Abstract
Objective
To purify and characterize a specific enzyme from a commercial pectinase for the production of steviol from stevioside (Ste) without adding organic solvent and to improve steviol production.
Results
Commercial Sumizyme PX converted Ste to steviol with a yield of 98%. β-Glucosidase from Sumizyme PX (βglyPX) was purified in three steps with 12.5-fold purification and 51% yield. The specific activity of the purified βglyPX was 141 U/mg. The molecular weight of βglyPX was ~ 116 kDa on SDS-PAGE. Its optimum activity was at pH 3.5 and 65 °C. It was stable for 12 h up to 55 °C and for 24 h at pH 2–9.5. K m values of βglyPX for pNPGal, oNPGlc, lactose, and Ste were 2.4, 0.7, 18, and 7.8 mM, respectively. The optimum conditions for steviol production were 55 °C, 900 U/ml, 80 mg Ste/ml, 12 h.
Conclusion
βglyPX contains great potential for industrial steviol production from Ste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
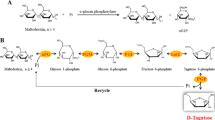
Stevioside (Ste, 13-O-β-sophorosyl-19-O-β-D-glucosyl-steviol) is a noncaloric natural sweetener isolated from leaves of Stevia rebaudiana (Bertoni). It is about 200 times sweeter than sucrose (Prakash et al. 2008). Therefore, it can be used as a non-caloric sugar substitute in various drinks and foods categories. Ste can be degraded to its aglycone steviol [(5β,8α,9β,10α,13α)-13-hydroxykaur-16-en-18-oic acid] by intestinal microflora found in various animals including humans (Koyama et al. 2003a). Ste is a large neutral molecule with both polar and hydrophobic regions whereas steviol contains a hydrophobic ring with one negative charge on the carboxyl group (Srimaroeng et al. 2005). Both compounds have potential roles as antihyperglycemic agents by stimulating insulin secretion from pancreatic beta cells (Jeppesen et al. 2000). Ste cannot be effectively absorbed into everted sacs of rat intestine whereas steviol can be rapidly absorbed (Koyama et al. 2003b). Steviol can inhibit the accumulation of p-amino-hippurate in rat renal cortical slices (Toskulkao et al. 1994) and human organic anion transporters 1 and 3 (Srimaroeng et al. 2005). Thus, it has the potential to diminish renal clearance of anionic drugs and their metabolites (Srimaroeng et al. 2005). Steviol is rare in nature. To produce steviol, a chemical method involving hydrolysis of Ste under extremely acidic conditions has been used (Kohda et al. 1976). However, steviol produced in that way will rearrange itself into isosteviol automatically (Kohda et al. 1976). NaIO4 and NaOH (Wan et al. 2012) have also been used to produce steviol. Such process, however, requires a highly diluted system with large amounts of expensive sodium periodate to achieve useful yields (Ko et al. 2013).
Production of steviol by enzymatic methods using crude pancreatin, pancreatic lipase, Aspergillus niger (Milagre et al. 2009), β-glucosidase from Sulfolobus solfataricus (Nguyen et al. 2016; Wan et al. 2012), or Penicillium decumbens (Ko et al. 2013) has been reported. Milagre et al. (2009) reported that by using ethanol as organic solvent, different products were produced (steviol or isosteviol) with low Ste hydrolysis yields (4.2–12.4%). Using toluene as organic solvent, 94% Ste was hydrolyzed to isosteviol and 21% Ste was hydrolyzed to steviol over 7 days. A. niger gave a yield of 21% Ste hydrolysis to steviol (Milagre et al. 2009) but without any characterization and optimization. From our previous study, a commercial pectinase could hydrolyze Ste to steviol without organic solvents (Nguyen et al. 2014). Commercial pectinases are complexes of pectinases, cellulases, hemicellulases, and glycosidases (Capek et al. 1995). Most studies have used crude enzymes produced from A. niger for the hydrolysis of Ste to steviol (Milagre et al. 2009; Nguyen et al. 2014; Wan et al. 2012) but only with low yields (Milagre et al. 2009). In this study, we purified and characterized a specific enzyme from the commercial pectinase for the production of steviol from Ste without adding organic solvent. Steviol production yields were improved by optimization of enzyme reaction conditions, enzyme concentration, Ste concentration and reaction temperatures.
