Abstract
Monoclonal antibodies represent the major class of biopharmaceutical products (for therapeutics and diagnostics) with an increasing demand that reaches several tons per year worldwide. Traditional large-scale manufacturing processes are based on stirred tank bioreactors for the growth of Chinese Hamster Ovary cells (CHO) which requires high initial investments and production costs. Therefore, there is an urgent need for alternative production platforms that can at least act as a complement to the over-exploited mammalian fermentation systems. In this perspective, the use of plants for the large-scale production of biopharmaceuticals (‘Molecular farming’) represents an interesting and mature technology that has already proved its benefits in terms of safety, scalability, rapidity and reduced manufacturing costs. Here we discuss the recent advances in the production of monoclonal antibodies (mAbs) in plant-based platforms such as transgenic plants, tissue and cell cultures and transient expression systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monoclonal antibodies (mAbs) are useful tools in medicine, biology and biochemistry due to their binding specificity to different molecular targets and their stability both in vivo and in vitro. They represent the most successful biopharmaceuticals on the market with more than 50 mAbs currently approved and sales expected to increase to $125 billion by 2020 (Shukla et al. 2017; Ecker et al. 2015). Mammalian cell cultures mainly based on Chinese Hamster Ovary cells (CHO) are still the favored system for the production of commercial mAbs, even if the increasing demand (also linked to the growing market of follow‐on biologics -biosimilars-) is promoting the development of alternative expression platforms. Indeed, the facilities necessary for large-scale production in mammalian cells require high initial investments and their operating and maintenance costs are high (Ecker et al. 2015). Among alternative expression systems, plants represent promising bioreactors for the large-scale production of recombinant proteins and antibodies. They offer an attractive expression platform with several advantages such as the absence of potential human pathogens, possibility to engineer a tailored antibody glycosylation profile, possibility to scale-up production by simply increasing the number of plants and competitiveness of manufacturing costs. Nevertheless, expression of recombinant proteins in plants still presents several bottlenecks mainly related to purification and downstream processing as well as to regulatory issues (Fischer et al. 2015; Drake et al. 2017).
The estimated costs of mAb production in transgenic plants are calculated at about 100 €/g (Buyel et al. 2017) which are comparable to the average costs of 50–100 €/g reported in CHO cells (Lim et al. 2010; Kelley 2009). To strengthen the potentiality of this alternative production system a recent milestone was achieved by the private company Protalix Biotherapeutics Inc. (Israel) that developed a biopharmaceutical drug in carrot cells to treat a rare human metabolic disease (Gaucher’s disease). ELELYSO™, the commercial name of the glucorebrosidase enzyme used in replacement therapy, was the first plant-cell drug to receive the human use approval from FDA in 2012 (Mor 2015). This success story gave the impulse to other companies to invest in plant-based manufacturing facilities. For example, Medicago Inc. (Canada and US-based biopharmaceutical company) developed a quadrivalent seasonal influenza vaccine that has recently completed Phase III clinical trials (Efficacy, Safety, and Immunogenicity of a Plant-Derived Quadrivalent Virus-Like Particles Influenza Vaccine in Adults, https://clinicaltrials.gov/ct2/show/NCT03301051?term=medicago&rank=2) (Pillet et al. 2016). Until today, a wide range of recombinant proteins such as antibodies, enzymes and vaccines have been produced using plant-based expression systems and demonstrated efficacy and safety in pre-clinical as well as clinical studies (Yao et al. 2015). The results of some clinical evaluations are detailed in Table 1. In the next paragraphs, we will illustrate the major differences between glycoproteins produced in plants or mammalian cells and the most recent technologies used to efficiently produce mAbs in different systems such as whole plants, cell suspensions, hairy root cultures and microalgae.
Plant-made versus mammalian cells derived glycoproteins
Post-translational modifications of proteins occurring in plant cells are essentially similar to those in animal cells and the correct assembly of complex molecules, such as antibodies, are assisted by chaperones that mediate the folding and formation of disulfide bonds while the addition of N-glycans is performed by specific cellular glycosyltransferases. The N-glycans are those accounting for the most significative differences between plant-derived glycoproteins and their mammalian counterpart and are considered to be a major issue for the use of plant-derived pharmaceuticals in human therapy due to possible immunogenic reactions (Jin et al. 2008). In fact, while core N-glycans are similar in plants and mammals, complex N-glycans show substantial differences with sialic acid and β 1,4-linked galactose residues typical of animal cells and β1,2-xylose and α1,3-fucose of plants (Bosch et al. 2013). Potential immunogenicity of plant-typical sugar residues is still a controversial issue and until now clinical trials performed with plant-derived glycoproteins have never evidenced adverse effects on humans (Tusé et al. 2015). However, to overcome possible biosafety regulatory constraints this issue has been successfully addressed by ‘optimizing’ the N-glycosylation profile of plant-derived molecules by using different strategies such as the inactivation of endogenous plant-specific glycosyltransferases and/or their complementation by heterologous human glycosyltransferases (Steinkellner and Castilho 2015; Montero-Morales and Steinkellner 2018). For example, fucose and xylose-free glycoproteins were successfully obtained in whole plants or plant cell lines either using RNA interference or recent genome editing approaches (Jansing et al. 2018; Hanania et al. 2017). In many cases, mAbs produced in glyco-engineered plant systems have shown far better biological activity compared to those obtained in animal cells showing enhanced antibody‐dependent cellular cytotoxicity (ADCC) and Fc-γ receptor binding (Marusic et al. 2018; Qiu et al. 2014). Overall, recent advances in plant glyco-engineering allowed the production of ‘human-like’ recombinant glycoproteins with a highly homogeneous glycosylation pattern and plant expression systems can be considered versatile platforms for the manufacturing of mAbs with improved characteristics (‘glycobetters’).
