Abstract
Cancer cell lines of human tissue origin have been extensively used to investigate antiproliferative activity and toxicity of herbal extracts, isolated compounds, and anticancer drugs. These cell lines are genetically and/or epigenetically well characterized to determine the altered expression of proteins within given cellular pathways and critical genes in cancer. Human derived hepatoma (HepG2) cell line has been extensively exploited to examine cytoprotective, antioxidative, hepatoprotective, anti-hepatoma, hypocholesterolemic, anti-steatosis, bioenergetic homeostatic and anti-insulin resistant properties. Moreover, mechanism of action of various botanicals and bioactive constituents has been reported using these cells. HepG2 cells have significant differences as compared to primary hepatocytes with respect to expression of cytochrome P450 enzymes and xenobiotic receptors in conventional in vitro culture conditions. Therefore, strategies have been employed to overcome limitations of two dimensional (2D) in vitro HepG2 cell culture in order to recognize functional biomarkers more accurately and to boost its predictive value in clinical research. In consequence, three dimensional (3D) human hepatoma cell culture models are being developed as a resource to achieve these goals of simulating the in vivo tumor microenvironment. It is assumed that bioengineered 3D hepatoma cell culture models can provide significant assistance in scrutinizing the molecular response of herbal natural products to recognize novel prognostic targets and crucial biomarkers in treatment strategies for cancer patients in near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Human cancer cell lines are a coherent tool to explore mechanisms associated with proliferation, deregulation, apoptosis, somatic alterations, cancer therapeutics, and pharmacogenomics. These cell lines are characterized by a broad range of mutations in cancers of different tissue origin, exhibit interrelated chromosomal losses/gains with exclusive mRNA expressions. This, in turn, allows effective extrapolation of discovered results to accurately hypothesize clinical response (Ferreira et al. 2013; Niu and Wang 2015). Cancer cell lines are generally materialized as in vitro experimental model for cancer research since these cells grow as monolayer homogeneous cell population. Other approaches such as the use of primary tumors and tumors derived from the xenografts represent mixed cell types and warrant certain manipulations to investigate DNA methylation and transcriptional profiling. Furthermore, in vivo drug testing is practically difficult and necessitates ethical clearance to execute in animals (Ferreira et al. 2013). Thus, two dimensional (2D) cell cultures are preferred in vitro models to uncover various biomolecular mechanisms that underlie cancer cells in vivo.
In 2D culture process, cell lines are usually generated through tumor tissue or by genetic transformation of primary human cells (e.g., liver) via modulating agents such as SV40, considering their proliferative potential and static metabolism. Generally, these cells are seeded on collagen treated culture plates to form confluent monolayers for development of a physiologically and pathologically specific 2D model. 2D model does not only consider cell line as selection criteria, but other elements such as, culture platform (glass or polystyrene) for mechanical assistance, growth factors requirement, utilization of essential nutrients in media, and other biophysical and/or biochemical cues also play an important role in generating a reliable disease model (Duval et al. 2017).
Traditionally 2D human hepatocarcinoma HepG2 cell cultures have been used as in vitro liver model for toxicity studies. These hepatoma epithelial cells were derived from a 15 year old Caucasian American male with well differentiated hepatocellular carcinoma (HCC) with modal chromosome number 55 and cultured in Dulbecco’s modified Eagle’s medium (ATCC 2017). Genetic profile of these cells along with activation of Wnt signaling and deregulation of cell survival pathways resemble embryonal and fetal hepatoblastoma. This cell line is a well recognized and established gold standard to probe studies on drug metabolism, cytotoxicity, genotoxicity, hepatotoxicity and tool for chemical risk assessment in vitro. HepG2 cells are comparable to human hepatocytes for evaluation of CYP1A2, CYP2B6, CYP3A4 induction at mRNA and protein levels credited to their phenotypic stability, high differentiation and immortality advocating their use as a cell based model in drug discovery.
