Abstract
Objectives
To produce and deliver Helicobacter pylori lipoprotein Lpp20 via using Lactococcus lactis with aim of developing an efficient way to use this protective antigen in vaccine formulation.
Results
An engineered L. lactis strain carrying the lpp20 gene from H. pylori was constructed. The inducible expression of Lpp20 in L. lactis was detected as a 20 kDa intracellular protein by SDS-PAGE. Lpp20 constituted 10 % of the L. lactis cellular proteins. The expression product was highly immunoreactive, as demonstrated by western blot assays using mouse anti-H. pylori sera. Animal experimentation showed that oral vaccination with the engineered strain excited significantly elevated levels of serum Lpp20-specific IgG antibodies in BALB/c mice (P < 0.05).
Conclusions
This report presents the first efficient expression and delivery of whole Lpp20 protein to the immunization sites by using L. lactis, demonstrating an efficient utilization mode of Lpp20 in anti-H. pylori vaccination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colonization of Helicobacter pylori in the human stomach induces gastric and even extra-gastric lesions involving certain malignancies (Zhang et al. 2016a, b). Vaccination is the most promising prevention measure for this infection (Zeng et al. 2015). A potential target of a vaccine should be surface exposed and conserved with a high immunogenicity (Keenan et al. 2000). H. pylori Lpp20 is such a conserved lipoprotein. Rabbit anti-H. pylori antisera recognizes Lpp20 as the major antigen (Kostrzynska et al. 1994). Lpp20 is released omto extracellular spaces by membrane vesicle formation. Intragastric administration of the outer membrane vesicles (OMV) induced immune protection in mice, and hybridoma backpacks producing Lpp20-specific antibodies could significantly reduce bacterial loads in mice (Keenan et al. 2000). As confirmed in mice, H. pylori colonization levels were correlated with the anti-Lpp20 monoclonal antibodies (Keenan et al. 2002). Intranasal administration of either recombinant Lpp20 or the OMV to mice elicited remarkable humoral immune responses, which were also related to the H. pylori loads (Keenan et al. 2003). The recombinant eukaryotic expression plasmid, pcDNA3.1(+)/lpp20, expressing Lpp20, as a DNA vaccine, induced strong cellular and humoral immunity in mice (Yu et al. 2010). The synthesized peptides composed of Lpp20 epitopes activated Th1 cells and proliferation of CD4+ T lymphcytes, oral vaccination with the peptide consisting of Lpp20 epitopes and cholera toxin subunit B (CTB) exerted protection against H. pylori challenges (Li et al. 2012). Taken together, increasing evidence indicates that Lpp20 is a promising antigen candidate for H. pylori vaccines (Keenan et al. 2000).
Although certain attenuated pathogens have proved effective in vaccine formulation by animal experimentation, their application in humans is limited by their safety (Michetti et al. 1999). To circumvent this issue, certain probiotics such as L. lactis have been exploited for H. pylori vaccine delivery (Lee et al. 2001). However, the effects of the lactococcal adjuvant were insufficient to protect immunized animals from H. pylori infection when using a weak immunogen like UreB (Lee et al. 2001). Thus greater protective antigens should be employed for enhancing the immune effect.
Here, a L. lactis strain was genetically modified to express and deliver H. pylori Lpp20 protein. The expression products were identified using SDS-PAGE and western blot assays. The immune efficacy was evaluated by oral immunization of mice with the engineered L. lactis. This report offers an efficient utilization mode of Lpp20 in anti-H. pylori vaccination and a novel promising vaccine candidate against H. pylori.
Materials and methods
Plasmids and their hosts
Bacteria and plasmids used are shown in Supplementary Table 1. Escherichia coli MC1061 and TB1 were cultivated at 37 °C using lysogeny broth (LB). L. lactis NZ3900 was grown at 30 °C using M17 medium (Difco) containing 5 g glucose l−1 (GM17). Chloramphenicol (10 mg/l) was used for cultivation of bacteria harboring pNZ8110-lpp20 AND ampicillin (100 mg/l) for the strain harboring pMAL-c2x-lpp20.
