Abstract
Objectives
To engineer Yarrowia lipolytica for improving the heterologous production of campesterol (a key precursor to manufacture pharmaceutical steroids).
Results
By screening 7-dehydrocholesterol reductase (DHCR7) from diverse species, DHCR7 from Danio rerio was the best candidate for campesterol synthesis. Overexpression of ACL (ATP: citrate lyase) or POX2 (peroxisome acyl-CoA oxidase 2) were key to improving campesterol production. The highest yield of campesterol was 942 mg/l was with the strain overexpressing POX2 in a 5 l bioreactor via high cell density fermentation process with a restricted supply of carbon sourc, sunflower seed oil.
Conclusions
A promising platform to synthesize downstream steroid drugs was established. Efficient approaches were provided to improve the production of desired molecules in Y. lipolytica with high oil utilization efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yarrowia lipolytica has gained attention for the synthesis of heterologous terpenoids (Yang et al. 2016). Among those, campesterol is an important precursor to manufacture pharmaceutical steroids (Du et al. 2016). In our previous study, campesterol biosynthesis was realized in Y. lipolytica firstly by introducing heterologous 7-dehydrocholesterol reductase (DHCR7) along with disrupting the bypass of ergosterol formation (Du et al. 2016). Although the titer (453 mg/l) is far more than other reported productions achieved in Saccharomyces cerevisiae (Lecain et al. 1996; Duport et al. 1998; Souza et al. 2011), it still does not satisfy the requirement for production (Yao et al. 2013) and should be further improved.
Screening enzymes plays an important role to enhance the productivity of heterologous pathway (Chen et al. 2016). Our previous study increased campesterol production by selecting DHCR7 s only from three sources (Du et al. 2016). Thus, the species have been extended in this study. Selection of the critical targets involved in the formation and distribution of acetyl-CoA is crucial to the production of its derivatives like campesterol. For acetyl-CoA formation, overexpression of ACS (acetyl-CoA synthetase in PDH bypass) and ATP: citrate lyase (ACL), which are responsible to supply cytoplasmic acetyl-CoA, should be tested for campesterol overproduction, although manipulating these would not necessarily bring positive effects in Y. lipolytica (Zhou et al. 2012; Wang et al. 2016). For acetyl-CoA distribution, lipid formation and campesterol synthesis competes the same precursor acetyl-CoA, whereas lipid accumulation can also provide store spaces for campesterol to avoid the cell burden (Du et al. 2016). Y. lipolytica strains only accumulate significant quantities of cellular lipids when cultivated on hydrophobic substrates (Friedlander et al. 2016). Therefore, the essential targets to campesterol precursors (mevalonic acid, MVA) synthesis as well as to fatty acid formation and degradation should also be investigated. In this study, campesterol production has been improved through screening DHCR7 sources as well as identifying the targets. These targets involve the rate limited enzymes in carbon metabolism, lipid metabolism and the MVA pathway and would be overexpressed mainly under when cells are growing with plant oils.
Materials and methods
Strains, media and culture condition
All the strains used are summarized in Supplementary Table 1. Escherichia coli DH5α, which was used for plasmids construction and replication, was cultivated at 37 °C in lysogeny broth (LB) complete medium with 50 mg kanamycin/l or 100 mg ampicillin/l for selection. Engineered Y. lipolytica strains were selected and grown on synthetic complete drop-out (SC) medium with supplementation of appropriate amino acids. For campesterol production, Y. lipolytica strains were cultured at 28 °C in YP medium supplemented with appropriate carbon source, glucose or sunflower seed oil, according to Du et al. (2016). In shake-flask culture, there was 50 g glucose/l in the media. Sunflower seed oil concentration was standardized in moles of carbon equivalent to 50 g glucose/l (i.e., 28.86 g/l) according to Sestric et al. (2014). For the 5 l bioreactor experiment, 250 ml seed culture was transferred into 2.25 l YP medium supplemented with 28.86 g sunflower seed oil/l as carbon source. The oil concentration was restricted below 2 g/l by adjusting the feeding rate after the batch oil was depleted (Du et al. 2016). Samples of 15 ml were collected to determine oil concentration, cell density and campesterol production.