Despite the fact that conventional 2D HepG2 cells have provided a wealth of information, it has been shown in recent reports that such conventional 2D planar HepG2 cell cultures represent disparity to primary hepatocytes in certain structural and functional characteristics. Therefore, three dimensional (3D) human hepatoma cell models are being developed as a resource to overcome functional aberrations associated with the 2D culturing method and to achieve more accuracy in simulating the in vivo tumor microenvironment. In this regard, bioengineered 3D hepatic models provide more realistic microenvironment (biomolecular, biochemical and biomechanical conditions) and maintain the fluidity of model. Overall, 3D platform optimizes predictive value, metabolic stability and reliability to scrutinize molecular response of herbals in drug testing and is clearly expected to be a sought after useful reference for research in hepatoma modeling.
Identification of botanicals employing two dimensional (2D) hepatoma cell cultures
HepG2 mediated bioactivities of herbal plants and their active principles are represented in Table 1. For instance, a cumulative effect of decoction of Nigella sativa seeds, Hemidesmus indicus root, and Smilax glabra rhizome holds traditional repute as a curative measure for cancer and other diseases. These three herbal species account for their cytotoxic action against HepG2 cells via silencing incorporation of [14C]-leucine into protein and [3H]-thymidine into DNA of cells eventually leading to the decreased synthesis of DNA and proteins. This in turn induces cell necrosis and/or apoptosis of hepatic cancer cells. Further studies corroborate anti-hepatocarcinogenic effect of herbal formulation by inducing cell apoptosis. This is characterized by induction of DNA fragmentation, nuclear condensation, membrane blebbing, and formation of apoptotic bodies escorting enhancement in p53, p21, caspase-3, caspase-9, expression of Bcl-2-associated X protein (Bax) and suppression of Bcl-2 (Samarakoon et al. 2012). Huanglian Jiedu formulation has been valued clinically for treatment against HCC. It comprises berberine, geniposide, palmatine, and baicalin as bioactive constituents. This herbal formulation represses nascent protein synthesis in HCC cells by inactivation of eukaryotic elongation factor 2 (eEF2) via activating phosphorylation of elongation factor 2 (EF2) kinase (Wang et al. 2015). The study advocates suppression of proliferation, angiogenesis, and invasion of HCC cells without deregulation of initiation factors in translation. Furthermore, an amalgamation of Curcuma longa rhizome, Piper nigrum fruit, and Allium sativum bulb synchronizes bioenergetic mechanism in HepG2 cells. This synergism of three spices relapse activity of lactate dehydrogenase, elevates pyruvate dehydrogenase level, regulates the expression of p53, MYC mRNAs, HIF-1α, VEGF and thus, revert cancer cells to normal via modification of lactic acid fermentation to oxidative phosphorylation (Ramachandran and Menon 2016). A Japanese–Chinese traditional herbal formula, comprising roots of Scutellaria baicalensis, Coptis japonica, Gardenia jasminoides, and Phellodendron amurense, reveals the deterrent activity of acyl-coenzyme A: cholesterol acyltransferase expression in HepG2 cells emphasizing prominence of formulation in inhibiting lipid biosynthesis and maintenance of cholesterol homeostasis (Yotsumoto et al. 1997). Qushi Huayu decoction hampers triglyceride content, restrains P-IκB and TNF-α expression, thereby slumping secretion of TNF-α and suggests the curative action of this herbal mixture on hepatic steatosis. HPLC analysis of the herbal combination elucidated the presence of chlorogenic acid, resveratrol, jasminoidin and polygonin which might be accountable for its preventive efficacy against hepatic lipotoxicity (Feng et al. 2013). Dai-saiko-to decoction, a combination of seven herbals, reduces the level of VLDL and triglyceride synthesis in plasma and hence exerts hypocholesterolemic potential. Addition of another herbal, i.e., Citrus aurantium to this decoction regulates the level of plasma lipids due to rise in the expression level of LDL receptor and SREBP-2 (Iizuka et al. 2013). A Chinese herbal decoction, Wu-Zi-Yan-Zong-Wan, renders cytoprotection against oxidative injury exaggerated by ethanol in cytochrome P450 2E1 cDNA-transfected HepG2 (E47) cells. This five component decocted extract unveils presence of marker compounds such as betaine, quercetin, schisandrin, hyperin, schisandrin B and deoxyschisandrin as active ingredients. This herbal formula turns down lactate dehydrogenase leakage, reactive oxygen species production, lipid peroxidation and raises content of reduced glutathione, membrane potential, precludes DNA ladder formation and hence affords protection in opposition to oxidative damage (Chen et al. 2010). Ethyl acetate extract of an herbal formula known as Jiedu Xiaozheng Yin decoction has been reported to arrest the growth of HepG2 cells at G0/G1 phase via increasing cyclin D and E expression (Cao et al. 2013). These various combinatory decoction studies indicate that HepG2 cells can be effectively employed to determine their antiproliferative activity by inducing apoptosis and suppressing survival genes such as Bcl-2.