Production of mAbs in whole plants
The first pioneering study on the production of a full-size IgG in plants dates back to almost 30 years ago (Hiatt et al. 1989). Since then different antibody formats have been successfully expressed in plants such as secretory IgA, Fab fragments, single-chain antibody fragments (scFvs), minibodies, single variable domains, antibody fusion proteins (immunocytokines), scFv-Fc antibodies and camelid heavy-chain antibodies (Fig. 1). These molecules were expressed in plants with different aims such as to confer virus resistance, to modulate plant cell processes but also for the production of valuable biopharmaceuticals including therapeutic mAbs (Lomonossoff and D’Aoust 2016).
Production of antibodies in whole plants was obtained using two different expression strategies based either on the stable transformation of the nuclear genome or on transient expression systems exploiting viral or Agrobacterium tumefaciens transfer DNA (T-DNA) expression vectors (Sheshukova et al. 2016). Among the bottlenecks of producing pharmaceutical proteins in whole plants, there is the necessity to consider the environmental impact and regulatory compliances, which often require growing plants in controlled conditions (contained greenhouses). This can be a limitation for the cost-effectiveness of this production platform since it greatly reduces the scaling-up potential compared to growing in the open field. Conversely, in the case of transient expression systems that generally provide much higher yields and rapidity of recombinant protein accumulation, the production in contained greenhouses still allows an efficient scale-up and cost competitiveness of the manufacturing process. In the next paragraphs, the production of antibodies using either transgenic plants or transient expression systems will be illustrated.
Transgenic plants
Plants producing whole mAbs were traditionally obtained by cross-pollinating two transgenic lines separately transformed with the antibody heavy (HC) or light chain (LC) genes. This strategy was generally time-consuming but allowed the production of correctly assembled IgG molecules and more complex immunoglobulins such as secretory IgAs (Hiatt et al. 1989; Ma et al. 1995). More efficient and rapid strategies are now available based on the use of binary vectors containing both HC and LC coding sequences in the same T-DNA that allow to generate transgenic plants expressing complete IgGs in a single transformation event (De Muynck et al. 2010). Several plant species can be efficiently engineered for the production of mAbs such as Nicotiana benthamiana (a wild relative species of tobacco), Arabidopsis thaliana, lettuce, potato and maize, but most antibodies reported in literature have been expressed in transgenic tobacco (N. tabacum) generally reaching levels of several milligrams per kg (Yusibov et al. 2016). Interesting results have also been obtained in the small aquatic plant Lemna minor (duckweed) in which a glycoengineered version of a mAb against human CD30 was obtained (Cox et al. 2006). The first antibody in Europe entering clinical trials was the 2G12 mAb produced in transgenic tobacco plants to be used as a microbicide against HIV infections (Ma et al. 2015). Other examples of antibodies that reached Phase II in the US were the CaroRx™ (secretory IgA) for the treatment of dental caries also obtained in tobacco (Weintraub et al. 2005) and Avicidin, an IgG against the epithelial cell adhesion molecule (EpCAM) for the treatment of refractive cancers (colon, lung and prostate) that was produced in maize (Gavilondo and Larrick 2000). Production and accumulation of recombinant proteins in seeds, a natural plant storage compartment, is an interesting alternative strategy that has several advantages such as the reduction of typical plant contaminants normally present in the leaves (e.g. alkaloids, phenolic compounds etc.) and the possibility to enhance long-term protein stability without the need of storing the plant material at low temperatures (Boothe et al. 2010). An interesting example of this strategy was the expression of the neutralizing anti-HIV-1 2G12 antibody in seeds of transgenic maize which allowed to obtain a yield of 75 mg per kg of dry seed weight (Rademacher et al. 2008).
Transient expression systems based on agroinfiltration technology
Transient or epichromosomal transformation differs from stable transformation in that the exogenous sequence is not inherited by the progeny thus reducing the risk of environmental biosafety issues linked to the dissemination of the transgene through seeds or pollen. This approach generally provides high protein yields in a very short period of time (few days to weeks) which is not achievable via stable transformation (Komarova et al. 2010). Transient expression can be obtained using either vectors or plasmids containing T-DNA gene cassettes derived from A. tumefaciens bearing a strong constitutive promoter or vectors carrying appropriately modified genomic sequences of plant viruses inserted in T-DNA cassettes (viral vectors) (Kopertekh and Schiemann 2017).