DNA manipulation and plasmids construction
Primers and plasmids used are given in Supplementary Tables 1 and 2. All the endogenous genes employed in this study (Supplementary Table 3) were amplified from the genomic DNA of Y. lipolytica ATCC201249. The genes encoding heterologous DHCR7 were codon optimized (Supplementary Table 3) and synthesized by Genscript Inc. (China). They were carried by plasmid pUC57-Kan (Supplementary Table 1).
All the genes mentioned above were cloned into integrative plasmid pINA1269 via In-Fusion assembly, obtaining pINA1269 series plasmids (Supplementary Table 1). These genes were under the control of a strong constitutive promoter hp4d. Before transformed into Y. lipolytica strains, all these plasmids were linearized by NotI. Y. lipolytica transformation was conducted with a kit Frozen-FZ yeast transformation II (Zymo Research, USA). The engineered cells were selected on SC medium plates without leucine added and further verified via PCR with primer pINA1269_SEQ-F/pINA1269_SEQ-R.
Plasmids pEASY-blunt-101, pEASY-blunt-102 and pUC57-Simple-101 from our previous study (Du et al. 2016) were also applied to disrupt the gene ERG5 (encoding C-22 sterol desaturase) and simultaneously introduce the DHCR7 expression cassette (URA3-EXP1p-DHCR7-XPR2t). Plasmids, pUC57-Simple-DHCR7_Dr, pEASY-Blunt-101 and pEASY-Blunt-102 were digested by NotI and co-transformed into the wild-type Y. lipolytica ATCC201249, giving strain SyBE_Yl02060056. The clones were selected on SC medium plates without uracil and further verified via PCR with primers of Yl-DHCR7_Dr-F/Yl-DHCR7_Dr-R and Yl-DHCR7_Dr-SEQ-F/Yl-chorosomeA-R.
Measurement of biomass and sterols compounds
Biomass determination, samples extraction and steroids analysis were performed according to our previous study (Du et al. 2016). Before measuring the dry cell weight (DCW), the harvested cells were washed three times with hexane followed by distilled water to remove residual oil (Papanikolaou et al. 2001). Saponification was required in sample treatment before GC-TOF/MS (Waters Corp., USA) analysis (Du et al. 2016). Standards of campesterol and ergosterol were purchased from Sigma. Ergosta-5,22-dieneol was identified by Nist library search 2006 (the mass fragment 69, 255, 380 and 470 m/z, Supplementary Fig. 1) and semi-quantitatively analyzed based on the standard curve of ergosterol. Data was collected from at least three replicate samples.
Results and discussion
Danio rerio was the best DHCR7 source for campesterol production
Screening enzymes from diversity sources is an efficient strategy to improve the productivity of heterologous pathway (Chen et al. 2016). In our previous study, DHCR7 from X. laevis (DHCR7_Xl) achieved the highest campesterol titer than other DHCR7 s from Rattus norvegicus and Oryza saliva (Du et al. 2016). Here, to extend the enzyme sources, DHCR7-Xl and other five newly selected DHCR7 s (Fig. 1b) were further screened initially in the host cells without gene ERG5 deletion for higher campesterol production. As a result, only DHCR7 from Danio rerio (DHCR7_Dr) and from Waddlia chondrophila (DHCR7_Wc) achieved higher campesterol yield than DHCR7_Xl (228% increase for DHCR7_Wc and 432% increase for DHCR7_Dr, Fig. 1a). DHCR7_Dr was selected to construct strain SyBE_Yl02060056 with ERG5 deletion, to further avoid the bypass of ergosterol formation (Supplementary Fig. 1). As expected, campesterol was the unique detectable sterol compound in the product (Supplementary Fig. 2). The campesterol yield in strain SyBE_Yl02060056 reached to 19.3 ± 2.8 mg/g of DCW, which was 156% increase compared with strain SyBE_Yl01070028 harboring DHCR7_Xl with the same genetic manipulation (Fig. 1c). This result verified that D. rerio was the best DHCR7 source for campesterol overproduction. Strain SyBE_Yl02060056 would therefore be the candidate to use for further optimization.
