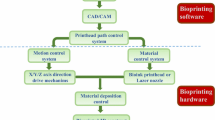
Abstract
With the advances of stem cell research, development of intelligent biomaterials and three-dimensional biofabrication strategies, highly mimicked tissue or organs can be engineered. Among all the biofabrication approaches, bioprinting based on inkjet printing technology has the promises to deliver and create biomimicked tissue with high throughput, digital control, and the capacity of single cell manipulation. Therefore, this enabling technology has great potential in regenerative medicine and translational applications. The most current advances in organ and tissue bioprinting based on the thermal inkjet printing technology are described in this review, including vasculature, muscle, cartilage, and bone. In addition, the benign side effect of bioprinting to the printed mammalian cells can be utilized for gene or drug delivery, which can be achieved conveniently during precise cell placement for tissue construction. With layer-by-layer assembly, three-dimensional tissues with complex structures can be printed using converted medical images. Therefore, bioprinting based on thermal inkjet is so far the most optimal solution to engineer vascular system to the thick and complex tissues. Collectively, bioprinting has great potential and broad applications in tissue engineering and regenerative medicine. The future advances of bioprinting include the integration of different printing mechanisms to engineer biphasic or triphasic tissues with optimized scaffolds and further understanding of stem cell biology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Langer and Vacanti (1993) first defined tissue engineering as an approach of seeding cells to the pre-formed solid and rigid biomaterial scaffolds for tissue fabrication. However, the term ‘tissue engineering’ was introduced even earlier by Dr. Fung of the University of California at San Diego in 1985 (Auger et al. 2013). In the conventional tissue engineering approach, the autologous cells are first cultured as a monolayer to expand the cell numbers. The cultured cells are then collected and seeded into the pre-formed porous scaffolds. The scaffolds used for tissue engineering should be biocompatible and degradable. The seeded cells on the scaffold are kept alive and can penetrate or migrate inside the scaffolds instead of staying on the surface. Therefore, the scaffolds should be highly porous with inter-connected pores and safe to the seeded cells. In addition, a customized bioreactor mimicking in vivo environment and stimulation is usually desired to maturate the fabricated organ construct before implantation. The goal of tissue engineering is to create the replacements for the lost or diseased organs and eventually solve the crisis of organ donor shortage. Some successes have been achieved in engineering thin and hollow organs (Jain et al. 2005; Atala et al. 2006). These tissues can survive in vivo through nutrients diffusion from the host vasculature. However, more than 90 % of organs, such as kidney, liver, and heart, are thick and complex (Matas et al. 2015; Kim et al. 2015; Colvin-Adams et al. 2015). When the size of the engineered tissue exceeds 400 μm in any dimension, it will surpass the O2 diffusion limitation. In this case, functional vasculature must be enabled in the engineered constructs to supply the cells with O2 and nutrients, and also to remove the waste products generated by the tissue (Levenberg et al. 2005).
Unfortunately, the conventional tissue engineering approaches fail to generate these thick, complex and vascularized tissues due to the following limitations:
-
(a)
The effectiveness of cell seeding and penetration to the biomaterial scaffold is still limited. Although scaffold design has been significantly improved to enhance the cell seeding and migration, the uniform of tissue formation or maturation throughout the scaffold is still far from optimal (Ma and Choi 2001; Kang et al. 2012; Hu et al. 2012; Lozano et al. 2015).
-
(b)
Organs with complex structure are usually composed by multiple cell types and biological factors. However, the precise delivery of cells and biological factors to the desired 3D positions is still far from being resolved.
-
(c)
Thick tissues possess complex vascular system (Nerem and Seliktar 2001), which should be enabled within the scaffold. However, the conventional tissue engineering approach has difficulties to construct vascular system within the pre-formed 3D scaffolds.
Additive manufacturing or 3D-printing is driving significant innovations in manufacturing, engineering, education and medicine. 3D-Bioprinting, which was derived by combing biotechnology and 3D-printing, promises to solve the critical issues mentioned above. As one of the most advanced enabling technology in tissue engineering, 3D-bioprinting combines solid free-form fabrication and precise placement of cells and other biological factors to the desired 2D and 3D positions. It is described as a precise approach of delivering biomaterials, cells and supporting biological factors to the targeted locations with spatial control to fabricate functional 3D constructs. The key elements of realizing functional bioprinting include the capacity of precise positioning, printable biomaterials, and cell sources. In addition, vascularization, innervation, and maturation are also crucial to engineer functional tissues. Bioprinting has promising applications in the field of regenerative medicine, personalized medicine, clinical diagnosis and medicinal development. Although the concept of bioprinting was introduced more than 10 years ago, the current progress of bioprinting is still in its initial stage and far from industrial applications.