Materials and methods
Enzyme and materials
Sumizyme PX from Aspergillus niger was provided by Oak Zone Biotech Corporation (Oak Zone, Seoul, Korea). Cytolase PCL5 from A. niger was provided by Bision Corporation (Bision, Gyunggi-do, Korea). Ste (≥ 95%) was purchased from Daepyung Co., Ltd. (Gyeonggi-do, Korea). Each crude enzyme (30%, v/v) was reacted with 2% (w/v) Ste at 55 °C and 20 mM sodium acetate buffer (pH 4.5) for 0–24 h. Steviol was detected and determined as described in our previous study (Nguyen et al. 2016) with steviol as standard (Supplementary Fig. 1).
Purification of β-glucosidase from Sumizyme PX
β-Glucosidase from Sumizyme PX was loaded onto a DEAE-Sepharose fast flow ion exchange chromatography column (2 × 40 cm) equilibrated with 20 mM sodium acetate buffer (pH 4.5) containing 20 mM NaCl. The column was washed with the same buffer and eluted with 20–1000 mM NaCl in 20 mM sodium acetate buffer (pH 4.5). Fractions with high Ste-hydrolyzing β-glucosidase activity were pooled, concentrated by ultrafiltration, and then held at 60 °C for 20 min. To obtain purified enzyme for kinetic characterization, the supernatant after heating was reloaded onto a 2nd DEAE-Sepharose fast flow column (2 × 40 cm). Fractions with Ste-hydrolyzing β-glucosidase activities were pooled, concentrated by ultrafiltration, analyzed by SDS-PAGE. The purified enzyme was used for further study. Protein was determined with the Bradford method using crystalline bovine serum albumin as standard.
βglyPX activity was determined with 3 mM p-nitrophenyl-β-glucoside (pNPGlc) and 1.1 µg enzyme in 20 mM sodium acetate buffer (pH 4) at 55 °C for 1–20 min. The increase in absorbance at 410 nm caused by the release of p-nitrophenol was measured to calculate glucosidase activity. One unit (U) of β-glucosidase activity was defined as the amount of enzyme required to release 1 µmol p-nitrophenol per min under the above reaction conditions.
Effect of temperature and pH on activity and stability of βglyPX
The activity of βglyPX was assayed from 20–80 °C. Stability of βglyPX was assessed from 28–80 °C over 12 h. The activity and stability of βglyPX was assayed from pH 2–8 with the following buffers for 15 min: 20 mM glycine/HCl (pH 2–3), 20 mM sodium acetate buffer (pH 3–5.5), 50 mM sodium phosphate buffer (pH 6–6.5) and 50 mM Tris/HCl buffer (pH 7–8). The pH stability was assayed from pH 2–11 for 24 h in the same buffers above at 4 °C. The remaining activity was then determined as described in the enzyme assay.
βglyPX kinetic assay
βglyPX kinetics were assayed as in our previous study using lactose (0.5–50 mM), oNPGal (0.05–10 mM), and pNPGlc (0.05–10 mM) as substrates (Nguyen et al. 2016). The K m values were calculated from Lineweaver–Burk plots using SigmaPlot program (SPSS, San Diego, CA, USA).
Production of steviol from rubusoside (Ru), stevioside, and rebaudioside A (Reb A) by βglyPX
Ru was prepared as described previously (Nguyen et al. 2014). RebA (≥ 95%) and stevioside glucosides with α-1-4 linkages (SG) were obtained from Daepyung Co., Ltd. (Gyeonggi-do, Korea). βglyPX (500 U β-glucosidase/ml) was reacted with 40 mg/ml of Ru, Ste, RebA, or SG in 20 mM sodium acetate buffer (pH 3.5) at 55 °C for 12 h. The reaction digest was centrifuged at 12,000×g for 10 min. Steviol was detected and determined as described in our previous study (Nguyen et al. 2016). Kinetic parameters of the hydrolysis of Ste by βglyPX were obtained using 0.5–50 mM Ste for 30 min. Glucose was analyzed with a glucose-oxidase kit. Apparent K m and k cat were measured as described previously (Nguyen et al. 2016).