Experiments conducted by Bhatia et al. (2015) reflects pronounced efficiency of aqueous extract of Origanum dayi and Ochradenus baccatus plants in promoting apoptosis via intrinsic mitochondrial pathway by regulating mRNA expression of P450 enzymes, caspases (3, 7 and 9), Bax, Bcl-2-associated death promoter (Bad) and poly(adenosine diphosphate-ribose) polymerase (PARP). Terminalia arjuna aqueous extract impedes alterations in redox status incited by tertiary butyl hydroperoxide in cultured hepatoma cells via upregulating level of antioxidant enzymes by its bioactive components (Shivananjappa et al. 2013). In a similar trend, aqueous extract of Barringtonia racemosa (leaf and stem) and ethyl acetate extract of Lavandula coronopifolia (aerial parts) sustains cellular homeostasis and grants protection against toxic response initiated by reactive damaging entities (Kong et al. 2016; Farshori et al. 2015). Available literature thus reflects the imperative utility of HepG2 cells to be employed as oxidative stress model as these cells preserve endogenous functions and expression of diverse antioxidative enzymes. HepG2 cells provide an excellent tool for appraising oxidative damages incited by various toxins and to discover vital mechanisms associated with protection presented by numerous botanicals against oxidative insults. Herbal extracts have also been estimated for their anti-insulin resistant action using dexamethasone incited insulin resistant HepG2 cells as an evaluation model. Plants enhance extracellular glucose consumption and reduce intracellular glycogen content underscoring their potential to amplify insulin sensitivity (Ma et al. 2014). These results suggest that HepG2 in 2D conventional culturing conditions can be successfully employed to determine hepatoprotective, antioxidant enzymatic gene expression and redox alterations against oxidative stress. These cells can also be employed for insulin regulation in energy metabolism studies.
There are numerous studies where purified known isolated compounds can also be evaluated in this culture model. For example, pre-exposure of HepG2 cells with 3″-O-methylcrenatoside, leucoseceptoside A, crenatoside, and martynoside isolated from Incarvillea compacta roots fortifies antioxidative enzymatic machinery and downregulates transactivation of NF-κB in luciferase reporter gene assay. Thus these phenylethanoid glycosides operate as hepatoprotectants on carbon tetrachloride aggravated damage in HepG2 cells (Shen et al. 2015). Gardenoside, an extraction product of G. jasminoides, intervenes with hepatic lipotoxicity induced in HepG2 cells by free fatty acid mixture (oleic and palmitic acid mixture, 2:1). This compound effectively attenuates secretion of inflammatory cytokines and averts translocation of NF-κB into nucleus preventing its activation and renders protection against cellular steatosis (Liang et al. 2015). Quercetin also modulates alterations in expression of antioxidant biomarkers in response to oxidative damage aggravated in human hepatoma cells by tert-butyl hydroperoxide (Alia et al. 2006). Fatsioside A (baccharane-type triterpenoid glycoside) extracted from Fatsia japonica fruit activates AMPK signaling, increases acetyl-CoA carboxylase expression and promotes hepatoma cell death (Zheng et al. 2015). A steroidal saponin, known as polyphyllin I extorted from the rhizome of Paris polyphyllin exhibits repression ability against constitutive and cisplatin provoked phosphorylation of p65 protein and c-Myc, Bcl-2 and VEGF expression level (Han et al. 2015). These results indicate dual nature of HepG2 cells, facilitating studies on hepatoprotective action by regulation of NF-κB and anti-hepatoma activities via modulation of VEGF and Bcl-2 expression. Overall, HepG2 mediated cumulative biomodulatory actions of herbal plants and their active principles have been represented in Fig. 1. The figure clearly illustrates the range of 2D HepG2 culturing techniques for evaluation of hepatoprotective and anti-hepatoma studies. 2D model can also be employed for evaluation of cholesterol homeostasis and insulin regulation.