Animals and ethics statements
The SPF BALB/c mice, aged six weeks, were purchased from Henan Experimental Animal Center, China. This project was approved by the Institutional Review Board at Zhengzhou University. The animal experimentation was performed complying with the ARRIVE guidelines.
PCR
Oligonucleotide primers used to amplify H. pylori lpp20 gene were as follows: 5′-GCGCCATGGGCATGAAAAATCAAGTTA-3′ (forward, containing a NcoI site) and 5′-GCCCTGCAGCTACTTTTTAACCATGC-3′ (reverse, containing a PstI site). The lpp20 gene was amplified from genomic DNA of H. pylori MEL-Hp27. The PCR conditions were: 30 cycles of 94 °C for 1 min, 55 °C for 1 min and 72 °C for 3 min. The products were analyzed via electrophoresis using 10 g agarose l−1 gel.
Construction of recombinant L. lactis
Nuclease digestion, DNA ligation and plasmid isolation were carried out as instructed by the suppliers. The lpp20 gene and plasmid pNZ8110 underwent restriction enzyme digestion and ligation reaction, generating the recombinant plasmid pNZ8110-lpp20. The pNZ8110-lpp20 was transferred into E. coli MC1061 and L. lactis NZ3900 by heat shock and electroporation, respectively. The transformants were selected via using the chloramphenicol resistant gene in pNZ8110, and identified by PCR and gene sequencing, as described previously (Chen et al. 2011; Zhang et al. 2015).
Preparation of anti-H. pylori antisera
Mouse anti-H. pylori antisera were prepared by using the methods previously reported except for using H. pylori cellular lysate antigens (0.25 g l−1) instead of purified UreB protein (0.1 g l−1) as immunogens (Zhang et al. 2009). In brief, H. pylori MEL-27 cells, cultivated on Brucella agar plate for three days, were harvested and underwent ultrasonic disruption and centrifugation, and the supernatant was separated as the cellular lysate antigens. BALB/c mice were injected subcutaneously with the cellular lysate antigens plus Freund’s adjuvant according to the reported immunization schedule (Zhang et al. 2009). The antisera were obtained by orbital blood sampling on day 7 post immunization, the titers of H. pylori specific antibodies were assayed by double immunodiffusion test and indirect ELISA as reported elsewhere (Zhang et al. 2009).
Expression and immunological identification of Lpp20
The expression of Lpp20 was induced by addition of 25 μg nisin l−1 to the NZ3900/pNZ8110-lpp20 culture at OD600 = 0.3–0.4 and incubation for 5 h. The expression product was identified by SDS-PAGE analysis, and its immunoreactivity was demonstrated by western blotting using mouse antisera against H. pylori (Chen et al. 2011).
Oral inoculation of mice
BALB/c mice, aged 6 weeks, were randomly assigned into three groups (six mice per group). Lpp20, pNZ8110 and PBS groups were treated with 200 µl L. lactis NZ3900/pNZ8110-lpp20 (5 × 1014 CFU/l), NZ3900/pNZ8110 (5 × 1014 CFU/l) and PBS, respectively, by gavage at 7 day intervals for 5 times. Sera and intestinal juice of the mice were collected on day 14 post immunization as ELISA samples (Zhang et al. 2009; Zhang et al. 2016a, b).
Measurement of specific antibodies
Expression of Lpp20 in E. coli TB1/pMAL-c2x-lpp20 was induced by addition of 0.3 mM IPTG to the culture medium at OD600 = 0.4. The expression product, designated as rLpp20, was purified by amylose affinity chromatography, as instructed by the supplier (New England Biolabs, UK). In brief, after inducement, the cells were harvested by centrifugation at 4000×g for 20 min, and resuspended in Column Buffer (pH 7.4, 20 mM Tris/HCl, 200 mM NaCl, 1 mM EDTA, 10 mM β-mercaptoethanol). The cells were held at −20 °C for approx. 12 h, and then underwent sonication and centrifugation. The supernatant was loaded on an amylose resin column, and the rLpp20 was eluted using Column Buffer containing 10 mM maltose.