Screening 7-dehydrocholesterol reductase (DHCR7) sources for campesterol biosynthesis in Y. lipolytica. a The campesterol production in ERG5 undisrupted strains with DHCR7 from different species. These strains were cultured in shake-flask under glucose condition and campesterol yield for each strain was determined by GC-TOF/MS. Xl, X. laevis, Hs, H. sapiens, Gg, G. gallus, Dr, D. rerio, At, A. thaliana, Wc, W. chondrophila. b Phylogenetic analysis of DHCR7 protein sequences. c Comparison of campesterol yield in strain SyBE_Yl01070028 (DHCR7_Xl) and SyBE_Yl02060056 (DHCR7_Dr) with disrupted EGR5
Overexpression of ACL or POX2 under oil condition was the key point to improve campesterol production
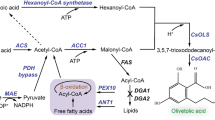
Acetyl-CoA is the key molecule channeling the central metabolism and heterologous synthesis. The key enzymes associated with acetyl-CoA formation and distribution were selected (Fig. 2) and overexpressed in strain SyBE_Yl02060056 growing with sunflower seed oil. When the oil was used as the carbon source, overexpression of HMGR (3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase) did not significantly increase campesterol yield (Fig. 2). Overexpression of MAE (malic enzyme) decreased campesterol yield by 43% (Fig. 2). According to Blazeck et al. (2014), MAE overexpression might enhance lipid synthesis which would draw more acetyl-CoA flux away from MVA pathway. Here, only overexpressing ACL and POX2 (peroxisome acyl-CoA oxidase, POX) significantly increased the campesterol yield (from 19.3 ± 2.8 mg/g of DCW to 25.3 ± 0.1 mg/g DCW for ACL overexpression and to 24.7 ± 0.1 mg/g DCW for POX2 overexpression, Fig. 2). Overexpression of these two genes wAS aimed to enhance cytoplastic acetyl-CoA supply by improving citrate cleavage or enhancing β-oxidation (Fig. 2). Since ACL is generally inhibited by exogenous fatty acids in the medium (Papanikolaou and Aggelis 2011), thus overexpression of ACL might be of benefit to the output of citrate for cytoplastic acetyl-CoA supply under oil growth conditions. By contrast, overexpressing CS (citrate synthase) might enhance the TCA cycle activity which directs more carbon towards to energy metabolism when the activity of ACL is low. POX1–6, which catalyze the first step of β-oxidation, exhibited different activities and substrate specificities in Y. lipolytica (Mlickova et al. 2004). Among POX1–6, POX2 is the only enzyme processing highly catalytic activity and specificity to long-chain fatty acids (Luo et al. 2002). Since long-chain fatty acids (C16–C18) are the main components of sunflower seed oil, selection of the POX gene(s) should be targeted on the constituents of the substrate oil. In Y. lipolytica, MFE (multifunctional β-oxidation protein) and POT (peroxisomal oxoacyl thiolase) were reported to regulate the size and number of peroxisomes, which then related to the level of β-oxidation proteins and the rate of cell division, respectively (Smith et al. 2000). At this stage, it is hard to explain why overexpression of these two β-oxidation enzymes dramatically decreased campesterol yield (Fig. 2). In the future, transcriptional analysis of these targets might further elucidate the mechanism. Fine-tuning of the expression level of these essential targets would be required to improve campesterol synthesis. Strains SyBE_Yl02060065 and SyBE_Yl02060069 overexpressing ACL and POX2, respectively, would be employed in further fermentation optimization experiments.