Programming of 3D hepatoma cell culture
Foregoing studies on medicinal plants advocate 2D monolayered hepatoma cells as in vitro reference model to determine their initial activities. However, some studies propose that functions and expression of certain genes in HepG2 cells do not resemble primary hepatocytes as former represents feeble expression of cytochrome P450 enzymes and xenobiotic receptors in conventional 2D culturing method (Ramaiahgari et al. 2014). There is rapid dedifferentiation of hepatocytes with degradation in phenotypic characteristics, collapse of biological functionalities such as loss of cell–matrix and cell–cell interactions, poor secretion of plasma proteins and alterations in cell signaling pathways. Thus, HepG2 cells cultured on rigid plastic surfaces have metabolic incompetence to envisage drug-induced hepatic injury.
Keeping in view, problems associated with the 2D cellular model, 3D in vitro culture approaches to cultivate HepG2 cells have been developed. In 3D modeling, cells and their extracellular matrix are considered as functional units to improvise drug development. An extensive source of cells for 3D modeling including stem cells, cell lines, allogenic cells, autologous cells, xenogenic cells, animal derived primary cells, and their genetically modified variants have been employed to generate 3D models. The basic procedure engrosses generation of aggregates or spheroids using scaffolds/matrices, which may be biological or synthetic in nature. Cells are plated on an acellular matrix or dispersed within the matrix and then solidified or polymerized. Other scaffold-free system entails forced floating, hanging or agitation-based methods in which 3D spheroids are grown in suspensions (Edmondson et al. 2014). In addition, these static methods are being integrated with bioreactors to regulate environmental circumstances obligatory for cell cultivation.
In 3D culture study, HepG2 spheroids generated on extracellular matrix loaded hydrogels have been proposed as a suitable model in toxicity and safety evaluation studies. These hepatic spheroids mimic tissues of actual liver structurally and demonstrate elevation in metabolic expression of key regulators viz., phase I enzymes (CYP1A2, CYP3A4, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP4F3), phase II enzymes (UGT1A1, UGT1A3, UGT1A6, UGT2B4, SULT2A1), phase III drug transporters (OAT7, OAT2) and nuclear receptors (CAR, AhR, PXR) involved in drug metabolism for longer duration (Ramaiahgari et al. 2014). However, several small spheroids readily unite to form spheroids of bigger diameter with cellular necrosis in the core due to lack of oxygen and nutrient insufficiency. Therefore, HepG2 cells have been cultivated in micro space culture plates as this methodology is effective in maintaining the diameter of spheroids below the level of survival threshold (Nakamura et al. 2011). These results suggest that 3D HepG2 spheroidal culture can be successfully utilized to determine effects of experimental extract or chemicals on the metabolic profile of hepatoma cells. Further, nano-cultured cells represent even high susceptibility to CYP2E1 expression and this model surrogate mechanistic characteristics, i.e., decline in mitochondrial membrane potential, glutathione content and phosphorylation of JNK associated with paracetamol provoked hepatic damage (Aritomi et al. 2014). In a 2D–3D hybrid system, encapsulation of HepG2 cells in alginate hydrogel propped up by poly-carbonate platform and co-cultured with MCF-7 cells respond with raised sensitivity to acetaminophen, rifampin, diclofenac, and quinidine (Lan and Starly 2011). 3D multicellular heterospheroids prepared in collagen hydrogel culture also demonstrate resistance to doxorubicin over monolayered and homospheroid cells. This might be due to the creation of oxygen gradient in the spheroidal core that replicates hypoxic environment observed within solid tumors and modulates cells to defy diffusion of drugs and thus confirm better viability (Yip and Cho 2013). 3D cell models incorporate varied configuration systems to overcome shortcomings in 2D monolayered cultures. Moreover, these systems require microfabricated 3D scaffolds with complex architecture to replicate in vivo like microenvironment to withstand requisite distribution and circulation of oxygen and other molecules (Derda et al. 2009). Application of scaffolds (natural or synthetic polymers or their admixture) that impersonate extra-cellular matrices in permeability, porosity and mechanical consistency illustrate better biochemical and biophysical interventions for expression of biomarkers (Ravi et al. 2015). HepG2 cells developed via 3D porous polystyrene substrate display lower predisposition towards cell death at higher concentrations of methotrexate (hepatotoxin), increase the formation of bile canaliculi and regulate structural integrity as compared to 2D configuration (Bokhari et al. 2007). However, 3D microfabrication involves a time intense advanced engineering specialization and high cost instruments for accurate outcomes. An affordable approach of stacking hydrogel impregnated chromatography papers for microfabrication of 3D cell cultures can be useful substitute method (Derda et al. 2009). In this method, cells impregnated papers can be stacked on cellulose acetate or poly(dimethylsiloxane) (PDMS) films with altering permeability for oxygen and water. Stacked and destacked layers of impregnated chromatography papers regulate oxygen and nutrient gradient thus, enabling determination of viable cells distribution in three dimension facilitating analysis of molecular and genetic outcomes in better recapitulating in vivo scenario. This modeling technique might be a reasonable approach for exploration of prime areas in tissue engineering and biotechnology.