Using rLpp20 as the detector antigen, ELISA was performed to test for Lpp20-specific antibodies in sera and intestinal juice from the mice. The ELISA methods and the secondary antibodies used were as described previously (Zhang et al. 2016a, b).
Statistical analysis
Quantitative data were presented as average ± standard deviation. Statistical analysis was performed with the aid of SPSS17.0 package. Difference among the groups was determined via using one-way analysis of variance and Bonferroni tests. Statistical significance was inferred at P < 0.05.
Results
PCR product of lpp20
The PCR product of lpp20 gene was a DNA fragment of 548 bp in length. The sequence of the fragment was identical to the published lpp20 sequence (Genbank accession No.: AY294021), as confirmed by sequentially gene sequencing.
Construction of recombinant L. lactis expressing Lpp20
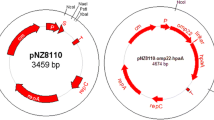
The lpp20 gene was cloned into the expression vector pNZ8110, downstream of the nisin controlled promoter Pnis, generating pNZ8110-lpp20. pNZ8110-lpp20 was introduced into E. coli, producing the recombinant strain MC1061/pNZ8110-lpp20. The plasmid pNZ8110-lpp20 was obtained from MC1061/pNZ8110-lpp20, and then used to transform L. lactis NZ3900, generating NZ3900/pNZ8110-lpp20. It was shown by gene sequencing that the lpp20 gene had been properly cloned in pNZ8110. Figure 1 shows the sketch maps of pNZ8110 and pNZ8110-lpp20, while Figs. 2 and 3 the identification results of the transformants.
Detection of mouse anti-H. pylori antibodies
The double immunodiffusion tests showed that the sera of the mice subcutaneously immunized with H. pylori cellular lysates were capable of recognizing the H. pylori somatic antigens, while no co-immunoprecipitation lines were observed between H. pylori antigens, PBS and sera of non-immunized mice. As confirmed by indirect ELISA, titers of the antisera reached 1:800. These results showed that the polyclonal antisera could be used for identification of H. pylori antigens.
Induced expression of Lpp20
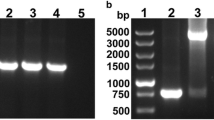
SDS-PAGE analysis gave a distinct protein band at ~20 kDa that was visible in the cell lysate samples of NZ3900 harboring pNZ8110-lpp20, (Fig. 4a). The percentage of Lpp20 in the cellular protein extract of the engineered L. lactis was 10.2 %. Western blotting showed that the Lpp20 expressed in L. lactis was recognized by the antisera against H. pylori. The positive immunoreaction was detected on the 20 kDa band in cell lysate samples of the engineered L. lactis strain, but not in those of NZ3900/pNZ8110 and NZ3900 (Fig. 5).
Electrophoresis results of cell lysates of the L. lactis strains (a) and rLpp20 purified from E. coli strain (b). In a, 1, NZ3900; 2, NZ3900/pNZ8110; 3, NZ3900/pNZ8110-lpp20; 4, protein markers. In b, 1, whole-cell proteins of E. coli TB1; 2, whole-cell proteins of TB1/pMAL-c2x-lpp20; 3, supernatant proteins of TB1/pMAL-c2x-lpp20 cell lysates; 4, rLpp20 purified from supernatant of TB1/pMAL-c2x-lpp20 cell lysates; 5, protein markers
Evaluation of Lpp20-specific antibodies
The purified rLpp20 was obtained with an approximately 90 % purity (Fig. 4b), and used in ELISA assays of the Lpp20 specific antibodies. The results are shown in Fig. 6.