The three most common bioprinting mechanisms are inkjet bioprinting (Cui and Boland 2008, 2009; Cui et al. 2010, 2011, 2012a, b, c, d, 2013, 2014; Gao et al. 2014, 2015a, b), extrusion bioprinting (Cohen et al. 2006; Iwami et al. 2010; Shor et al. 2009; Hsieh et al. 2015), and laser bioprinting (Barron et al. 2004; Guillemot et al. 2010a, b; Zhang et al. 2015).
Extrusion bioprinting is a contact printing process and typically uses temperature-controlled polymerized materials for scaffold fabrication. This printing process usually causes high cell casualty so it is frequently used in acellular material printing. Sometimes extrusion bioprinting also applies in cell spheroids deposition. This approach does not demand high printing resolution and it is more likely a dispensing process instead of printing. In addition, this approach has difficulties of managing singe cell which is critically important for neuron regeneration or fabricating functional tissues with higher degree of cell organization of specific anatomic structures (Mironov et al. 2009; Moon et al. 2010). Laser bioprinting offers higher cell viability and printing resolution. Instead of moving cells directly, laser bioprinting uses laser energy to vaporize the solution of biological samples and eject the remaining substances (Barron et al. 2004; Zhang et al. 2015). This approach may cause over-drying leading to the failure for biological systems. Furthermore, the much higher cost of laser printing equipment, as well as the exceedingly low printing efficiency inhibit its application in regenerative medicine (Odde and Renn 1999, 2000; Zhang et al. 2015). Thus, it is mostly applied in the basic research field when single or multiple cell manipulation is needed, instead of tissue construction or other clinical applications demanding higher throughput.
Inkjet printing is also known as drop-on-demand printing. It is a non-contact printing technology that reproduces digital patterns onto a substrate using tiny ink drops (Mohebi and Evans 2002). Inkjet printing is based on thermal, piezoelectric, or electromagnetic mechanisms (Beeson 1998). In thermal inkjet printers, small air bubbles generated by heating in the printhead collapse to provide pressure pulses to eject tiny drops out of the nozzle (Hock et al. 1996; Canfield et al. 1997; Hudson et al. 2000). The droplet size varies from 10 to 150 pl, which is determined by the applied temperature gradient, frequency of current pulses, and viscosity of the ink (Hock et al. 1996; Canfield et al. 1997; Hudson et al. 2000).
As for piezoelectric inkjet printers, the actuator of polycrystalline piezoelectric ceramic in each nozzle provides the transient pressure to eject the ink drops (de Jong et al. 2006). These printing technologies have already been widely used in printing electronic materials and complex integrated circuits in industry (Sirringhaus et al. 2000). Although biological substances are usually considered sensitive, fragile DNA molecules have been directly printed using commercially-available inkjet printers for high-density DNA microarray fabrication (Okamoto et al. 2000; Goldmann and Gonzalez 2000).
Challenges still exist when printing cells using inkjet technology. The working frequency of piezoelectric inkjet printers is 15–25 kHz, which is within the well-documented sonification damage to the cell membrane (Seetharam and Sharma 1991). Although the heating element in thermal inkjet printers raises the local temperature to 300 °C (Hudson et al. 2000), the ejected mammalian cells are only heated for 2 µs with a temperature raise of 4–10 °C above ambient and an average cell viability over 90 % (Cui et al. 2010). In addition, the development, operation, and maintenance of thermal inkjet is usually more convenient than piezoelectric printing. Therefore, the majority successes in tissue bioprinting are based on thermal inkjet printing instead of piezoelectric inkjet printing.
One limitation of inkjet bioprinting is the strict requirement of bioink viscosity. This issue has recently been minimized by using water based biomaterials or combination of various printing technologies. Water-based bioinks allow the printer to freely deliver cells from single to multiple cells by simply adjusting the bioink concentration and the digital patterns. Cells are usually well-protected in the aqueous environment during the printing process therefore it is assumed to be the safest strategy to deliver living systems.
Based on the discussion above, bioprinting based on thermal inkjet printing is so far the most appropriate approach for regenerative medicine and tissue engineering applications. Researchers keep developing this technology as an optimal approach for cell delivery and scaffold fabrication. Therefore, we will mainly focus on the advancement and applications using this bioprinting technology in this review.
Cell printing
Although the term of bioprinting can be used on printing any biological systems, it usually involves living cell patterning in tissue engineering and regenerative medicine applications. Therefore, the capacity of printing living cells is critical to evaluate a bioprinting platform or system.