Recent considerations
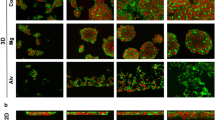
An innovative coordination of microphysiological liver system encompassing four categories of human hepatic cells structured into a 3D microfluidic sinusoidal complex to detect mechanism of drug toxicity, based on fluorescence biosensors, has been devised recently (Vernetti et al. 2017). Fabrication of hepatoma spheroidal culture in a microfluidic environment using PDMS decreases the resistance of 5-fluorouracil with an increase in spheroidal size due to the release of death signals such as TNF-α by innermost necrotic cells (Zuchowska et al. 2017). Such cell-on-a chip micro device can be successfully employed to evaluate the anticancer proficiency of new drugs. Another liver-on-a-chip based model of HepG2/C3A spheroids entrapped in methacrylate gelatin hydrogel results in increased expression of ceruloplasmin, transferrin, MRP2, cytokeratin 18, ZO-1 and displays toxic response to acetaminophen with similar in vivo scenario (Bhise et al. 2016). HepG2 cells have been soaked in alginate to fabricate 3D bioprinted mCherry-HepG2 hydrogel structures followed by cross linking with calcium chloride. These newly generated cells hold significant promise in advancing gene expression profiling and put forward alternative innovative options to circumvent surgical complications and donor scarcity (Jeon et al. 2017). Hepatospheroids adhered with hybrid scaffolds are prepared by cross-linking collagen with gum arabic. These non-cellular adherent scaffolds favor cell–cell interaction and formation of spheroids in comparison to cellular matrix substrate. HepG2 cells seeded in collagen–gum arabic hybrid substrates are cultured under microgravity conditions simulated by using rotating bioreactor. Cells cultivated under such conditions promote secretion of urea and albumin. Further, development of biological substitutes would be of high impact for in vitro safety evaluation of drugs and in the engineering of bioartificial hepatic devices (Sarika et al. 2016). HepG2 cylindroids within hollow fibers of polyvinylidene fluoride represent better sensitivity at short exposure duration to lower concentrations of acrylamide and sodium nitrite. The rough inner surface of fibers endorses cells to adhere, propagate and express hepatic genes via micropores that facilitate the exchange of materials between inner hepatic cells and culture media. This study urges high throughput toxicity testing of chemical compounds in food manufacturing and processing industries (Chu et al. 2017). HepG2 cells cultured on a liquid–solid interface (2 days) and afterward on a liquid–gas interface (1 day) in collagen vitrigel membrane chamber comprising of high-density collagen fibrils with continuous oxygenation have been considered as the most suitable model for analyzing absorption, distribution, metabolism, and excretion behavior of chemicals. This model reproduces functional characteristics of hepatocytes along with expression of efflux transporters such as MRP2, MRP3, and MRP4 (Luckert et al. 2017). These results clearly prove that 3D HepG2 model or liver cell on chip model is superior to conventional 2D HepG2 cell culture model to understand the efficacy of drugs on the survival of cancer cells, toxicity, and microenvironment. However, in a recent study, HepG2 cells cultured in 2D monolayers for 21 days asserted similarity to 3D culture system comprising of alvetex scaffolds, matrigel matrix, collagen sandwich in the analysis of biochemical markers, hepato-oriented gene expression profile and cellular morphological features (Zuchowska et al. 2017). The rationale for this pronounced resemblance is that during long cultivation for 21 days, cells revealed primary differentiation due to maturation and secondary differentiation during confluency phase. Thus, protracted culture duration is accountable for better and increased HepG2 metabolic capability regardless of 2D or 3D culture. Overall structural integrity and viability in HepG2 3D cultures increase with better in vivo like expression as shown in Fig. 2, which also illustrates that 3D model articulates better biomolecular expressions when 2D cell culture model is compared to the development of specialized 3D engineering system.