The Agrobacterium-mediated expression systems based on T-DNA gene cassettes are typically made up of vectors carrying multiple cloning sites, a strong constitutive promoter, selectable marker genes and plasmid replication components for E. coli and A. tumefaciens. These vectors exploit the ability of A. tumefaciens to transfer the T-DNA bearing the gene of interest in the plant cells. This methodology, known as agroinfiltration, is based on the permeation of the agrobacterium containing solution in the intercellular spaces of the leaves by using a needleless syringe or through vacuum (Chen and Lai 2015). These methods provide synchronous gene expression because agrobacterium is known to simultaneously infect almost 100% of the cells in the infiltrated leaves. Transient expression based on leaf agroinfiltration was successfully used to produce fully functional mAbs in different plant species such as N. benthamiana, tobacco, lettuce and tomato (De Muynck et al. 2010). Moreover, to enhance antibody accumulation in plant tissues, the co-expression of plant virus gene-silencing suppressor proteins (e.g. P19 from Artichoke mottled crinckle virus -AMCV-) proved to be an effective strategy increasing protein expression levels up to tenfold (Circelli et al. 2010). A further advantage of this technology is also the rapid scalability of the process which gives the possibility to handle large numbers of plants by using automated vacuum-agroinfiltration systems on large-scale under Good Manufacturing Practice (GMP) Regulations (Chen et al. 2013).
Most recent and efficient transient expression systems, also based on T-DNA gene cassettes, rely on the use of virus-based expression vectors delivered in plant tissues by agroinfiltration. In the first generation of viral vectors the gene of interest is inserted as an additional Open Reading Frame in the functional genome of the plant virus (“full virus” strategy) (Lico et al. 2008). To overcome the limitations of this strategy, mostly concerning the size of the foreign gene tolerated by the viral genome, a second generation of virus expression vectors was engineered. These include only the viral elements (strong promoters, terminators, enhancers, silencing suppressor genes) required for the expression of the foreign gene and are referred to as the “deconstructed virus” strategy (Gleba et al. 2007). This allowed developing a technique called ‘Magnifection’ that drastically facilitated large-scale manufacturing processes and most importantly enhanced protein yields up to 100 times. The expression platform commercially known as ‘MagnICON’ proved to be very effective for the high-yield production of mAbs in N. benthamiana (Giritch et al. 2006; Klimyuk et al. 2014). A successful example of the exploitation of this technology is the Phase 1 clinical study conducted on chimeric antibodies for the treatment of B cell follicular lymphoma essentially by using these molecules as individualized idiotype vaccines created from patient’s own cancer cells. The rapid and high-level expression offered by plants permitted to proceed from biopsy to the individualized vaccine in less than 12 weeks (Tusé et al. 2015). Another advanced application of the ‘Magninfection’ technology led to the production of a cocktail of three neutralizing mAbs (ZMapp) developed for the treatment of Ebola virus infection in glycoengineered N. benthamiana plants with knocked-down plant-specific glycosyl transferases (β1,2-xylosyltransferase; and α1,3-fucosyltransferase) (Qiu et al. 2014; Strasser et al. 2008). The ZMapp cocktail was administered to 71 patients during the 2015 Ebola outbreak in West Africa under compassionate-use protocols. In this randomized, controlled trial the estimated effect of ZMapp appeared to be beneficial although the result did not meet the statistical threshold for efficacy (PREVAIL II Writing Group et al. 2016). Recently, the ‘Magninfection’ technology allowed to reach the highest results in terms of antibody production yield in whole plants with levels of 2 g per kg of fresh weight (Zischewski et al. 2016).
Another efficient viral-derived expression system is based on the Cowpea mosaic virus (CPMV). This virus has a bipartite RNA which has been used to develop deconstructed expression vectors for the production of antibodies (Sainsbury et al. 2010). A recent development of this technology is the ‘hyper-translatable’ Cowpea mosaic virus-based protein expression system (‘CPMV-HT’) that allows the expression of multimeric proteins, encoded by genes in a single T-DNA construct, using the agroinfiltration system (Saxena et al. 2016). This viral vector was successfully used to accumulate high levels of the anti-HIV mAb 2G12 in N. benthamiana plants and the purified antibody showed to be functionally equivalent to the same molecule produced in mammalian cells (Sainsbury et al. 2010). In recent years, several biotechnology companies operating mainly in the United States, have built semi-automated systems for the large-scale production of plant-derived bio-pharmaceuticals using agroinfiltration-based transient expression platforms (Holtz et al. 2015).
Production in plant cells and tissue cultures
Plant tissues and plant cell-based platforms represent a valid system for the production of heterologous proteins thanks to the possibility of producing in sterile contained conditions with low risks of contamination by ‘human pathogens’ (Schillberg et al. 2013). Furthermore, transformed plant cells and organs can be propagated indefinitely and the protein of interest can be secreted into the culture medium facilitating the product recovery and purification (Magy et al. 2014). The following paragraphs summarize the most promising plant cell and tissue culture systems used for the production of biopharmaceuticals as well as future perspectives and challenges.