Identification of the critical targets for campesterol synthesis in Y. lipolytica under oil condition. Acetyl-CoA associated metabolism under oil condition was divided into acetyl-CoA formation (from central metabolism or from β-oxidation) and acetyl-CoA distribution (to MVA pathway for sterol synthesis or to lipids accumulation). The data for overexpressing the targets was presented besides the associated pathway. The endogenous proteins were indicated as blue, while the heterologous enzyme DHCR7 was presented by red. Solid arrows represented the one-step conversions, while dashed arrows represented multiple steps. Line thickness represented metabolic flow. Significance levels of Student t test: *P < 0.05, **P < 0.01, ***P < 0.001. CS citrate synthase, ACL, ATP citrate lyase, ACS acetyl-CoA synthetase, POX1–6 peroxisome acyl-CoA oxidase 1–6; MFE multifunctional β-oxidation protein, POT peroxisomal oxoacyl thiolase, HMGR 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase, MAE malic enzyme, TCA tricarboxylic acid cycle, TAG triacylglycerol, FFA free fatty acid
High cell density fermentation in bioreactor achieved the highest campesterol production
The high cell density fermentation was conducted in a 5 l bioreactor under a carbon source restriction strategy. The initial oil concentration in batch medium was set as 28.86 g/l to be consistent with that in the shake-flask. After the initial oil was depleted after 46 h, oil was fed into the medium to keep its concentration lower than 2 g/l (Fig. 3a, b). At this point, all the strains have entered their stationary phase with biomasses of 20.2 ± 1.6 mg/g of DCW and 26.4 ± 1.1 mg/g of DCW for ACL and POX2 overexpressed strains, respectively (Fig. 3a, b). Therefore, the supplementary oil would mainly be used to accumulate campesterol or lipid bodies. Eventually, the highest campesterol reported titer of 942 ± 50.1 mg/l was achieved after 142 h cultivation in strain SyBE_Yl02060069 was overexpressing POX2 (Fig. 3b). This is 18% higher than that in the strain with overexpressed ACL (801 ± 36.4 mg/l, Fig. 3a). Moreover, compared with the data in shake-flask, the biomass conversion yields (YX/S) was decreased by 21%, while the product yields (YP/S) was increased by 20% in bioreactor for the ACL-overexpressed strain SyBE_Yl02060065. For the POX2 overexpressing strain, SyBE_Yl02060069, YX/S was nearly equal, while YP/S was enhanced by 45% through the carbon source restriction strategy (Fig. 3c).
Bioreactor fermentation under carbon source restriction strategy. Y. lipolytica strains SyBE_Yl02060065 (with overexpressed ACL, a) and SyBE_Yl02060069 (with overexpressed POX2, b) were cultured in a 5 l bioreactor with sunflower seed oil as the carbon source. The oil concentration (green line) was maintained less than than 2 g/l by controlling the oil feeding rate. Biomass and campesterol production were indicated by blue line and red line, respectively. YX/S (yield of total biomass achieved per unit of substrate consumed) and YP/S (yield of product synthesized per unit of substrate consumed) for fed-batch bioreactor cultures were compared with those from the shake-flask experiments (c)
The alteration on YX/S and YP/S indicated that the cell growth had been effectively controlled in the batch fermentation, directing more carbon metabolic flux towards the desired product. The YX/S and YP/S (46.5 ± 1.8 and 1.7 ± 0.1%) achieved by strain SyBE_Yl02060069 in bioreactor here was higher than that in our previous study [39.9 and 0.8%, (Du et al. 2016)]. Through further metabolomics analysis along with culture condition optimization, it would obtain a more balance state between biomass formation and product accumulation for higher campesterol production.