Optimization of steviol production by βglyPX
Production of steviol from Ste was evaluated after incubating 500 U βglyPX/ml with 30 mg Ste/ml for 12 h at 28–80 °C. Also βglyPX, from 10 to 1500 U/ml, was assayed with 50 mg Ste/ml in 20 mM sodium acetate buffer (pH 3.5) at 55 °C for 12 h. The effect of Ste (10–150 mg/ml) on the reaction containing 900 U βglyPX/ml in 20 mM sodium acetate buffer (pH 3.5) at 55 °C for 12 h was also assayed.
Results
Production of steviol from stevioside
Among the pectinases tested in our previous study (Nguyen et al. 2014), Sumizyme PX and Cytolase PCL5 were the best for conversion of Ste to steviol (Supplementary Fig. 2). Steviol yields used Sumizyme PX and Cytolase PCL5 were 99% (7.8 mg steviol/ml) and 90% (7.2 mg steviol/ml), respectively. Based on these results, Sumizyme PX was selected for purification of β-glucodisase.
Purification and characterization of βglyPX
Sumizyme PX is a crude commercial enzyme from A. niger. Purification of βglyPX from crude Sumizyme PX is summarized in Table 1. The fold purification of the purified enzyme was 12.5. The overall yield was 51%. It had a specific activity of 141 U/mg protein towards pNPGlc (Table 1). The purified protein showed a single band on 10% SDS-PAGE with an approximate MW of 116 kDa (Fig. 1).
The optimum temperature of βglyPX was 65 °C (Supplementary Fig. 3a). The enzyme was stable below 55 °C with over 90% activity. It maintained 80% of its activity at 60 °C but only 16% of its activity at 65 °C after 12 h (Supplementary Fig. 3a). The optimum pH of βglyPX was 3.5 (Supplementary Fig. 3b). The purified βglyPX maintained over 90% activity at pH 2–9.5 after 24 h at 4 °C (Supplementary Fig. 3b). The enzyme maintained only 19 and 3% activity at pH 10 and 11, respectively.
Kinetic characterization of βglyPX
The Michaelis–Menten constant (K m), turnover number (k cat), and catalytic efficency (k cat/K m) values of βglyPX for oNPGal were 2.4 mM, 2042 s−1, and 1,060 s−1 mM−1 (Table 2, Supplementary Fig. 4a, b). K m, k cat, and k cat/K m values of βglyPX for pNPGlc were 0.7 mM, 795 s−1, and 1240 s−1 mM−1 (Table 2, Supplementary Fig. 4c, d). K m, k cat, and k cat/K m values of βglyPX for lactose were 18 mM, 82.4 s−1, and 4.6 s−1 mM−1 (Table 2, Supplementary Fig. 4e-f). The apparent K m, k cat, and k cat/K m values of βglyPX for Ste were 7.8 mM, 17.9 s−1, and 2.27 s−1 mM−1, respectively (Table 2, Supplementary Fig. 4g, h).
Production of steviol from steviol glucosides
βglyPX hydrolyzed 40 mg Ste/ml to 15.3 mg steviol/ml after 12 h with 96% theoretical yield and 40 mg SG/ml to 6.1 mg steviol/ml after 12 h (Supplementary Fig. 5). No steviol was produced when Ru or RebA was used as substrate (Supplementary Fig. 5).
