fig. 1a). The resulting recombinant plasmid pMG36e–VP1 was first chemically transformed into E. coli. DH5α competent cells for amplification, and then introduced into L. lactis MG1363 cells via electroporation following a standard protocol. Single colony of positive L. lactis MG1363 strains harboring pMG36e–VP1 was selected on GM17 plate with 1.5% (w/v) agar containing EM and further verified by DNA sequencing. Finally, L. lactis MG1363 strain carrying pMG36e–VP1 was designated as MG–VP1. L. lactis MG1363 cells carrying the empty vectors (parental plasmid pMG36e) were termed MG–NC and used as negative control.
Secretory expression of VP1 in L. lactis. a Schematic diagram for construction of expression plasmid pMG36e–VP1. “SPusp45” indicates the signal peptide of Usp45. “Cla I” and “Sal I” represent restriction endonuclease recognition sites. b Western blot analysis of secretory expression of VP1. “M” indicates the protein marker. Lanes 1 and 2 represent the culture supernatant of L. lactis MG–VP1 and L. lactis MG–NC (negative control), respectively. VP1 was only detectable in the supernatant of MG–VP1; no corresponding band was detected in the supernatant of MG–NC. This indicated that MG–VP1 could express and secrete VP1 into the extracellular environment
SDS-PAGE and Western blot
To analyze the expression of VP1, overnight cultures of MG–VP1 grown in fresh GM17 medium supplemented with EM at 30 °C were collected by centrifugation at 12000×g for 15 min. Next, the obtained supernatant was concentrated 10-fold with ultrafiltration membrane and subject to 12% SDS-PAGE and Western blot analysis. In short, VP1 was probed by rabbit anti-VP1 hyperimmune sera produced in house by immunizing rabbit twice with purified E. coli-expressed VP1. HRP-conjugated goat anti rabbit IgG was used as the secondary antibody. Each step was performed at room temperature for 1 h and the resulting bands were visualized via an imaging system.
Immunization of BALB/c mice
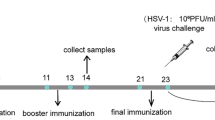
A prime-boost scheme was performed to evaluate the immunogenicity of MG–VP1 (Fig. 2). MG–VP1 and MG–NC were individually cultured, centrifuged, and then resuspended in sterile phosphate buffered saline (PBS) to 1010 cfu/mL. 6-week-old female BALB/c mice were randomly assigned to three treatment groups (MG–VP1, MG–NC, and PBS) with six mice in each group. Mice in MG–VP1 group were initially immunized with 109 MG–VP1 cells by oral route on three consecutive days (day 0–2) and boosted twice with the same amount of MG–VP1 cells on day 14–16 and 28–30, respectively. By contrast, mice treated with MG–NC or PBS were orally administered with equivalent dose of MG–NC or sterile PBS following the same immunization protocol. Serum samples (orbital sinus) and fecal extracts were collected before immunization (day 0) and at 2 weeks after each immunization (day 16, 30 and 44) for further analysis.
Illustration of the immunization scheme. Three groups of 6-week-old female BALB/c mice (n = 6) were immunized with MG–VP1, MG–NC or PBS, respectively, three times at 2-week intervals. Each mice was orally administered with 109 cells or sterile PBS on three consecutive days. Sera and fecal extracts were collected before immunization (day 0) and at 2 weeks after each immunization (day 16, 30 and 44) for analysis
Antibody measurement
An indirect ELISA was performed to test VP1-specific serum IgG and fecal IgA on day 0, 16, 30 and 44 after initial immunization. Briefly, 96-well plates were coated with 100 ng/well purified VP1 at 4 °C overnight. As primary antibodies, serial dilutions of serum samples or fecal extracts including negative and blank controls were added to the plates and then incubated at 4 °C overnight. Subsequently, HRP-conjugated goat anti mouse IgG antibody and goat anti mouse IgA antibody were used to detect mouse IgG and IgA in the sample, respectively. Plates were washed thrice with PBS containing 0.05% (v/v) Tween-20 between each step. 3,3′,5,5′-Tetramethylbenzidine was added as substrate for color development and optical density at 450 nm (OD450) was measured after stopping the reaction with 2 M H2SO4.