Plant cell suspension cultures
For more than two decades undifferentiated plant cell suspension cultures have been used for the production of heterologous proteins. Plant cell suspensions are usually prepared from callus tissue in shaker flasks or fermenters to form single cells and small aggregates. The most used plant cell lines are derived from N. tabacum (such as BY2 or NT1 cells) while some others originate from edible crop species such as rice, soybean, carrot and tomato (Santos et al. 2016; Xu and Zhang 2014). Cultured plant cells require simple nutrients to grow and the purification and downstream process is easy because they generally allow the accumulation of the recombinant protein in the culture medium even if size and composition of the foreign protein may influence its secretion capacity (Schillberg et al. 2013). A weakness of this system is the low yield of secreted protein (in the range of milligrams per liter of culture) that is also influenced by the proteolytic activity in the culture medium (Magy et al. 2014). The first human protein expressed in N. tabacum cell suspension cultures dates back to 1990s when the correctly processed human serum albumin was obtained (Sijmons et al. 1990) but several other proteins such as cytokines, hormones, vaccine antigens and antibodies have been produced since then (Schillberg et al. 2013). A striking successful example of the exploitation of plant cell-based bioreactors was the production of the drug ELELYSO™ to treat Gaucher’s Disease in carrot cells by using a cost-effective plant cell culture platform called ProCellEx™ that significantly reduced the manufacturing costs of this drug (Tekoah et al. 2015). The same culture platform was recently used to produce the enzyme alpha-galactosidase-A (‘Fabrazyme’) in tobacco cells for the treatment of the rare genetic Fabry disease (Kizhner et al. 2015).
Antibodies remain the most expressed proteins because of their diagnostic and pharmaceutical value. An example is the tumour targeting monoclonal antibody M12 that was obtained in BY-2 cells using a 200 litres disposable bioreactor with yields of 20 mg l−1 (Raven et al. 2015) while the optimization of the culture medium allowed raising the yield of this antibody to 100 mg l−1 (Vasilev et al. 2013). Among the advantages of using cell suspension cultures, there is also the possibility to optimize the glycosylation profile of proteins by engineering the glycan metabolism of plant cells. An example was the establishment of stably engineered BY-2 cell lines that co-expressed the human β-1,4-galactosyltransferase enzyme or in which the β(1,2)-xylosyltransferase and the α(1,3)-fucosyltransferase genes were inactivated. These lines were successfully used to produce antibodies with a human-like glycosylation profile (Mercx et al. 2017; Navarre et al. 2017). Recently, a novel expression system was developed based on plant cell suspension packs (or cell ‘cookies’). These cell ‘cookies’ are directly transformed with a recombinant Agrobacterium bearing the gene of interest and incubated for a few days until the desired level of protein has been reached (Rademacher 2013).
Hairy root cultures
Among cultured plant tissues, hairy roots (HR) represent an interesting novel platform for the production of heterologous proteins. HR are neoplastic tissues that originate upon infection of monocot or eudicot plant species with Agrobacterium rhizogenes (now revised as Rhizobium rhizogenes) a gram-negative soil bacterium of the family Rhizobiaceae that is naturally able to introduce into the genome of the infected plant a T-DNA segment from its root-inducing (Ri) plasmid (White et al. 1985). This T-DNA carries a set of oncogenes that promote the formation of proliferating HR that can be stably grown in sterile cultures for several years. Since decades HR have found numerous applications in the production of bioactive secondary metabolites but just recently they have been considered as possible bioreactors for the manufacturing of biopharmaceutical proteins (Mehrotra et al. 2015). HR offer several advantages such as genetic stability, growth in sterile contained conditions, fast biomass accumulation and the possibility to secrete the heterologous proteins in the culture medium. On the other hand, the low protein yields (in the range of mg per liter of culture) and the difficulties in setting up large-scale production in bioreactors represent the major challenges for the future exploitation of this plant expression platform. Yet there are many examples in the literature of different classes of heterologous proteins that have been successfully produced in HR such as enzymes, vaccine antigens, growth factors, interleukins and antibodies (Xu et al. 2012). The first biopharmaceutical protein to be expressed in HR was the Anti-Streptococcus mutans mAb Guy’s 13 that was successfully secreted in the culture medium (Wongsamuth and Doran 1997). A recent example of a mAb expressed in HR, is the anti-vitronectin mAb M12 tumour-targeting antibody (Häkkinen et al. 2014). In this work, an optimised induction protocol for the cultivation of tobacco HR secreting the mAb M12 in the culture medium enhanced antibody yield by 30-fold and about 57% of the antibody produced was secreted in the medium. Characterization of the purified antibody showed that it possessed a typical plant glycosylation pattern, which still represents a major issue for plant-derived antibodies to be used in human therapy. In a recent approach, tumour-targeting anti-CD20 and anti-tenascin C antibodies and immunocytokines were expressed in glyco-engineered N. benthamiana HR cultures in which β(1,2)-xylosyltransferase and the α(1,3)-fucosyltransferase genes were silenced (Lonoce et al. 2016, 2018). These examples demonstrated that it is possible to produce fully functional anticancer antibodies with a human compatible glycosylation profile in HR.
An advantage of hairy roots compared to expression platforms based on whole plants is the possibility of secreting the recombinant proteins in the culture medium. It was demonstrated that antibody purification from HR culture medium could be carried out using a simple two-step procedure composed by a first clarification of the medium by filtration and an affinity chromatography step using Protein A resin. In the case of anti-CD20 antibodies, Lonoce and colleagues reported a 60% protein recovery with final yields of 20 mg l−1 of purified product (Lonoce et al. 2018).