Effect of temperature, enzyme, and stevioside concentration on steviol production yield
The optimum temperature for steviol production from Ste by using βglyPX was 55 °C (Fig. 2a). An increase in βglyPX concentration up to 900 U βglyPX/ml increased steviol formation which was maintained at up to 1500 βglyPX/ml (Fig. 2b). An increase in Ste concentration up to 80 mg Ste/ml also increased steviol formation which was maintained at up to 150 mg Ste/ml. When Ste concentration was at 80 mg Ste/ml, 96% Ste was hydrolyzed (Fig. 2c). The maximum steviol yield (30.2 mg steviol/ml) was obtained with 900 U βglyPX/ml, 80 mg Ste/ml, and 55 °C for 12 h (Fig. 2d and Table 3).
Effect of temperature a βglyPX concentration b stevioside concentration c, and reaction time d on steviol production using stevioside as substrate. a 500 U βglyPX/ml was reacted with 30 mg Ste/ml for 12 h at 28–80 °C. b 10–1500 U βglyPX/ml was reacted with 50 mg Ste/ml in 20 mM sodium acetate buffer (pH 3.5) at 55 °C for 12 h. c 10–150 mg Ste/ml was reacted with 900 U βglyPX/ml in 20 mM sodium acetate buffer (pH 3.5) at 55 °C for 12 h. d Reaction time on steviol production from 0 to 12 h was determined with 900 U βglyPX/ml, 80 mg Ste/ml, and 55 °C. Closed circle; Relative steviol formation (%), open circle; relative stevioside hydrolysis (%)
Discussion
A. niger is widely used in fermentations due to its capability of producing various hydrolyzing exo enzymes such as pectinases, cellulases, hemicellulase, β-glucosidases, and β-galactosidases (Capek et al. 1995; Dekker 1986; Peshin and Mathur 1999). Aspergillus species secrete some distinct β-glucosidases. Their major forms have MW of 100–130 kDa belonging to family 3 of glycoside hydrolase (GH3) and some of their minor forms have MW of 40–50 kDa (Zhao et al. 2013) (Table 2). βglyPX has similar molecular mass to that of the major form of β-glucosidase from Aspergillus. The optimum temperature of βglyPX was 65 °C, similar to that of Au0847 β-glucosidase (Gong et al. 2014). It has lower temperature for stability (55 °C) than previously reported β-glucosidases from Aspergillus (60 °C) due to different incubation time (Gong et al. 2014). The optimum pH of βglyPX (pH 3.5) was more acidic than β-glucosidase from other A. niger strains (pH 4–5), A. aculeatus (pH 5), Penicillium decumbens (pH 4), or Streptomyces Sp. GXT6 (pH 6.5) (Table 2). The K m of βglyPX for pNPGlc (K m = 0.7 mM) was higher than K m of β-glucosidase from A. aculeatus (K m = 0.05 mM), similar to that of β-glucosidase of A. niger NL1 (Zhao et al. 2013) but lower to that of β-glucosidase from A. niger Au0847 (K m = 2.7 mM) (Gong et al. 2014), A. niger CCRC31494 (K m = 21.7 mM) (Yan and Lin 1997), β-glucosidase from P. decumbens, Streptomyces sp. GXT6, or S. solfataricus (Ko et al. 2012, 2013; Wang et al. 2015) (Table 2). The K m of βglyPX for Ste (K m = 7.8 mM) was higher than that of β-glucosidae from A. aculeatus (K m = 3.6 mM) (Ko et al. 2012) and Streptomyces sp. GXT6 (K m = 1.5 mM) (Wang et al. 2015), but lower to that of β-glucosidase from P. decumbens and S. solfataricus (Ko et al. 2013; Nguyen et al. 2016). The optimum temperature of βglyPX activity was at 65 °C. However, the optimum temperature for steviol production from Ste using βglyPX was at 55 °C due to βglyPX was less-stable (84% lost activity) at 65 °C after 12 h.