Although bioprinting based on thermal inkjet printing technology has many successful applications, there were concerns that the printing process may cause damage or cell death. A small print-head nozzle size is necessary for high printing resolution. Due to the thermal heat and mechanical stresses applied to the cells during printing, it is possible that the cells may be damaged or their phenotype may be altered (Tirella et al. 2011). Therefore, a comprehensive evaluation of cell viability, apoptosis, heat shock proteins production, cell membrane damages of the printed cells is desired to confirm the bioprinting safety. Using a modified Hewlett-Packard thermal inkjet printer, cell viability at various cell concentrations was between 85 and 95 %. No significant difference in apoptosis and heat shock protein expression was observed between printed and non-printed cells (Cui et al. 2010). Quantitative cell seeding can be achieved by adjusting the cell concentration in bioink. The inkjet printing process does alter the cell membrane of printed cells. Fluorescent labeled dextran dye with molecular weight (MW) up to 40,000 can penetrate into the printed cells. No dye was found in the non-printed cells even with the lowest MW (3000). The cell membrane pore size was estimated as 105 Å according to the Stokes diameter of these dye molecules (Cui et al. 2010).
The pores developed during printing were transient and can be repaired by the cells in just a couple hours. The transient nature of the cell membrane pores as well as the self-repair mechanism can be utilized for targeted gene delivery during the printing process (Cui et al. 2010).
Microvasculature printing
Although the concept of tissue engineering was introduced more than two decades ago, the current tissue engineering strategies still cannot create fully vascularized tissue constructs. The current tissue engineering paradigm is that successfully engineered thick tissues must include vasculature. As biological approaches alone, such as VEGF or co-culture of vessel cells, have fallen short of their promises, one may look for an engineering approach to build microvasculature. Layer-by-layer approaches for customized fabrication of cell and scaffold constructs have shown great potential in building complex 3D structures (Catros et al. 2012). With the advent of cell printing, one may be able to build precise human microvasculature with suitable bioink. Human microvascular endothelial cells (HMVECs) and fibrin scaffold were utilized as bioink for microvasculature construction (Cui and Boland 2009).
A standard inkjet printer was modified to simultaneously deposit HMVECs and fibrin scaffold to form the microvasculature. The bioink and biopaper components for fibrin bioprinting were carefully evaluated for optimal condition of simultaneous deposition of cells and scaffold (Cui and Boland 2009). The printed microvasculature was incubated for 10–15 min after the printing to finalize the crosslinking and enhance the cell attachment. After 3 weeks in culture, the printed HMVECs aligned themselves in the fibrin channel and proliferated to form a confluent lining. Confocal laser scanning images at the z-axis demonstrated tubular structure of the printed human microvasculature. The endothelial cells were forming a vessel-like structure in the printed fibrin channel (Cui and Boland 2009). This demonstrates the printed and proliferated endothelial cells possessed the crucial angiogenesis function. The simultaneous deposition of endothelial cells and fibrin using thermal inkjet printing technology can be used for human microvasculature fabrication.
Muscle printing
Biological microelectromechanical system (Bio-MEMS) devices conjugated with biological components are promising for the development of novel bioengineering microdevices, such as motors and actuators (Xi et al. 2005), heart pumps (Tanaka et al. 2007), and biosensors (Harms et al. 2006). Muscle cells have been widely used in these applications by generating force activated by actin-myosin motors regulated by excitation–contraction coupling (Bers 2002). These muscle powered microdevices utilizing energy generated by biochemical reaction are promising to save energy, resources, and spaces (Asano et al. 2012). C2C12 skeletal muscle cells possess the advantages of infinite proliferation and differentiation into multinucleated myotubes (Yaffe and Saxel 1977). As a well established cell line, the overall properties of C2C12 cells cultured and differentiated in vitro have been tested to closely mimic the properties of skeletal muscle in vivo (Miller 1990).
Although C2C12 cells have been widely used to incorporate with bio-microdevices for many applications, it is important that the muscle cells and microdevices are consistently conjugated to produce reliable and reproducible results. The traditional methodology for Bio-MEMS fabrication is to manually seed cells on or into the microdevices (Fujita et al. 2010). However, the randomly deposited cells through this approach were uneven and further affected the cell proliferation and differentiation. Therefore, it is critical to incorporate a precise cell seeding technology to develop the Bio-MEMS constructs with consistent cell arrangement.