Moss cultures
Moss cultures have received increasing interest as a novel system for the production of biopharmaceutical proteins in bioreactors (Decker et al. 2014). Moss cells have the ability to photosynthesize and can be grown in self‐contained systems like bioreactors. Furthermore, recombinant proteins can be secreted into the culture medium simplifying the purification process. The moss Physcomitrella patens has been widely used for the production of different classes of biopharmaceuticals mostly due to its almost complete genetic characterization which makes it an ideal organism for precise gene targeting via homologous recombination. This type of genetic manipulation allowed, for example, glyco-engineering approaches for the elimination of plant-typical immunogenic residues leading to the production of safe therapeutic proteins. This approach was used for the production of the glycoengineered version of the tumour-targeting lewis Y-specific mAb MB314 which proved to be far more efficient in ADCC than the CHO‐derived antibody (Kircheis et al. 2012). A recent achievement was made by the Greenovation Biopharmaceuticals company that developed a moss culture-based production platform (Bryo-Technology) allowing high-quality proteins to be produced on large-scale under GMP conditions using disposable wave reactors (Reski et al. 2015; Shen et al. 2016).
Microalgae cultures
Plants are not the only photosynthetic organisms that can be exploited for the production of recombinant proteins. Microalgae, for example, are unicellular species with a size range of a few micrometers (µm) to 100 µm that can be used as biofactories of different molecules (Potvin and Zhang 2010). The major advantage of algae is their high growth rate and rapid accumulation of biomass, ease of cultivation in contained bioreactors and their ability to carry out post-translational modifications of proteins such as glycosylation. The major drawback is that the yields of pharmaceutical proteins obtained with this system are still very low compared to transient expression systems using whole plants and glycoengineering attempts to produce proteins with human-like glycosylation profiles have not been successful so far (Mathieu-Rivet et al. 2014; Vanier et al. 2017). Nevertheless, there are many examples in the scientific literature of the use of several species of microalgae (Chlamydomonas reinhardtii, Phaeodactylum tricornutum, Dunaliella salina and Schizochytrium sp) for the expression of different classes of pharmaceutical proteins such as vaccines, enzymes and antibodies (Doron et al. 2016). Chlamydomonas reinhardtii is certainly the most widely used green microalgae for recombinant protein production due to its low manufacturing costs and the ease in scaling up the production process. Furthermore, the genome of this species has been sequenced and different genetic tools are available for its transformation facilitating recombinant protein production (Shamriz and Ofoghi 2016). The first example of the expression of a recombinant antibody molecule in microalgae was obtained in the chloroplast of C. reinhardtii in which a functional single-chain antibody (HSV8-lsc) directed against glycoprotein D of the herpes simplex virus accumulated in a functional form (Mayfield et al. 2003). Recently, the diatom P. tricornutum was engineered to express a full mAb directed against the Hepatitis B virus surface antigen (HBsAg), either secreted or retained in the endoplasmic reticulum (Hempel and Maier 2012; Vanier et al. 2018). These examples show the potential of microalgae to produce fully assembled and functional antibodies, although the production yields are still far from being competitive with other plant systems.
Conclusions and future scenarios
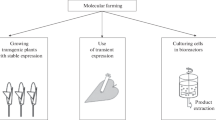
In this review, we highlighted the extremely diversified scenarios of biopharmaceuticals plant-based production platforms (Fig. 2). The different manufacturing strategies proved their advantages in the development of new drugs some of which were recently approved for human therapy. These success stories rely on peculiar features of plant-based platforms that in specific cases can outcompete with traditional animal-based systems. An example is the production of the seasonal influenza vaccine for which manufacturing promptness is an essential prerequisite to obtain an efficacious drug against the most diffuse viral isolates. For this reason plant transient expression systems based on agroinfiltration technology, thanks to their production flexibility and rapidity, proved to be particularly suitable for the preparation of such vaccines. Another example is the production of the ZMapp antibody cocktail against Ebola. In this case, only a very flexible manufacturing system could have allowed the simultaneous production of three different viral neutralizing antibodies to be used in passive immunotherapy. Probably, the most unique feature of plant-based systems is the possibility of producing ‘glycobetter’ proteins. The commercial success of the glucorebrosidase enzyme (ELELYSO™) to treat Gaucher’s disease relies mainly on its high mannose glycosylation profile that gives superior quality compared to the equivalent animal cell-derived drug and could only be obtained by using plant cells. Also in the case of immunotherapeutic antibodies, we have shown that the use of glyco-engineered plants allowed the production of tailor-made mAbs with enhanced biological activity compared to their mammalian derived counterparts. Finally, future scenarios envisage a dramatic escalation of the demand of certain biopharmaceuticals such as mAbs to treat common diseases (Alzheimer’s disease, HIV, rheumatoid arthritis etc.) which will require production on a very large scale (hundreds of tons per year). If this would be the case, production using transgenic plants that can be easily and cost-effectively up-scaled by just increasing the cultivated area could be an ideal alternative to animal-based systems.