Future prospects
Bioengineered 3D culture methods are under expansion to address the urgent need of predictive in vitro hepatic model in drug discovery and research. Although considerable interest has been focused in this area employing hydrogels, microfluidics and bioreactors, the major bottleneck is a deficiency of standardized approach. 3D models have high flexibility and can overcome shortcomings observed with other platforms. However, there is a dilemma in replicating in vivo physiological tissue conditions due to variance in tissue origin, tissue–tissue intervention, their response to mechanical and physical microenvironment, the spatiotemporal gradient of oxygen, nutrients, metabolites and other signaling molecules (Duval et al. 2017). Hence, more efforts are desired to develop, optimize and validate cultivation conditions to mimic in vivo tissue scenario more precisely with cost effective strategies. One of the possible solutions to current challenges could be the development of a combinatorial model with an integration of biological, topographical and mechanical factors with the low cost set-up. This could be achieved by use of natural biological substrates to construct a versatile model for diverse cell types. Natural biosubstitutes could steer signaling cascades associated with cancer cells and may offer constructive research outcome that may improve strategies for 3D disease modeling design.
Conclusion
Numerous studies on the investigation of herbal bioactivities have been carried out by using HepG2 cell cultures. However, HepG2 cells in 2D culture conditions have been reported to display inadequate expression of different cytochromes, albumin, and α-1 antitrypsin in comparison to primary human hepatic cells. In this context, it is assumed that more studies on biomimetic 3D hepatoma cell cultures can better reflect their expression to reiterate in vivo tumor microenvironment and can be employed to decipher molecular response of herbal isolates to recognize novel prognostic targets for pre-clinical drug discovery studies. Innovative bioengineered 3D hepatoma models hold promise to identify crucial biomarkers in herbal botanicals associated with different diseases to begin tailoring treatment strategies for individual patients.
References
ATCC. https://www.atcc.org/products/all/HB-8065.aspx#characteristics. Accessed 24 Nov 2017
Alia M, Ramos S, Mateos R, Granado-Serrano AB, Bravo L, Goya L (2006) Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol Appl Pharmacol 212:110–118
Aritomi K, Ishitsuka Y, Tomishima Y, Shimizu D, Abe N, Shuto T, Irikura M, Kai H, Irie T (2014) Evaluation of three-dimensional cultured HepG2 cells in a nano culture plate system: an in vitro human model of acetaminophen hepatotoxicity. J Pharmacol Sci 124:218–229
Bhatia D, Mandal A, Nevo E, Bishayee A (2015) Apoptosis-inducing effects of extracts from desert plants in HepG2 human hepatocarcinoma cells. Asian Pac J Trop Biomed 5:87–92
Bhise NS, Manoharan V, Massa S, Tamayol A, Ghaderi M, Miscuglio M, Lang Q, Shrike Zhang Y, Shin SR, Calzone G, Annabi N, Shupe TD, Bishop CE, Atala A, Dokmeci MR, Khademhosseini A (2016) A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 8:014101. https://doi.org/10.1088/1758-5090/8/1/014101
Bokhari M, Carnachan RJ, Cameron NR, Przyborski SA (2007) Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J Anat 211:567–576
Cao Z, Lin W, Huang Z, Chen X, Zhao J, Zheng L, Ye H, Liu Z, Liao L, Du J (2013) Ethyl acetate extraction from a Chinese herbal formula, Jiedu Xiaozheng Yin, inhibits the proliferation of hepatocellular carcinoma cells via induction of G0/G1 phase arrest in vivo and in vitro. Int J Oncol 42:202–210