Virus neutralization assay
To determine the presence of neutralizing antibodies in MG–VP1-immunized mice, a RD cell-based micro-neutralization assay was conducted in 96-well plates. In brief, serum and fecal samples collected on day 44 after initial immunization were heat-inactivated at 56 °C for 30 min and twofold serially diluted. Then, 50 μl of diluted samples were mixed with equal volume of 102 TCID50 of EV71/BrCr in each well. After incubation at 37 °C for 1 h, 104 RD cells were added to each well for continuous incubation at 37 °C with 5% CO2 for 4 days. Finally, the cells were checked under a microscope and the neutralizing titer was defined as the highest dilution that fully protects the cells from CPE. Samples from MG–NC or PBS inoculated mice were used as negative control.
Statistical analysis
Data were analyzed using GraphPad Prism version 5 and expressed as mean ± SD. One-way analysis of variance (ANOVA) with post hoc test was performed to analyze the differences between means across groups. Comparison between different time course and treatment groups was statistically determined by two-way ANOVA with post hoc analysis. Values of p < 0.05 were considered statistically significant.
Results
Secretory expression of VP1
As shown in Fig. 1b, secretory expression of VP1 (~ 30 kDa) in L. lactis MG1363 was confirmed by Western blot. VP1 could only be detected by rabbit anti-VP1 hyperimmune sera in supernatant from recombinant L. lactis MG–VP1.
Detection of VP1-specific antibodies
The obtained MG–VP1 was competent for inducing considerable serum IgG and fecal IgA responses in mice after oral immunization. Further details were given in Fig. 3.
Detection of VP1-specific antibodies in mice by ELISA. a Detection of IgG titers in serum samples. b Detection of IgA titers in fecal extracts. As it was shown, statistically higher levels of serum IgG and fecal IgA were detected in mice immunized with MG–VP1 (p < 0.05), while no detectable antibody was detected in samples collected from mice that were immunized with MG–NC (negative control) or PBS (blank control). For MG–VP1 immunized mice, obvious increase of serum IgG levels was observed as immunization proceeded and the highest titers were reached on day 44 after the initial vaccination. In consistent with the production of IgG, there was a significant growing in IgA response. But the presence of highest fecal IgA titers appeared on day 30 rather than on day 44 after the initial vaccination with no statistical difference (p > 0.05). Moreover, both IgG and IgA titers in MG–VP1 immunized mice after each booster immunization were significant higher than that following primary immunization (p < 0.05). The experiments were performed in triplicate and data were presented as the mean ± SD from six mice
Determination of neutralizing antibody titers
Figure 4 showed that MG–VP1 was able to elicit the production of neutralizing antibodies in mice.
Analysis of neutralizing antibodies against EV71. An in vitro micro-neutralization assay was used to measure the ability of neutralizing antibodies in samples (day 44) to inhibit EV71 replication in RD cells. It was found that both serum and fecal samples from either MG–NC or PBS vaccinated mice showed no inhibition of virus infection, even at the minimum dilution. The sera from MG–VP1 immunized mice displayed significant neutralizing activities (p < 0.05), whereas neutralizing titers in fecal samples appeared to be slightly higher, but not statistically different from those of the control groups (p > 0.05). Data represent the mean ± SD from six mice
Discussion
EV71 is one of the main causative agents of HFMD which remains a major public health concern across the Asian-Pacific region. Since the common transmission of EV71 in infants and young children is through fecal–oral route, oral mucosa is supposed be the primary site of EV71 infection. Therefore, oral immunization might be an attractive strategy for developing novel effective vaccines against EV71. Over the past years, LAB, as live vehicles to deliver preventive and therapeutic molecules, have been used to control a variety of viral diseases (Al Kassaa et al. 2014). In particular L. lactis MG1363 strains have been extensively used in developing oral vaccines against human or animal pathogens (Li et al. 2018; Zhou et al. 2018). In consideration of these, recombinant L. lactis MG1363 expressing VP1 of EV71 was produced and its possibility to be used as an oral vaccine against EV71 infection in mice was evaluated.