Bioprinting was able to print and align C2C12 cells onto the tiny cantilevers at a resolution at 300 dpi (85 μm). In order to control cell proliferation and differentiation with minimal variations, the same amount of cells was printed to evenly cover each cantilever of the microdevices. The viability of printed C2C12 cells was 91.2 ± 2.6 % and the printed cells aligned closely with each other forming confluent myotubes on almost all the cantilevers. Conjugated myotube and cantilever constructs responded synchronously to the electric pulses of 2 V with 40 ms duration up to 5 Hz (Cui et al. 2013). This showed the bioprinted microdevices possessed equal or even better physiological properties comparing to the conventionally fabricated constructs in term of the spontaneous responses to the stimulation with significantly less culture time. Moreover, the bioprinted myotubes can also be used for muscle exercise studies with electric stimulations at various frequencies, which demonstrates the versatility of this work.
Cartilage printing
Cartilage defects resulting from osteoarthritis, aging, and joint injury are a major cause of joint pain and chronic disability (Mow and Hayes 1997). Mature cartilage cannot heal spontaneously because of its avascular, aneural, and alymphatic nature. The most common clinical treatments for cartilage repair include microfracture, osteochondral transfer, and autologous chondrocyte implantation. All these invasive and complicated treatments are still not able to restore the long lasting healthy cartilage (Rasanen et al. 2007). Although articular cartilage was predicted to be one of the first tissues to be successfully engineered (Brittberg et al. 1994), the current cartilage tissue engineering strategies still cannot fabricate new tissue that is indistinguishable from native cartilage with respect to the zonal organization, extracellular matrix (ECM) composition, and mechanical properties (Hunziker 2002). In addition, most current cartilage repair strategies involve removing healthy cartilage tissue around the lesion site to create artificial defects for further treatment (Kalson et al. 2010). This procedure in fact causes additional necrosis to the existing healthy cartilage and leads to ultimate cartilage degeneration and failure of implanted tissue (Shapiro et al. 1993).
Inkjet bioprinting is able to directly repair cartilage tissue with closely mimicked native cartilage anatomy to the lesion site without additional damage. The ideal implanted tissue is expected to integrate with existing native cartilage and to repair lesions of different sizes and thicknesses. The multifaceted nature of this challenge requires a technique adaptable to variable physical dimensions and properties for tissue repair; bioprinting technology, based on inkjet printing, provides the necessary capabilities.
A standard thermal inkjet printer was modified to precisely deposit human articular chondrocytes and poly(ethylene) glycol dimethacrylate (PEGDMA; MW, 3400) layer-by-layer into a cartilage defect within an osteochondral plug for cartilage repair. For a representative defect of 4 mm diameter and cartilage thickness of 2 mm, a nominal 0.23 µl bioink, estimated to contain 1140 human chondrocytes (5 × 106 cells/ml) was printed and photopolymerized for each layer to repair the cartilage defect in a layer-by-layer assembly. The thickness of each printed layer was about 18 μm. Total firing time of printhead was 1.1 s and the whole printing process completed less than 2 min. Compared to manual zonal cartilage fabrication which requires at least 11 min for UV exposure (Kim et al. 2003), bioprinting reduced UV exposure to the cells by 80 %. The viability of human chondrocytes printed with simultaneous photopolymerization increased 40 % than that when exposed to the same UV light source continuously for 10 min in manual fabrication (Cui et al. 2012b).
Printed cartilage implant attached firmly with existing tissue and greater proteoglycan deposition was also observed at the interface of implant and native cartilage. Printed cartilage in 3D biopaper had elevated glycosaminoglycan (GAG) content comparing to that without biopaper. This study indicates the importance and feasibility of direct cartilage repair with the bioprinting successfully controlling placement of individual cells, preserving cell viability, maintaining chondrogenic phenotype, and demonstrating integration with host tissue (Di et al. 2015).
Bone printing
Although bone is well known for its self-healing capacities (Mourino and Boccaccini 2010), the body cannot completely heal a bone defect without intervention when it is beyond the critical size (Jones et al. 2007; Seitz et al. 2005). Large-scale bone loss resulting from tumor resections and high impact trauma is the major cause for bone repair and implantation in clinic. The availability and functionality of bone autografts and allografts are limited to restore the normal operations. The inert implants fail over time due to repetitive loading. Therefore, tissue-engineered bone which can ideally be remodeled into new bone to restore, maintain or improve its functions is becoming increasingly attractive (Rezwan et al. 2006).