Chen ML, Ip SP, Tsai SH, Ko KM, Che CT (2010) Biochemical mechanism of Wu-Zi-Yan-Zong-Wan, a traditional Chinese herbal formula, against alcohol-induced oxidative damage in CYP2E1 cDNA-transfected HepG2 (E47) cells. J Ethnopharmacol 128:116–122
Chu Q, Zhao Y, Shi X, Han W, Zhang Y, Zheng X, Zhu J (2017) In vivo-like 3-D model for sodium nitrite- and acrylamide-induced hepatotoxicity tests utilizing HepG2 cells entrapped in micro-hollow fibres. Sci Rep 7:14837. https://doi.org/10.1038/s41598-017-13147-z
Derda R, Laromaine A, Mammoto A, Tang SK, Mammoto T, Ingber DE, Whitesides GM (2009) Paper-supported 3D cell culture for tissue-based bioassays. Proc Natl Acad Sci USA 106:18457–18462
Duval K, Grover H, Han L, Mou Y, Pegoraro AF, Fredberg J, Chen Z (2017) Modeling physiological events in 2D vs. 3D cell culture. Physiology 32:266–277
Edmondson R, Broglie JJ, Adcock AF, Yang L (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12:207–218
Farshori NN, Al-Sheddi ES, Al-Oqail MM, Hassan WH, Al-Khedhairy AA, Musarrat J, Siddiqui MA (2015) Hepatoprotective potential of Lavandula coronopifolia extracts against ethanol induced oxidative stress-mediated cytotoxicity in HepG2 cells. Toxicol Ind Health 31:727–737
Feng Q, Gou XJ, Meng SX, Huang C, Zhang YQ, Tang YJ, Wang WJ, Xu L, Peng JH, Hu YY (2013) Qushi Huayu decoction inhibits hepatic lipid accumulation by activating AMP-activated protein kinase in vivo and in vitro. Evid Based Complement Altern Med 2013:184358. https://doi.org/10.1155/2013/184358
Ferreira D, Adega F, Chaves R (2013) The importance of cancer cell lines as in vitro models in cancer methylome analysis and anticancer drugs testing. In: César LC, Elena AO (eds) Oncogenomics and cancer proteomics—novel approaches in biomarkers discovery and therapeutic targets in cancer. InTech Publishers, Rijeka, pp 139–166
Han W, Hou G, Liu L (2015) Polyphyllin I (PPI) increased the sensitivity of hepatocellular carcinoma HepG2 cells to chemotherapy. Int J Clin Exp Med 8:20664–20669
Iizuka A, Yoshie F, Amagaya S, Yasuda T, Iizuka M, Yamaguchi H, Nagumo S, Kondo K (2013) Effect of Dai-Saiko-To (Da-Chai-Hu-Tang) on LDL-Receptor gene expression in human hepatoma cell line (HepG2). Am J Plant Sci 4:454–459
Jeon H, Kang K, Park SA, Kim WD, Paik SS, Lee SH, Jeong J, Choi D (2017) Generation of multilayered 3D structures of HepG2 cells using a bio-printing technique. Gut Liver 11:121–128
Kong KW, Mat-Junit S, Aminudin N, Hassan FA, Ismail A, Aziz AA (2016) Protective effects of the extracts of Barringtonia racemosa shoots against oxidative damage in HepG2 cells. PeerJ 4:e1628. https://doi.org/10.7717/peerj.1628
Lan SF, Starly B (2011) Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicol Appl Pharmacol 256:62–72
Liang H, Zhang L, Wang H, Tang J, Yang J, Wu C, Chen S (2015) Inhibitory effect of Gardenoside on free fatty acid-induced steatosis in HepG2 hepatocytes. Int J Mol Sci 16:27749–27756
Luckert C, Schulz C, Lehmann N, Thomas M, Hofmann U, Hammad S, Hengstler JG, Braeuning A, Lampen A, Hessel S (2017) Comparative analysis of 3D culture methods on human HepG2 cells. Arch Toxicol 91:393–406
Ma JZ, Yang LX, Shen XL, Qin JH, Deng LL, Ahmed S, Xu HX, Xue DY, Ye JX, Xu G (2014) Effects of traditional Chinese medicinal plants on anti-insulin resistance bioactivity of DXMS-induced insulin resistant HepG2 cells. Nat Prod Bioprospect 4:197–206