Currently, secretory expression and cell surface-display are two main mechanisms frequently used to deliver exogenous antigens via L. lactis. In this study, we preferred the former strategy because secretory expression might potentially produce higher levels of protein than cell wall anchored display (Cano-Garrido et al. 2015). Our data confirmed that recombinant L. lactis MG–VP1 was able to secrete VP1 to the extracellular environment with the aid of the signal peptide SPusp45. Meanwhile, MG–VP1 was competent in eliciting robust VP1-specific IgG levels in mouse sera (p < 0.05) and considerable VP1-specific IgA levels in feces (p < 0.05) upon oral immunization. As neutralizing antibody plays a key role in in vivo protection against lethal EV71 infection (Lee and Chang 2010), we also tested the neutralizing ability of the serum and fecal samples to assess the immunogenicity of MG–VP1. The presence of neutralizing ability was mainly found in serum samples (p < 0.05), indicating that MG–VP1 was capable of eliciting neutralizing antibodies in mice. This was in accordance with the reports that the major neutralizing epitopes have been positioned on VP1 of EV71. However, neutralizing titers detected in fecal samples were slightly higher but statistically different from that of the control groups (p > 0.05). This might indicate that MG–VP1 without appropriate adjuvant could not elicit sufficient neutralizing antibodies in mouse mucosal system under the tested conditions. Based on previous study, the use of agonists for toll-like receptors is expected to enhance the immunogenicity of MG–VP1, but more comprehensive studies are needed to validate our hypothesis.
Conclusion
In this study, recombinant L. lactis expressing the major antigen VP1 of EV71 was produced for the first time and its immunogenicity in BALB/c mice was investigated via oral administration. The data demonstrated that the engineered L. lactis was able to secrete VP1 to the extracellular environment and elicit considerable VP1-specific IgG and IgA and neutralizing antibodies in mice. Although preliminary, it can provide new insights and experimental evidences for further development of effective and needle-free vaccines to protect children from EV71 infection.
References
Al Kassaa I, Hober D, Hamze M, Chihib NE, Drider D (2014) Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicrob Proteins 6:177–185
Cano-Garrido O, Seras-Franzoso J, Garcia-Fruitos E (2015) Lactic acid bacteria: reviewing the potential of a promising delivery live vector for biomedical purposes. Microb Cell Fact 14:137
Gao F, Wang Y, Mao Q, Yao X, Liu S, Li F, Zhu F, Yang J et al (2012) Enterovirus 71 viral capsid protein linear epitopes: identification and characterization. Virol J 9:26
Ku Z, Ye X, Shi J, Wang X, Liu Q, Huang Z (2015) Single neutralizing monoclonal antibodies targeting the VP1 GH loop of enterovirus 71 inhibit both virus attachment and internalization during viral entry. J Virol 89:12084–12095
Lee MS, Chang LY (2010) Development of enterovirus 71 vaccines. Expert Rev Vaccines 9:149–156
Li L, Qiao X, Chen J, Zhang Y, Zheng Q, Hou J (2018) Surface-displayed porcine reproductive and respiratory syndrome virus from cell culture onto gram-positive enhancer matrix particles. J Ind Microbiol Biotechnol 45:889–898
Mao QY, Wang Y, Bian L, Xu M, Liang Z (2016) EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev Vaccines 15:599–606
Peng X, Fang X, Li J, Kong L, Li B, Ding X (2016) Enhancing immune responses of EV71 VP1 DNA vaccine by co-inoculating plasmid IL-12 or GM-CSF expressing vector in mice. Cell Mol Biol 62:35–41
Ventarola D, Bordone L, Silverberg N (2015) Update on hand-foot-and-mouth disease. Clin Dermatol 33:340–346
Wang X, Peng W, Ren J, Hu Z, Xu J, Lou Z, Li X, Yin W et al (2012) A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol 19:424–429
Wyszynska A, Kobierecka P, Bardowski J, Jagusztyn-Krynicka EK (2015) Lactic acid bacteria–20 years exploring their potential as live vectors for mucosal vaccination. Appl Microbiol Biotechnol 99:2967–2977
Yi EJ, Shin YJ, Kim JH, Kim TG, Chang SY (2017) Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res 6:4–14
Yuan J, Tang X, Yin K, Tian J, Rui K, Ma J, Mao C, Chen J et al (2015) GITRL as a genetic adjuvant enhances enterovirus 71 VP1 DNA vaccine immunogenicity. Immunol Res 62:81–88
Zhou BY, Sun JC, Li X, Zhang Y, Luo B, Jiang N, Liu MC (2018) Analysis of immune responses in mice orally immunized with recombinant pMG36e-SP-TSOL18/Lactococcus lactis and pMG36e-TSOL18/Lactococcus lactis vaccines of taenia solium. J Immunol Res 2018:9262631
Acknowledgements
This work was supported by the Key Scientific Research Project of Universities of Henan Province, China (Grant Nos. 19A330004 and 18B360012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, P., Wang, Y., Tao, L. et al. Recombinant lactococcus lactis secreting viral protein 1 of enterovirus 71 and its immunogenicity in mice. Biotechnol Lett 41, 867–872 (2019). https://doi.org/10.1007/s10529-019-02695-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02695-1