Thermal inkjet bioprinting has been developed as an enabling technology to simultaneously deposit cells, growth factors, and biomaterial scaffolds to the desired 2D and 3D locations (Cui and Boland 2008, 2009; Cui et al. 2010, 2011, 2012a, b, c, d, 2013, 2014). The drops of ejected ink coming through the nozzles are smaller than 0.03 mm diameter, which guarantees excellent printing resolution (Hock et al. 1996; Canfield et al. 1997). Many inkjet printed scaffolds were natural hydrogels for the enhanced biocompatibility to the cells (Cui and Boland 2008, 2009; Boland et al. 2006a, b; Deitch et al. 2008). These scaffolds usually lacked mechanical strength due to the properties of material and crosslinking methods, limiting their applications to soft tissues. Bone grafts have also been created using natural hydrogels such as fibrin or alginate (Catelas et al. 2006; Spalazzi et al. 2006, 2008; Khanarian et al. 2011). Although the cells proliferated and differentiated well in these natural hydrogels, the compressive modulus of these scaffolds is less than 5 kPa even after 4 weeks in culture, which is not ideal for bone tissue engineering (Spalazzi et al. 2006, 2008; Khanarian et al. 2011).
A 3D-bioprinting platform with simultaneous photopolymerization using a synthetic polymeric hydrogel was developed. The compressive modulus of the printed PEGDMA using layer-by-layer assembly exceeded 500 kPa, which is 100 times more than the compressive modulus of the natural hydrogels (Cui et al. 2012b, c) and is of the same order of magnitude as human musculoskeletal tissue (Hoenig et al. 2011). In addition, PEG hydrogel has been demonstrated to maintain cell viability and promote ECM production (Cui et al. 2012b, c; Bryant and Anseth 2002; Elisseeff et al. 2000).
Bone marrow-derived stem cells can migrate to the skeletal sites, proliferate and differentiate at the local injured area. Isolated human mesenchymal stem cells (hMSCs) can maintain their osteogenic potential during monolayer cell expansion in vitro (Bruder et al. 1997). These cells are therefore commonly used to reconstruct skeletal tissues in orthopedic tissue engineering (Caplan and Bruder 2001; Triffitt 2002; Oreffo and Triffitt 1999). hMSCs isolated from bone marrow or adipose tissue can be induced for osteogenic differentiation and form bone tissue when stimulated by ceramic scaffold (Leboy et al. 1991; Rickard et al. 1996; Kon et al. 2000). Bioactive glass (BG) and hydroxyapatite (HA) also can promote bone tissue formation (Jiang et al. 2010; Khanarian et al. 2011).
In bone printing, the approaches mentioned above were integrated into a novel bioprinting setup in which hMSCs and PEGDMA combined with BG or HA or both BG and HA nanoparticles were simultaneously printed to form the homogeneous bone constructs in a layer-by-layer approach. Biochemical analysis showed significantly higher total collagen production and alkaline phosphatase (ALP) activity in hMSCs printed within PEG-HA scaffold. The higher collagen production in PEG-HA scaffold was also observed in histology studies, which was consistent with the previous work by Patel et al. (2010) that HA presence increased cell ALP activity and promoted osteogenesis. Collectively, HA in PEG hydrogel maintained hMSCs viability, promoted hMSCs osteogenic differentiation and biosynthetic function.
This work demonstrates the feasibility of fabricating a neobone tissue by delivering hMSCs and osteogenic factors, such as HA and BG nanoparticles, in a strong PEG scaffold for bone tissue engineering. Using layer-by-layer assembly, the deposited hMSCs were fixed at their initially deposited positions using simultaneous photopolymerization with reduced phototoxicity. HA in the scaffold significantly stimulated hMSCs osteogenic differentiation as well as osteogenic ECM production with minimal cell toxicity. Combined with the previous success in cartilage bioprinting (Cui et al. 2012b), it is promising to construct an osteochondral interface, which is one of the most important and difficult subjects in bone tissue engineering (Hunziker and Driesang 2003).
The future
Taken together, bioprinting based on thermal inkjet printing demonstrates the feasibility of printing living systems and the flexibility of printing various subjects from soft to hard tissues with minimal side effects. In fact, the benign effects to the printed cells can be used for many other attractive applications, such as gene transfection and targeted drug delivery. The bioprinting system is versatile for 2D and 3D tissue application as well as avascular and vascular tissue construction. One promising clinical application is to develop a hand-held printer or print-head with digital control for direct tissue repair. By using 3D reconstructions of scanned lesions, bioprinting is able to precisely deliver cells, growth factors, and biomaterial scaffolds to repair the lesion with various shape and thickness. One promising direction is to combine the bioprinting approaches based on various mechanisms to meet the different challenges. Ultimately, the successful application in microvasculature fabrication also revealed the bioprinting may be the only solution to engineer thick and complex tissues with fully functional vasculature and innervation.