References
Blazeck J, Hill A, Liu L, Knight R, Miller J, Pan A, Otoupal P, Alper HS (2014) Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun 5:3131
Chen Y, Xiao W, Wang Y, Liu H, Li X, Yuan Y (2016) Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb Cell Fact 15:113
Du HX, Xiao WH, Wang Y, Zhou X, Zhang Y, Liu D, Yuan YJ (2016) Engineering Yarrowia lipolytica for campesterol overproduction. PLoS ONE 11:e0146773
Duport C, Spagnoli R, Degryse E, Pompon D (1998) Self-sufficient biosynthesis of pregnenolone and progesterone in engineered yeast. Nat Biotechnol 16:186–189
Friedlander J, Tsakraklides V, Kamineni A, Greenhagen EH et al (2016) Engineering of a high lipid producing Yarrowia lipolytica strain. Biotechnol Biofuels 9:77
Kocharin K, Chen Y, Siewers V, Nielsen J (2012) Engineering of acetyl-CoA metabolism for the improved production of polyhydroxybutyrate in Saccharomyces cerevisiae. AMB Express 2:1
Lecain E, Chenivesse X, Spagnoli R, Pompon D (1996) Cloning by metabolic interference in yeast and enzymatic characterization of Arabidopsis thaliana sterol D7-reductase. J Biol Chem 271:10866–10873
Luo YS, Nicaud JM, Van Veldhoven PP, Chardot T (2002) The acyl–CoA oxidases from the yeast Yarrowia lipolytica: characterization of Aox2p. Arch Biochem Biophys 403:32–38
Mlickova K, Roux E, Athenstaedt K, d’Andrea S, Daum G, Chardot T, Nicaud JM (2004) Lipid accumulation, lipid body formation, and acyl coenzyme A oxidases of the yeast Yarrowia lipolytica. Appl Environ Microbiol 70:3918–3924
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. Eur J Lipid Sci Technol 113:1031–1051
Papanikolaou S, Chevalot I, Komaitis M, Aggelis G, Marc I (2001) Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa-butter substitute from industrial fats. Antonie Van Leeuwenhoek 80:215–224
Sestric R, Munch G, Cicek N, Sparling R, Levin DB (2014) Growth and neutral lipid synthesis by Yarrowia lipolytica on various carbon substrates under nutrient-sufficient and nutrient limited conditions. Bioresour Technol 164:41–46
Smith JJ, Brown TW, Eitzen GA, Rachubinski RA (2000) Regulation of peroxisome size and number by fatty acid beta-oxidation in the yeast Yarrowia lipolytica. J Biol Chem 275:20168–20178
Souza CM, Schwabe TM, Pichler H, Ploier B, Leitner E, Guan XL, Wenk MR, Riezman I, Riezman H (2011) A stable yeast strain efficiently producing cholesterol instead of ergosterol is functional for tryptophan uptake, but not weak organic acid resistance. Metab Eng 13:555–569
Wang G, Xiong X, Ghogare R, Wang P, Meng Y, Chen S (2016) Exploring fatty alcohol-producing capability of Yarrowia lipolytica. Biotechnol Biofuels 9:107
Yang X, Nambou K, Wei L, Hua Q (2016) Heterologous production of alpha-farnesene in metabolically engineered strains of Yarrowia lipolytica. Bioresour Technol 216:1040–1048
Yao K, Wang FQ, Zhang HC, Wei DZ (2013) Identification and engineering of cholesterol oxidases involved in the initial step of sterols catabolism in Mycobacterium neoaurum. Metab Eng 15:75–87
Zhou J, Yin X, Madzak C, Du G, Chen J (2012) Enhanced alpha-ketoglutarate production in Yarrowia lipolytica WSH-Z06 by alteration of the acetyl-CoA metabolism. J Biotechnol 161:257–264
Acknowledgements
This work was supported by the financial support from the International S&T Cooperation Program of China (2015DFA00960), the National Natural Science Foundation of China (21390203, 31570088 and 21622605) and Innovative Talents and Platform Program of Tianjin (16PTSYJC00050).
Supplementary information
Supplementary Table 1—Plasmids and strains used in this study.
Supplementary Table 2—Primers used in this study.
Supplementary Table 3—The Codon-optimized sequences of 7-dehydrocholesterol reductase (DHCR7) involved in this study.
Supplementary Fig. 1—Analysis of sterols profile of Y. lipolytica strains SyBE_Yl02060002 and SyBE_Yl02060004–08 for DHCR7 screening.
Supplementary Fig. 2—GC-TOF/MS analysis of campesterol producing strains SyBE_Yl02060006 and SyBE_Yl02060056.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, Y., Yao, M. et al. Improved campesterol production in engineered Yarrowia lipolytica strains. Biotechnol Lett 39, 1033–1039 (2017). https://doi.org/10.1007/s10529-017-2331-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2331-4