References
Boothe J, Nykiforuk C, Shen Y, Zaplachinski S, Szarka S, Kuhlman P, Murray E, Morck D, Moloney MM (2010) Seed-based expression systems for plant molecular farming. Plant Biotechnol J 8:588–606
Bosch D, Castilho A, Loos A, Schots A, Steinkellner H (2013) N-glycosylation of plant-produced recombinant proteins. Curr Pharm Des 19:5503–5512
Buyel JF, Twyman RM, Fischer R (2017) Very-large-scale production of antibodies in plants: the biologization of manufacturing. Biotechnol Adv 35:458–465
Chen Q, Lai H (2015) Gene delivery into plant cells for recombinant protein production. Biomed Res Int 2015:932161
Chen Q, Lai H, Hurtado J, Stahnke J, Leuzinger K, Dent M (2013) Agroinfiltration as an effective and scalable strategy of gene delivery for production of pharmaceutical proteins. Adv Tech Biol Med 1:103
Circelli P, Donini M, Villani ME, Benvenuto E, Marusic C (2010) Efficient Agrobacterium-based transient expression system for the production of biopharmaceuticals in plants. Bioeng Bugs 1:221–224
Cox KM, Sterling JD, Regan JT, Gasdaska JR et al (2006) Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat Biotechnol 24:1591–1597
De Muynck B, Navarre C, Boutry M (2010) Production of antibodies in plants: status after twenty years. Plant Biotechnol J 8:529–563
Decker EL, Parsons J, Reski R (2014) Glyco-engineering for biopharmaceutical production in moss bioreactors. Front Plant Sci 5:346
Doron L, Segal N, Shapira M (2016) Transgene expression in microalgae-from tools to applications. Front Plant Sci 7:505
Drake PMW, Szeto TH, Paul MJ, Teh AY-H, Ma JK-C (2017) Recombinant biologic products versus nutraceuticals from plants - a regulatory choice? Br J Clin Pharmacol 83:82–87
Ecker DM, Jones SD, Levine HL (2015) The therapeutic monoclonal antibody market. mAbs 7:9–14
Fischer R, Vasilev N, Twyman RM, Schillberg S (2015) High-value products from plants: the challenges of process optimization. Curr Opin Biotechnol 32:156–162
Gavilondo J V, Larrick JW (2000) Antibody engineering at the millennium. BioTechniques 29: 128–32, 134–6, 138 passim
Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y (2006) Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA 103:14701–14706
Gleba Y, Klimyuk V, Marillonnet S (2007) Viral vectors for the expression of proteins in plants. Curr Opin Biotechnol 18:134–141
Häkkinen ST, Raven N, Henquet M, Laukkanen M-L et al (2014) Molecular farming in tobacco hairy roots by triggering the secretion of a pharmaceutical antibody. Biotechnol Bioeng 111:336–346
Hanania U, Ariel T, Tekoah Y, Fux L et al (2017) Establishment of a tobacco BY2 cell line devoid of plant-specific xylose and fucose as a platform for the production of biotherapeutic proteins. Plant Biotechnol J 15:1120–1129
Hempel F, Maier UG (2012) An engineered diatom acting like a plasma cell secreting human IgG antibodies with high efficiency. Microb Cell Factories 11:126
Hendin HE, Pillet S, Lara AN, Wu C-Y, Charland N, Landry N, Ward BJ (2017) Plant-made virus-like particle vaccines bearing the hemagglutinin of either seasonal (H1) or avian (H5) influenza have distinct patterns of interaction with human immune cells in vitro. Vaccine 35:2592–2599
Hiatt A, Cafferkey R, Bowdish K (1989) Production of antibodies in transgenic plants. Nature 342:76–78
Holtz BR, Berquist BR, Bennett LD, Kommineni VJM, Munigunti RK, White EL, Wilkerson DC, Wong K-YI, Ly LH, Marcel S (2015) Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol J 13:1180–1190
Jansing J, Sack M, Augustine SM, Fischer R, Bortesi L (2018) CRISPR/Cas9-mediated knockout of six glycosyltransferase genes in Nicotiana benthamiana for the production of recombinant proteins lacking β-1,2-xylose and core α-1,3-fucose. Plant Biotechnol J. https://doi.org/10.1111/pbi.12981
Jin C, Altmann F, Strasser R, Mach L, Schähs M, Kunert R, Rademacher T, Glössl J, Steinkellner H (2008) A plant-derived human monoclonal antibody induces an anti-carbohydrate immune response in rabbits. Glycobiology 18:235–241
Kelley B (2009) Industrialization of mAb production technology: the bioprocessing industry at a crossroads. mAbs 1:443–452
Kircheis R, Halanek N, Koller I, Jost W, Schuster M, Gorr G, Hajszan K, Nechansky A (2012) Correlation of ADCC activity with cytokine release induced by the stably expressed, glyco-engineered humanized Lewis Y-specific monoclonal antibody MB314. mAbs 4:532–541
Kizhner T, Azulay Y, Hainrichson M, Tekoah Y, Arvatz G, Shulman A, Ruderfer I, Aviezer D, Shaaltiel Y (2015) Characterization of a chemically modified plant cell culture expressed human α-Galactosidase-A enzyme for treatment of Fabry disease. Mol Genet Metabol 114:259–267