Negligible amounts of steviol were produced from Ste using βglyPX without adding organic solvent at room temperature. βglyPX hydrolyzed 96% of 80 mg Ste/ml at 55 °C in this study. The optimized Ste concentration by βglyPX was lower than that of β-glucosidase from S. solfataricus (Nguyen et al. 2016), but higher than that of mutant β-glucosidase from S. solfataricus (Chen et al. 2014) (4.5 times), P. decumbens (2.1 times) (Ko et al. 2013), pancreatin, pancreatic lipase, fungal lipase or A. niger (Milagre et al. 2009) (Table 3). The specific hydrolytic activity of βglyPX was higher than β-glucosidase from S. solfataricus (Table 3). Immobilized enzyme can be used to reduce the cost of enzyme, and to improved enzyme stability and product yield. The steviol production by βglyPX (30.2 mg steviol/g Ste/h) was higher than mutant β-glucosidase from S. solfataricus (1.9 times higher), β-glucosidase from P. decumbens (2.9 times), pancreatin (63 times), pancreatic lipase (252 times) and Ste fermentation by A. niger (60.5 times). Thus, the steviol production yield of βglyPX was lower than that of β-glucosidase from S. solfataricus, but higher than those of β-glucosidase from P. decumbens, pancreatin, pancreatic lipase, fungal lipase and Ste fermentation by A. niger (Table 3).
In conclusion, for the first time we characterized βglyPX activity for the production of steviol using Ste as substrate. βglyPX was purified from commercial Sumizyme PX pectinase with yield of 51%. The optimum steviol concentration, reaction temperature, and enzymatic concentration for the production of steviol were determined. Therefore, βglyPX shows great potential for industrial steviol production from Ste.
References
Capek P, Renard CMGC, Thibault JF (1995) Enzymatic degradation of cell walls of apples and characterization of solubilized products. Int J Biol Macromol 17:337–340
Chen JM, Xia YM, Wan HD, Wang HJ, Liu X (2014) A complete specific cleavage of glucosyl and ester linkages of stevioside for preparing steviol with a beta-galactosidase from Sulfolobus solfataricus. J Mol Catal B-Enzyme 105:126–131
Dekker RF (1986) Kinetic, inhibition, and stability properties of a commercial beta-D-glucosidase (cellobiase) preparation from Aspergillus niger and its suitability in the hydrolysis of lignocellulose. Biotechnol Bioeng 28:1438–1442
Gong GH, Zheng ZM, Liu H, Wang L, Diao JS, Wang P, Zhao GH (2014) Purification and characterization of a beta-glucosidase from Aspergillus niger and its application in the hydrolysis of geniposide to genipin. J Microbiol Biotechnol 24:788–794
Jeppesen PB, Gregersen S, Poulsen CR, Hermansen K (2000) Stevioside acts directly on pancreatic beta cells to secrete insulin: actions independent of cyclic adenosine monophosphate and adenosine triphosphate-sensitive K + -channel activity. Metabolism 49:208–214
Ko JA, Kim YM, Ryu YB, Jeong HJ, Park TS, Park SJ, Wee YJ, Kim JS, Kim D, Lee WS (2012) Mass production of rubusoside using a novel stevioside-specific beta-glucosidase from Aspergillus aculeatus. J Agric Food Chem 60:6210–6216
Ko JA, Ryu YB, Kwon HJ, Jeong HJ, Park SJ, Kim CY, Wee YJ, Kim D, Lee WS, Kim YM (2013) Characterization of a novel steviol-producing beta-glucosidase from Penicillium decumbens and optimal production of the steviol. Appl Microbiol Biotechnol 97:8151–8161
Kohda H, Kasai R, Yamasaki K, Murakami K, Tanaka O (1976) New sweet diterpene glucosides from stevia-rebaudiana. Phytochemistry 15:981–983
Koyama E, Kitazawa K, Ohori Y, Izawa O, Kakegawa K, Fujino A, Ui M (2003a) In vitro metabolism of the glycosidic sweeteners, stevia mixture and enzymatically modified stevia in human intestinal microflora. Food Chem Toxicol 41:359–374