Nakamura K, Mizutani R, Sanbe A, Enosawa S, Kasahara M, Nakagawa A, Ejiri Y, Murayama N, Miyamoto Y, Torii T, Kusakawa S, Yamauchi J, Fukuda M, Yamazaki H, Tanoue A (2011) Evaluation of drug toxicity with hepatocytes cultured in a micro-space cell culture system. J Biosci Bioeng 111:78–84
Niu N, Wang L (2015) In vitro human cell line models to predict clinical response to anticancer drugs. Pharmacogenomics 16:273–285
Ramachandran S, Menon DB (2016) Synergistic effect of spices in a decoction regulates the energy metabolism in liver cancer cells. Int J Phytomed 8:29–36
Ramaiahgari SC, Den Braver MW, Herpers B, Terpstra V, Commandeur JN, van de Water B, Price LS (2014) A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch Toxicol 88:1083–1095
Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FD (2015) 3D cell culture systems: advantages and applications. J Cell Physiol 230:16–26
Samarakoon SR, Thabrew I, Galhena PB, Tennekoon KH (2012) Modulation of apoptosis in human hepatocellular carcinoma (HepG2 cells) by a standardized herbal decoction of Nigella sativa seeds, Hemidesmus indicus roots and Smilax glabra rhizomes with anti-hepatocarcinogenic effects. BMC Complement Altern Med 12:25. https://doi.org/10.1186/1472-6882-12-25
Sarika PR, James NR, Anilkumar PR, Raj DK, Kumary TV (2016) Microgravity as a means to incorporate HepG2 aggregates in polysaccharide–protein hybrid scaffold. J Mater Sci Mater Med 27:27. https://doi.org/10.1007/s10856-015-5638-5
Shen T, Li X, Hu W, Zhang L, Xu X, Wu H, Ji L (2015) Hepatoprotective effect of phenylethanoid glycosides from Incarvillea compacta against CCl4-induced cytotoxicity in HepG2 cells. J Korean Soc Appl Biol Chem 58:617–625
Shivananjappa MM, Mhasavade D, Joshi MK (2013) Aqueous extract of Terminalia arjuna attenuates tert-butyl hydroperoxide-induced oxidative stress in HepG2 cell model. Cell Biochem Funct 31:129–135
Vernetti LA, Vogt A, Gough A, Taylor DL (2017) Evolution of experimental models of the liver to predict human drug hepatotoxicity and efficacy. Clin Liver Dis 21:197–214
Wang N, Feng Y, Tana HY, Cheung F, Hong M, Lao L, Nagamatsu T (2015) Inhibition of eukaryotic elongation factor-2 confers to tumor suppression by a herbal formulation Huanglian-Jiedu decoction in human hepatocellular carcinoma. J Ethnopharmacol 164:309–318
Yip D, Cho CH (2013) A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem Biophys Res Commun 433:327–332
Yotsumoto H, Yanagita T, Yamamoto K, Ogawa Y, Cha JY, Mori Y (1997) Inhibitory effects of Oren-Gedoku-to and its components on cholesteryl ester synthesis in cultured human hepatocyte HepG2 cells: evidence from the cultured Hep2G cells and in vitro assay of ACAT. Planta Med 63:141–145
Zheng YS, Zhang JY, Zhang DH (2015) Fatsioside A-induced apoptotic death of HepG2 cells requires activation of AMP-activated protein kinase. Mol Med Rep 12:5679–5684
Zuchowska A, Kwapiszewska K, Chudy M, Dybko A, Brzozka Z (2017) Studies of anticancer drug cytotoxicity based on long-term HepG2 spheroid culture in a microfluidic system. Electrophoresis 38:1206–1216
Acknowledgements
The first author duly acknowledges the University Grants Commission (UGC), New Delhi for providing Scholarship under the Scheme MANF (vide Grant No. 201213-MANF-2012-13-SIK-PUN-16650).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, P., Robin, Mehta, R.G. et al. Progression of conventional hepatic cell culture models to bioengineered HepG2 cells for evaluation of herbal bioactivities. Biotechnol Lett 40, 881–893 (2018). https://doi.org/10.1007/s10529-018-2547-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-018-2547-y