References
Asano T, Ishizua T, Yawo H (2012) Optically controlled contraction of photosensitive skeletal muscle cells. Biotechnol Bioeng 109:199–204
Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB (2006) Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367:1241–1246
Auger FA, Gibot L, Lacroix D (2013) The pivotal role of vascularization in tissue engineering. Annu Rev Biomed Eng 15:177–200
Barron JA, Wu P, Ladouceur HD, Ringeisen BR (2004) Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed Microdevices 6:139–147
Beeson R (1998) Thermal (TIJ) or piezo? Who cares? IMI 7th annual ink jet printing conference
Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415:198–205
Boland T, Cui X, Aho M, Baicu C, Zile M (2006a) Image based printing of structured biomaterials for realizing complex 3D cardiovascular constructs. J Imaging Sci Technol 2:86–88
Boland T, Xu T, Damon B, Cui X (2006b) Application of inkjet printing to tissue engineering. Biotechnol J 1:910–917
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Bruder SP, Jaiswal N, Haynesworth SE (1997) Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem 64:278–294
Bryant SJ, Anseth KS (2002) Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res 59:63–72
Canfield B, Clayton H, Yeung KWW (1997) Method and apparatus for reducing the size of drops ejected from a thermal ink jet printhead. (US5673069)
Caplan AI, Bruder SP (2001) Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 7:259–264
Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B (2006) Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng 12:2385–2396
Catros S, Guillemot F, Nandakumar A, Ziane S, Moroni L, Habibovic P, van Blitterswijk C, Rousseau B, Chassande O, Amedee J, Fricain JC (2012) Layer-by-layer tissue microfabrication supports cell proliferation in vitro and in vivo. Tissue Eng Part C 18:62–70
Cohen DL, Malone E, Lipson H, Bonassar LJ (2006) Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng 12:1325–1335
Colvin-Adams M, Smith JM, Heubner BM, Skeans MA, Edwards LB, Waller CD, Callahan ER, Snyder JJ, Israni AK, Kasiske BL (2015) OPTN/SRTR 2013 annual data report: heart. Am J Transpl 15(S2):1–28
Cui X, Boland T (2008) Simultaneous deposition of human microvascular endothelial cells and biomaterials for human microvasculature fabrication using inkjet printing. NIP24/digital fabrication 2008: 24th international conference on digital printing technologies. Tech Prog Proc 24:480–483
Cui X, Boland T (2009) Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 30:6221–6227
Cui X, Dean D, Ruggeri ZM, Boland T (2010) Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol Bioeng 106:963–969
Cui X, Breitenkamp K, Finn MG, Lotz M, Colwell CW Jr (2011) Direct human cartilage repair using thermal inkjet printing technology. Osteoarthr Cartil 19:S47–S48
Cui X, Boland T, D’Lima DD, Lotz MK (2012a) Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul 6:149–155
Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD (2012b) Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 18:1304–1312
Cui X, Breitenkamp K, Lotz M, D’Lima D (2012c) Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation. Biotechnol Bioeng 109:2357–2368
Cui X, Hasegawa A, Lotz M, D’Lima D (2012d) Structured three-dimensional co-culture of mesenchymal stem cells with meniscus cells promotes meniscal phenotype without hypertrophy. Biotechnol Bioeng 109:2369–2380
Cui X, Gao G, Qiu Y (2013) Accelerated myotube formation using bioprinting technology for biosensor applications. Biotechnol Lett 35:315–321
Cui X, Gao G, Yonezawa T, Dai G (2014) Human cartilage tissue fabrication using three-dimensional inkjet printing technology. J Vis Exp 88:e51294. doi:10.3791/51294
de Jong J, de Bruin G, Reinten H, van den Berg M, Wijshoff H, Versluis M, Lohse D (2006) Air entrapment in piezo-driven inkjet printheads. J Acoust Soc Am 120:1257–1265
Deitch S, Kunkle C, Cui X, Boland T, Dean D (2008) Collagen matrix alignment using inkjet printer technology. Mater Res Soc Symp Proc 1094:52–57
Di BC, Fosang A, Donati DM, Wallace GG, Choong PF (2015) 3D-bioprinting of cartilage for orthopedic surgeons: reading between the Lines. Front Surg 2:39
Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R (2000) Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res 51:164–171
Fujita H, Shimizu K, Nagamori E (2010) Novel method for measuring active tension generation by C2C12 myotube using UV-crosslinked collagen film. Biotechnol Bioeng 106:482–489