Evaluation of mouse Lpp20-specific antibodies. This figure was processed using Microsoft Office Excel 2003. The mice of the Lpp20, pNZ8110 and PBS groups were treated by gavage with 200 µl of NZ3900/pNZ8110-lpp20 (5 × 1014 CFU/l), NZ3900/pNZ8110 (5 × 1014 CFU/l) and PBS, respectively, for five times with intervals of 1 week. The samples of sera and intestinal juice were collected two weeks post-immunization and tested via indirect ELISA. The asterisk indicates this value is statistically different from those of the other two groups (P < 0.05)
Discussion
Since Lpp20 was first identified as the major antigen recognized by the antisera of H. pylori whole cells immunized rabbits, it has been considered to be an excellent vaccine candidate (Kostrzynska et al. 1994; Li et al. 2016). However, since then, the application of this protein in vaccine formulation has progressed rather slowly. One of the possible reasons might be that except for obtainment of tiny Lpp20 from outer membrane vesicles (OMV), no methods for preparation of this protein with immune activity of its natural form have been successful. Lpp20 in OMV is immunogenic, while the recombinant form purified from an engineered E. coli strain was not, in absence of mucosal adjuvants (Keenan et al. 2003). Although the OMV were naturally produced and shed from H. pylori cells, no evidence demonstrated it to be a practical way of making vaccines by separation of Lpp20 from these membrane vehicles. As suggested, the sources of immunogenic Lpp20 might be the key factor influencing formulation of H. pylori vaccine with this crucial protective antigen. Additionally, increasing evidences indicated that L. lactis, through expression and delivery of antigens, could be a promising oral vaccine vehicle owing to its outstanding safety (Mierau et al. 2005; Gu et al. 2009). However, research on expression of Lpp20 in L. lactis has not been reported. Therefore, construction of a recombinant L. lactis expressing Lpp20 herein might be a considerable step towards the goal of developing more effective and safer oral vaccines against H. pylori.
Nisin is a safe and natural preservative, widely used as a food additive by industry. L. lactis NZ3900/pNZ8110 is a nisin-controlled expression system (NICE), using nisin as the inducer for gene expression. The present study made the lpp20 gene be inserted into pNZ8110 and controlled by nisA promoter. Nisin, added to the culture of this engineered strain, can activate the receptor and regulators encoded by the genes in the chromosome of NZ3900, and then initiate expression of Lpp20 in L. lactis (Mierau et al. 2005).
In the bacterial engineering, the ligation product of lpp20 fragment and pNZ8110 was firstly transferred into E. coli instead of L. lactis, the constructed plasmid pNZ8110-lpp20 was extracted from the E. coli transformants, and finally introduced into L. lactis NZ3900. The reason for this is that the probability of success in transformation of L. lactis by this way was much higher than that by using the ligation mixture to transform L. lactis directly, as observed in our studies (data not shown).
Studies demonstrated that the efficiency of L. lactis expression of heterologous protein could be much lower than expected, the expressed product was commonly detectable only by western blots, but not by SDS-PAGE (Gu et al. 2009; Zhang et al. 2015). SDS-PAGE analysis showed that the percentage of Lpp20 in the cell lysates of the engineered L. lactis was 10.2 %, much higher than those of other H. pylori proteins expressed in L. lactis (Lee et al. 2001; Gu et al. 2009; Chen et al. 2011; Zhang et al. 2015). The mechanism underlying this phenomenon is still uncertain. The Lpp20 expressed in L. lactis had a molecular weight of ~20 kDa, corresponding to the previous study (Kostrzynska et al. 1994). Western blotting showed that Lpp20 produced by the engineered L. lactis strain kept potent antigenicity.
The immunogenicity of the L. lactis strain producing Lpp20 was identified by oral vaccination of BALB/c mice. The engineered strain induced significantly elevated serum IgG levels, indicating that the Lpp20, through the L. lactis delivery, could be efficiently transferred to the mucosal immunization sites and provoke remarkable immune responses. The difference in intestinal SIgA levels between the immunized and control groups was considered as statistically insignificant (P > 0.05), possibly owing to the low sensitivity of the detection methods used and/or less sample sizes of the groups.
In conclusion, this is the first report that H. pylori Lpp20 has been efficiently expressed and delivered by using L. lactis. The engineered strain is a potential H. pylori vaccine candidate and presents a considerable basis for application of this crucial protective antigen.