Klimyuk V, Pogue G, Herz S, Butler J, Haydon H (2014) Production of recombinant antigens and antibodies in nicotiana benthamiana using ‘magnifection’ technology: GMP-compliant facilities for small- and large-scale manufacturing. Curr Top Microbiol Immunol 375:127–154
Komarova TV, Baschieri S, Donini M, Marusic C, Benvenuto E, Dorokhov YL (2010) Transient expression systems for plant-derived biopharmaceuticals. Expert Rev Vaccines 9:859–876
Kopertekh L, Schiemann J (2017) Transient production of recombinant pharmaceutical proteins in plants: evolution and perspectives. Curr Med Chem. https://doi.org/10.2174/0929867324666170718114724
Lico C, Chen Q, Santi L (2008) Viral vectors for production of recombinant proteins in plants. J Cell Physiol 216:366–377
Lim JAC, Patkar A, McDonagh G, Sinclair A, Lucy P (2010) Modeling bioprocess cost: process economic benefits of expression technology based on Pseudomonas fluorescens. BioProcess Int 8:62–70
Lomonossoff GP, D’Aoust M-A (2016) Plant-produced biopharmaceuticals: a case of technical developments driving clinical deployment. Science (New York, N.Y.) 353:1237–1240
Lonoce C, Salem R, Marusic C, Jutras PV, Scaloni A, Salzano AM, Lucretti S, Steinkellner H, Benvenuto E, Donini M (2016) Production of a tumour-targeting antibody with a human-compatible glycosylation profile in N. benthamiana hairy root cultures. Biotechnol J 11:1209–1220
Lonoce C, Marusic C, Morrocchi E, Salzano AM, Scaloni A, Novelli F, Pioli C, Feeney M, Frigerio L, Donini M (2018) Enhancing the secretion of a glyco-engineered anti-CD20 scFv-Fc antibody in hairy root cultures. Biotechnol J. https://doi.org/10.1002/biot.201800081
Ma JK, Hiatt A, Hein M, Vine ND, Wang F, Stabila P, van Dolleweerd C, Mostov K, Lehner T (1995) Generation and assembly of secretory antibodies in plants. Science (New York, N.Y.) 268:716–719
Ma JK-C, Drossard J, Lewis D, Altmann F et al (2015) Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol J 13:1106–1120
Magy B, Tollet J, Laterre R, Boutry M, Navarre C (2014) Accumulation of secreted antibodies in plant cell cultures varies according to the isotype, host species and culture conditions. Plant Biotechnol J 12:457–467
Marusic C, Pioli C, Stelter S, Novelli F, Lonoce C, Morrocchi E, Benvenuto E, Salzano AM, Scaloni A, Donini M (2018) N-glycan engineering of a plant-produced anti-CD20-hIL-2 immunocytokine significantly enhances its effector functions. Biotechnol Bioeng 115:565–576
Mathieu-Rivet E, Kiefer-Meyer M-C, Vanier G, Ovide C, Burel C, Lerouge P, Bardor M (2014) Protein N-glycosylation in eukaryotic microalgae and its impact on the production of nuclear expressed biopharmaceuticals. Front Plant Sci 5:359
Mayfield SP, Franklin SE, Lerner RA (2003) Expression and assembly of a fully active antibody in algae. Proc Natl Acad Sci USA 100:438–442
Mehrotra S, Srivastava V, Rahman LU, Kukreja AK (2015) Hairy root biotechnology—indicative timeline to understand missing links and future outlook. Protoplasma 252:1189–1201
Mercx S, Smargiasso N, Chaumont F, De Pauw E, Boutry M, Navarre C (2017) Inactivation of the β(1,2)-xylosyltransferase and the α(1,3)-fucosyltransferase genes in Nicotiana tabacum BY-2 Cells by a Multiplex CRISPR/cas9 strategy results in glycoproteins without plant-specific glycans. Front Plant Sci 8:403
Montero-Morales L, Steinkellner H (2018) Advanced plant-based glycan engineering. Front Bioeng Biotechnol 6:81
Mor TS (2015) Molecular pharming’s foot in the FDA’s door: protalix’s trailblazing story. Biotechnol Lett 37:2147–2150
Navarre C, Smargiasso N, Duvivier L, Nader J, Far J, De Pauw E, Boutry M (2017) N-Glycosylation of an IgG antibody secreted by Nicotiana tabacum BY-2 cells can be modulated through co-expression of human β-1,4-galactosyltransferase. Transgenic Res 26:375–384
Pillet S, Aubin É, Trépanier S, Bussière D, Dargis M, Poulin J-F, Yassine-Diab B, Ward BJ, Landry N (2016) A plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clin Immunol (Orlando, Fla.) 168:72–87
Potvin G, Zhang Z (2010) Strategies for high-level recombinant protein expression in transgenic microalgae: a review. Biotechnol Adv 28:910–918
PREVAIL II Writing Group, Multi-National PREVAIL II Study Team, Davey RT, Dodd L, Proschan MA, Neaton J et al (2016) A randomized, controlled trial of ZMapp for ebola virus infection. NE ngl J Med 375:1448–1456
Qiu X, Wong G, Audet J, Bello A et al (2014) Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514:47–53
Rademacher T (2013) Method for the generation and cultivation of a plant cell pack. Patent WO2013/113504
Rademacher T, Sack M, Arcalis E, Stadlmann J et al (2008) Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV-1 and contains predominantly single-GlcNAc N-glycans. Plant Biotechnol J 6:189–201