Gao G, Schilling AF, Yonezawa T, Wang J, Dai G, Cui X (2014) Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J 9:1304–1311
Gao G, Schilling AF, Hubbell K, Yonezawa T, Truong D, Hong Y, Dai G, Cui X (2015a) Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol Lett. doi:10.1007/s10529-015-1921-2
Gao G, Yonezawa T, Hubbell K, Dai G, Cui X (2015b) Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol J. doi:10.1002/biot.201400635
Goldmann T, Gonzalez JS (2000) DNA-printing: utilization of a standard inkjet printer for the transfer of nucleic acids to solid supports. J Biochem Biophys Methods 42:105–110
Guillemot F, Souquet A, Catros S, Guillotin B (2010a) Laser-assisted cell printing: principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine (Lond) 5:507–515
Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, Pippenger B, Bareille R, Remy M, Bellance S, Chabassier P, Fricain JC, Amedee J (2010b) High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater 6:2494–2500
Harms H, Wells MC, van der Meer JR (2006) Whole-cell living biosensors–are they ready for environmental application? Appl Microbiol Biotechnol 70:273–280
Hock SW, Johnson DA, Van Veen MA (1996). Print quality optimization for a color ink-jet printer by using a larger nozzle for the black ink only. US Patent: 5521622
Hoenig E, Winkler T, Mielke G, Paetzold H, Schuettler D, Goepfert C, Machens HG, Morlock MM, Schilling AF (2011) High amplitude direct compressive strain enhances mechanical properties of scaffold-free tissue-engineered cartilage. Tissue Eng Part A 17:1401–1411
Hsieh FY, Lin HH, Hsu SH (2015) 3D-bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 71:48–57
Hu C, Uchida T, Tercero C, Ikeda S, Ooe K, Fukuda T, Arai F, Negoro M, Kwon G (2012) Development of biodegradable scaffolds based on magnetically guided assembly of magnetic sugar particles. J Biotechnol 159:90–98
Hudson KR, Cowan PB, Gondek JS (2000). Ink drop volume variance compensation for inkjet printing. US Patent: 6042211
Hunziker EB (2002) Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil 10:432–463
Hunziker EB, Driesang IM (2003) Functional barrier principle for growth-factor-based articular cartilage repair. Osteoarthr Cartil 11:320–327
Iwami K, Noda T, Ishida K, Morishima K, Nakamura M, Umeda N (2010) Bio rapid prototyping by extruding/aspirating/refilling thermoreversible hydrogel. Biofabrication 2:014108
Jain RK, Au P, Tam J, Duda DG, Fukumura D (2005) Engineering vascularized tissue. Nat Biotechnol 23:821–823
Jiang J, Tang A, Ateshian GA, Guo XE, Hung CT, Lu HH (2010) Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng 38:2183–2196
Jones AC, Arns CH, Sheppard AP, Hutmacher DW, Milthorpe BK, Knackstedt MA (2007) Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials 28:2491–2504
Kalson NS, Gikas PD, Briggs TWR (2010) Current strategies for knee cartilage repair. Int J Clin Pract 64:1444–1452
Kang HW, Park JH, Kang TY, Seol YJ, Cho DW (2012) Unit cell-based computer-aided manufacturing system for tissue engineering. Biofabrication 4:015005
Khanarian NT, Jiang J, Wan LQ, Mow VC, Lu HH (2011) A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng Part A 18(5–6):533–545
Kim TK, Sharma B, Williams CG, Ruffner MA, Malik A, McFarland EG, Elisseeff JH (2003) Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthr Cartil 11:653–664
Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Synder JJ, Israni AK, Kasiske BL (2015) OPTN/SRTR 2013 annual data report: liver. Am J Transpl 15(S2):1–28
Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, Giardino R, Cancedda R, Quarto R (2000) Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res 49:328–337
Langer R, Vacanti JP (1993) Tissue engineering. Science 260:920–926
Leboy PS, Beresford JN, Devlin C, Owen ME (1991) Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol 146:370–378
Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R (2005) Engineering vascularized skeletal muscle tissue. Nat Biotechnol 23:879–884
Lozano R, Stevens L, Thompson BC, Gilmore KJ, Gorkin R, Stewart EM, in het Panhuis M, Romero-Ortega M, Wallace GG (2015) 3D-printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 67:264–273
Ma PX, Choi JW (2001) Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng 7:23–33
Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Israni AK, Kasiske BL (2015) OPTN/SRTR 2013 annual data report: kidney. Am J Transpl 15(S2):1–34