References
Chen SY, Zhang RG, Duan GC, Shi JX (2011) Food-grade expression of Helicobacter pylori ureB subunit in Lactococcus lactis and its immunoreactivity. Curr Microbiol 62:1726–1731
Gu Q, Song D, Zhu M (2009) Oral vaccination of mice against Helicobacter pylori with recombinant Lactococcus lactis expressing urease subunit B. FEMS Immunol Med Microbiol 56:197–203
Keenan J, Oliaro J, Domigan N, Potter H, Aitken G, Allardyce R, Roake J (2000) Immune response to an 18-kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect Immun 68:3337–3343
Keenan J, Neal S, Allardyce R, Roake J (2002) Serum-derived IgG1-mediated immune exclusion as a mechanism of protection against H. pylori infection. Vaccine 20:2981–2988
Keenan JI, Rijpkema SG, Durrani Z, Roake JA (2003) Differences in immunogenicity and protection in mice and guinea pigs following intranasal immunization with Helicobacter pylori outer membrane antigens. FEMS Immunol Med Microbiol 36:199–205
Kostrzynska M, O’Toole PW, Taylor DE, Trust TJ (1994) Molecular characterization of a conserved 20-kilodalton membrane-associated lipoprotein antigen of Helicobacter pylori. J Bacteriol 176:5938–5948
Lee MH, Roussel Y, Wilks M, Tabaqchali S (2001) Expression of Helicobacter pylori urease subunit B gene in Lactococcus lactis MG1363 and its use as a vaccine delivery system against H. pylori infection in mice. Vaccine 19:3927–3935
Li Y, Jiang Y, Xi Y, Zhang L, Luo J, He D, Zeng S, Ning Y (2012) Identification and characterization of H-2d restricted CD4+ T cell epitopes on Lpp20 of Helicobacter pylori. BMC Immunol 13:68
Li Y, Chen Z, Ye J, Ning L, Luo J, Zhang L, Jiang Y, Xi Y, Ning Y (2016) Antibody production and Th1-biased response induced by an epitope vaccine composed of cholera toxin B unit and Helicobacter pylori Lpp20 epitopes. Helicobacter 21:234–248
Mierau I, Kleerebezem M (2005) 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol 68:1–13
Yu W, Zhang Y, Jing J, Liu Z (2010) Construction of Helicobacter pylori Lpp20-IL2 DNA vaccine and evaluation of its immunocompetence in C57BL/6 mice. Wei Sheng Wu Xue Bao 50:554–559
Zeng M, Mao XH, Li JX et al (2015) Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386:1457–1464
Zhang XJ, Duan GC, Zhang RG, Fan QT (2009) Optimized expression of Helicobacter pylori ureB gene in Lactococcus lactis NICE system and experimental study on its immunoreactivity. Current Microbiol 58:308–314
Zhang XJ, Feng SY, Li ZT, Feng YM (2015) Expression of Helicobacter pylori hspA gene in Lactococcus lactis NICE system and experimental study on its immunoreactivity. Gastroenterol Res Pract 2015:750932
Zhang R, Duan G, Shi Q, Chen S, Fan Q, Sun N, Xi Y (2016a) Construction of a recombinant Lactococcus lactis strain expressing a fusion protein of Omp22 and HpaA from Helicobacter pylori for oral vaccine development. Biotechnol Lett. doi:10.1007/s10529-016-2173-5 Epub ahead of print
Zhang RG, Duan GC, Fan QT, Chen SY (2016b) Role of Helicobacter pylori infection in pathogenesis of gastric carcinoma. World J Gastrointest Pathophysiol 7:97–107
Acknowledgments
This study was funded by the Henan Innovation Center of Molecular Diagnosis and Laboratory Medicine (XTCX-2015-ZD2) and the China Postdoctoral Science Foundation (No. 200801273).
Supporting information
Supplementary Table 1—Plasmid vectors and bacterial strains used.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, R., Peng, X., Duan, G. et al. An engineered Lactococcus lactis strain exerts significant immune responses through efficient expression and delivery of Helicobacter pylori Lpp20 antigen. Biotechnol Lett 38, 2169–2175 (2016). https://doi.org/10.1007/s10529-016-2209-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2209-x