Koyama E et al (2003b) Absorption and metabolism of glycosidic sweeteners of stevia mixture and their aglycone, steviol, in rats and humans. Food Chem Toxicol 41:875–883
Milagre HMS, Martins LR, Takahashi JA (2009) Novel agents for enzymatic and fungal hydrolysis of stevioside. Braz J Microbiol 40:367–372
Nguyen TT, Jung SJ, Kang HK, Kim YM, Moon YH, Kim M, Kim D (2014) Production of rubusoside from stevioside by using a thermostable lactase from Thermus thermophilus and solubility enhancement of liquiritin and teniposide. Enzyme Microb Technol 64–65:38–43
Nguyen TTH, Kim SB, Kim NM, Kang C, Chung B, Park JS, Kim D (2016) Production of steviol from steviol glucosides using beta-glycosidase from Sulfolobus solfataricus. Enzyme Microb Tech 93–94:157–165
Peshin A, Mathur JMS (1999) Purification and characterization of beta-glucosidase from Aspergillus niger strain 322. Lett Appl Microbiol 28:401–404
Prakash I, Dubois GE, Clos JF, Wilkens KL, Fosdick LE (2008) Development of rebiana, a natural, non-caloric sweetener. Food Chem Toxicol 46(Suppl 7):S75–S82
Srimaroeng C, Chatsudthipong V, Aslamkhan AG, Pritchard JB (2005) Transport of the natural sweetener stevioside and its aglycone steviol by human organic anion transporter (hOAT1; SLC22A6) and hOAT3 (SLC22A8). J Pharmacol Exp Ther 313:621–628
Toskulkao C, Deechakawan W, Temcharoen P, Buddhasukh D, Glinsukon T (1994) Nephrotoxic effects of stevioside and steviol in rat renal cortical slices. J Clin Biochem Nutr 16:123–131
Wan HD, Tao GJ, Kim D, Xia YM (2012) Enzymatic preparation of a natural sweetener rubusoside from specific hydrolysis of stevioside with beta-galactosidase from Aspergillus sp. J Mol Catal B-Enzym 82:12–17
Wang Z, Wang J, Jiang M, Wei Y, Pang H, Wei H, Huang R, Du L (2015) Selective production of rubusoside from stevioside by using the sophorose activity of beta-glucosidase from Streptomyces sp GXT6. Appl Microbiol Biotech 99:9663–9674
Yan TR, Lin CL (1997) Purification and characterization of a glucose-tolerant beta-glucosidase from Aspergillus niger CCRC 31494. Biosci Biotechnol Biochem 61:965–970
Zhao LG, Zhou TC, Li X, Fan S, You LJ (2013) Expression and characterization of GH3 beta-Glucosidase from Aspergillus niger NL-1 with high specific activity, glucose inhibition and solvent tolerance. Microbiology 82:356–363
Acknowledgements
This work was partially supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program (1403-20160048) and Agriculture, Food and Rural Affairs Research Center Support Program funded by Ministry of Agriculture, Food and Rural Affairs, Republic of Korea (D. Kim) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A01056929; D. Kim, and 2015R1D1A4A01020522; T.T. Hanh Nguyen), under the framework of International Cooperation Program managed by the NRF (2016K1A3A1A19945059; 2016K2A9A2A08003613), and by OTTOGI Corporation through Research and Publication Projects.
Supporting information
Supplementary Fig—1 Steviol standard from thin layer chromatography
Supplementary Fig—2 Effect of time on steviol production from Ste by using crude Sumizyme PX and Cytolase PCL5
Supplementary Fig—3 Effect of temperature (A) and pH (B) on optimum activity and stability of βglyPX
Supplementary Fig—4 Primarily and Lineweaver Burk plots of βglyPX with different substrates
Supplementary Fig—5 Enzymatic hydrolysis of rubusoside, stevioside, rebaudioside, and stevioside glucosides using βglyPX by thin layer chromatography
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nguyen, T.T.H., Seo, C., Gu, BC. et al. Production of steviol from steviol glucosides using β-glucosidase from a commercial pectinase, Sumizyme PX. Biotechnol Lett 40, 197–204 (2018). https://doi.org/10.1007/s10529-017-2460-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2460-9