Raven N, Rasche S, Kuehn C, Anderlei T et al (2015) Scaled-up manufacturing of recombinant antibodies produced by plant cells in a 200-L orbitally-shaken disposable bioreactor. Biotechnol Bioeng 112:308–321
Reski R, Parsons J, Decker EL (2015) Moss-made pharmaceuticals: from bench to bedside. Plant Biotechnol J 13:1191–1198
Sainsbury F, Sack M, Stadlmann J, Quendler H, Fischer R, Lomonossoff GP (2010) Rapid transient production in plants by replicating and non-replicating vectors yields high quality functional anti-HIV antibody. PLoS ONE 5:e13976
Santos RB, Abranches R, Fischer R, Sack M, Holland T (2016) Putting the spotlight back on plant suspension cultures. Front Plant Sci 7:297
Saxena P, Thuenemann EC, Sainsbury F, Lomonossoff GP (2016) Virus-derived vectors for the expression of multiple proteins in plants. Methods Mol Biol (Clifton, N.J.) 1385:39–54
Schillberg S, Raven N, Fischer R, Twyman RM, Schiermeyer A (2013) Molecular farming of pharmaceutical proteins using plant suspension cell and tissue cultures. Curr Pharm Des 19:5531–5542
Shamriz S, Ofoghi H (2016) Outlook in the application of Chlamydomonas reinhardtii chloroplast as a platform for recombinant protein production. Biotechnol Genet Eng Rev 32:92–106
Shen J-S, Busch A, Day TS, Meng X-L et al (2016) Mannose receptor-mediated delivery of moss-made α-galactosidase A efficiently corrects enzyme deficiency in Fabry mice. J Inherit Metabol Dis 39:293–303
Sheshukova EV, Komarova TV, Dorokhov YL (2016) Plant factories for the production of monoclonal antibodies. Biochemistry 81:1118–1135
Shukla AA, Wolfe LS, Mostafa SS, Norman C (2017) Evolving trends in mAb production processes. Bioeng Trans Med 2:58–69
Sijmons PC, Dekker BM, Schrammeijer B, Verwoerd TC, van den Elzen PJ, Hoekema A (1990) Production of correctly processed human serum albumin in transgenic plants. Bio/technology (Nature Publishing Company) 8:217–221
Steinkellner H, Castilho A (2015) N-Glyco-engineering in plants: update on strategies and major achievements. Methods Mol Biol (Clifton, N.J.) 1321:195–212
Strasser R, Stadlmann J, Schähs M, Stiegler G, Quendler H, Mach L, Glössl J, Weterings K, Pabst M, Steinkellner H (2008) Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J 6:392–402
Tekoah Y, Shulman A, Kizhner T, Ruderfer I et al (2015) Large-scale production of pharmaceutical proteins in plant cell culture-the Protalix experience. Plant Biotechnol J 13:1199–1208
Tusé D, Ku N, Bendandi M, Becerra C et al (2015) Clinical safety and immunogenicity of tumor-targeted, plant-made Id-KLH conjugate vaccines for follicular lymphoma. Biomed Res Int 2015:648143
Vanier G, Lucas P-L, Loutelier-Bourhis C, Vanier J, Plasson C, Walet-Balieu M-L et al (2017) Heterologous expression of the N-acetylglucosaminyltransferase I dictates a reinvestigation of the N-glycosylation pathway in Chlamydomonas reinhardtii. Sci Rep 7:10156
Vanier G, Stelter S, Vanier J, Hempel F, Maier UG, Lerouge P, Ma J, Bardor M (2018) Alga-made anti-Hepatitis B antibody binds to human Fcγ receptors. Biotechnol J 13:e1700496
Vasilev N, Grömping U, Lipperts A, Raven N, Fischer R, Schillberg S (2013) Optimization of BY-2 cell suspension culture medium for the production of a human antibody using a combination of fractional factorial designs and the response surface method. Plant Biotechnol J 11:867–874
Weintraub JA, Hilton JF, White JM, Hoover CI, Wycoff KL, Yu L, Larrick JW, Featherstone JDB (2005) Clinical trial of a plant-derived antibody on recolonization of mutans streptococci. Caries Res 39:241–250
White FF, Taylor BH, Huffman GA, Gordon MP, Nester EW (1985) Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J Bacteriol 164:33–44
Wongsamuth R, Doran PM (1997) Production of monoclonal antibodies by tobacco hairy roots. Biotechnol Bioeng 54:401–415
Xu J, Zhang N (2014) On the way to commercializing plant cell culture platform for biopharmaceuticals: present status and prospect. Pharm Bioprocess 2:499–518
Xu J, Dolan MC, Medrano G, Cramer CL, Weathers PJ (2012) Green factory: plants as bioproduction platforms for recombinant proteins. Biotechnol Adv 30:1171–1184
Yao J, Weng Y, Dickey A, Wang KY (2015) Plants as Factories for human pharmaceuticals: applications and challenges. Int J Mol Sci 16:28549–28565
Yusibov V, Kushnir N, Streatfield SJ (2016) Antibody production in plants and green algae. Annu Rev Plant Biol 67:669–701
Zischewski J, Sack M, Fischer R (2016) Overcoming low yields of plant-made antibodies by a protein engineering approach. Biotechnol J 11:107–116
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Donini, M., Marusic, C. Current state-of-the-art in plant-based antibody production systems. Biotechnol Lett 41, 335–346 (2019). https://doi.org/10.1007/s10529-019-02651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02651-z