Miller JB (1990) Myogenic programs of mouse muscle cell lines: expression of myosin heavy chain isoforms, MyoD1, and myogenin. J Cell Biol 111:1149–1159
Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR (2009) Organ printing: tissue spheroids as building blocks. Biomaterials 30:2164–2174
Mohebi MM, Evans JRG (2002) A drop-on-demand ink-jet printer for combinatorial libraries and functionally graded ceramics. J Comb Chem 4:267–274
Moon S, Hasan SK, Song YS, Xu F, Keles HO, Manzur F, Mikkilineni S, Hong JW, Nagatomi J, Haeggstrom E, Khademhosseini A, Demirci U (2010) Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng Part C 16:157–166
Mourino V, Boccaccini AR (2010) Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J R Soc Interface 7:209–227
Mow VC, Hayes WC (1997) Basic orthopaedic biomechanics. Lippincott Williams & Wilkins, Philadelphia, p 275
Nerem RM, Seliktar D (2001) Vascular tissue engineering. Annu Rev Biomed Eng 3:225–243
Odde DJ, Renn MJ (1999) Laser-guided direct writing for applications in biotechnology. Trends Biotechnol 17:385–389
Odde DJ, Renn MJ (2000) Laser-guided direct writing of living cells. Biotechnol Bioeng 67:312–318
Okamoto T, Suzuki T, Yamamoto N (2000) Microarray fabrication with covalent attachment of DNA using bubble jet technology. Nat Biotechnol 18:438–441
Oreffo RO, Triffitt JT (1999) Future potentials for using osteogenic stem cells and biomaterials in orthopedics. Bone 25:5S–9S
Patel M, Patel KJ, Caccamese JF, Coletti DP, Sauk JJ, Fisher JP (2010) Characterization of cyclic acetal hydroxyapatite nanocomposites for craniofacial tissue engineering. J Biomed Mater Res A 94:408–418
Rasanen P, Paavolainen P, Sintonen H, Koivisto AM, Marja B, Ryynanen OP, Roine RP (2007) Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop 78:108–115
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR (2006) Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27:3413–3431
Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL (1996) Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res 11:312–324
Seetharam R, Sharma SK (1991) Purification and analysis of recombinant proteins. Marcel Dekker, New York, p 69
Seitz H, Rieder W, Irsen S, Leukers B, Tille C (2005) Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B 74:782–788
Shapiro F, Koide S, Glimcher MJ (1993) Cell origin and differentiation in the repair of full-thickness defects of articular-cartilage. J Bone Joint Surg Am 75(4):532–553
Shor L, Guceri S, Chang R, Gordon J, Kang Q, Hartsock L, An Y, Sun W (2009) Precision extruding deposition (PED) fabrication of polycaprolactone (PCL) scaffolds for bone tissue engineering. Biofabrication 1:015003
Sirringhaus H, Kawase T, Friend RH, Shimoda T, Inbasekaran M, Wu W, Woo EP (2000) High-resolution inkjet printing of all-polymer transistor circuits. Science 290:2123–2126
Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH (2006) Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng 12:3497–3508
Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH (2008) In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res A 86:1–12
Tanaka Y, Sato K, Shimizu T, Yamato M, Okano T, Kitamori T (2007) A micro-spherical heart pump powered by cultured cardiomyocytes. Lab Chip 7:207–212
Tirella A, Vozzi F, De MC, Vozzi G, Sandri T, Sassano D, Cognolato L, Ahluwalia A (2011) Substrate stiffness influences high resolution printing of living cells with an ink-jet system. J Biosci Bioeng 112:79–85
Triffitt JT (2002) Osteogenic stem cells and orthopedic engineering: summary and update. J Biomed Mater Res 63:384–389
Xi J, Schmidt JJ, Montemagno CD (2005) Self-assembled microdevices driven by muscle. Nat Mater 4:180–184
Yaffe D, Saxel O (1977) A myogenic cell line with altered serum requirements for differentiation. Differentiation 7:159–166
Zhang Z, Xiong R, Mei R, Huang Y, Chrisey DB (2015) Time-resolved imaging study of jetting dynamics during laser printing of viscoelastic alginate solutions. Langmuir 31:6447–6456
Acknowledgements
This work was funded by the Fundamental Research Funds for the Central Universities (WUT: 2015IB004), NSF 1011796, New York Capital Region Research Alliance grant, and Stemorgan Therapeutics R&D support (TERM002). The authors indicate no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, G., Cui, X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol Lett 38, 203–211 (2016). https://doi.org/10.1007/s10529-015-1975-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1975-1