Abstract
With the advances of stem cell research, development of intelligent biomaterials and three-dimensional biofabrication strategies, highly mimicked tissue or organs can be engineered. Among all the biofabrication approaches, bioprinting based on inkjet printing technology has the promises to deliver and create biomimicked tissue with high throughput, digital control, and the capacity of single cell manipulation. Therefore, this enabling technology has great potential in regenerative medicine and translational applications. The most current advances in organ and tissue bioprinting based on the thermal inkjet printing technology are described in this chapter, including vasculature , muscle , cartilage , and bone . In addition, the benign side effect of bioprinting to the printed mammalian cells can be utilized for gene or drug delivery, which can be achieved conveniently during precise cell placement for tissue construction. With layer-by-layer assembly, three-dimensional tissues with complex structures can be printed using converted medical images. Therefore, bioprinting based on thermal inkjet is so far the most optimal solution to engineer vascular system to the thick and complex tissues. Collectively, bioprinting has great potential and broad applications in tissue engineering and regenerative medicine. The future advances of bioprinting include the integration of different printing mechanisms to engineer biphasic or triphasic tissues with optimized scaffolds and further understanding of stem cell biology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Stem cell
- Bioprinting
- Additive manufacturing
- Tissue engineering
- Regenerative medicine
- Biomaterials
- Vasculature
- Muscle
- Cartilage
- Bone
1 Introduction
In 1993, Langer and Vacanti first defined tissue engineering as an approach of seeding cells to the pre-formed solid and rigid biomaterial scaffolds for tissue fabrication [1]. However, the term of tissue engineering was introduced even earlier by Dr. Fung of the University of California at San Diego in 1985 [2]. In conventional tissue engineering approach, the autologous cells are first cultured in monolayer to expand the cell numbers. The cultured cells are then collected and seeded into the pre-formed porous scaffolds. The scaffolds used for tissue engineering should be biocompatible and degradable. The seeded cells on the scaffold are kept alive and can penetrate or migrate inside the scaffolds instead of staying on the surface. Therefore, the tissue engineering scaffolds should be highly porous with inter-connected pores and safe to the seeded cells. In addition, a customized bioreactor mimicking in vivo environment and stimulation is usually desired to maturate the fabricated organ construct before implantation. The goal of tissue engineering is to create the replacements for the lost or diseased organs and eventually solve the crisis of organ donor shortage. Some successes have been achieved in engineering thin and hollow organs [3, 4]. These tissues can survive in vivo through nutrients diffusion from the host vasculature . However, more than 90 % demanding organs are thick and complex, such as kidney, liver, and heart (OPTN & SRTR Annual Data Report 2010). When the size of engineered tissue exceeds 400 μm in any dimension, it will surpass the oxygen diffusion limitation. In this case, functional vasculature must be enabled in the engineered constructs to supply the cells with oxygen and nutrients, and also to remove the waste products generated by the tissue [5]. Unfortunately, the conventional tissue engineering approaches failed to generate these thick, complex and vascularized tissues due to these limitations:
-
a.
The effectiveness of cell seeding and penetration to the biomaterial scaffold is still limited. Although scaffold design has been significantly improved to enhance the cell seeding and migration, the uniform of tissue formation or maturation throughout the scaffold is still far from optimal [6–8].
-
b.
Organs with complex structure are usually composed by multiple cell types and biological factors. However, the precise delivery of cells and biological factors to the desired 3D positions is still far from being resolved.
-
c.
Thick tissues possess complex vascular system [9], which should be enabled within the scaffold. However, the conventional tissue engineering approach has difficulties to construct vascular system within the pre-formed 3D scaffolds.
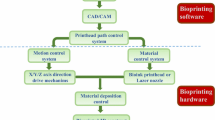
Additive manufacturing or 3D printing is driving significant innovations in manufacturing, engineering, education and medicine. 3D bioprinting , which was derived by combing biotechnology and 3D printing, is promising to solve these critical issues mentioned above. As one of the most advanced enabling technology in tissue engineering, 3D bioprinting combines solid freeform fabrication and precise placement of cells and other biological factors to the desired 2D and 3D positions. It is described as a precise approach of delivering biomaterials, cells and supporting biological factors to the targeted locations with spatial control to fabricate functional 3D constructs. The key elements of realizing functional bioprinting include capacity of precise positioning, printable biomaterials, and cell sources. In addition, vascularization, innervation, and maturation are also crucial to engineer functional tissues. Bioprinting has promising applications in the field of regenerative medicine , personalized medicine , clinical diagnosis and medicinal development. Although the concept of bioprinting was introduced more than 10 years ago, the current progress of bioprinting is still in its initial stage and far from industrial applications.
The three most common bioprinting mechanisms are inkjet bioprinting [10–21], extrusion bioprinting [22–24], and laser bioprinting [25–27]. Extrusion bioprinting is a contact printing process and typically uses temperature-controlled polymerized materials for scaffold fabrication. This printing process usually causes high cell casualty so it is frequently used in acellular material printing. Sometimes extrusion bioprinting also applies in cell spheroids deposition. This approach does not demand high printing resolution and it is more likely a dispensing process instead of printing. In addition, this approach has difficulties of managing singe cell which is critically important for neuron regeneration or fabricating functional tissues with higher degree of cell organization of specific anatomic structures [28, 29]. Laser bioprinting offers higher cell viability and printing resolution. Instead of moving cells directly, laser bioprinting uses laser energy to vaporize the solution of biological samples and eject the remaining substances [25]. This approach may cause over-drying leading to the failure for biological systems. Furthermore, the much higher cost of laser printing equipment, as well as the exceedingly low printing efficiency inhibit its application in regenerative medicine [30, 31] . Thus it is mostly applied in the basic research field when single or multiple cell manipulation is needed, instead of tissue construction or other clinical applications demanding higher throughput.
Inkjet printing is also known as drop-on-demand printing. It is a non-contact printing technology that reproduces digital patterns onto a substrate using tiny ink drops [32]. Inkjet printing is based on thermal, piezoelectric, or electromagnetic mechanisms [33]. In thermal inkjet printers, small air bubbles generated by heating in the printhead collapse to provide pressure pulses to eject tiny drops out of the nozzle [34–36]. The droplet size varies from 10 to 150 pL, which is determined by the applied temperature gradient, frequency of current pulses, and viscosity of the ink [34–36]. As for the piezoelectric inkjet printers, the actuator of polycrystalline piezoelectric ceramic in each nozzle provides the transient pressure to eject the ink drops [37]. These printing technologies have already been widely used in printing electronic materials and complex integrated circuits in industry [38]. Although biological substances are usually considered sensitive, fragile DNA molecules have been directly printed using commercially available inkjet printers for high-density DNA microarray fabrication [39, 40]. Challenges still exist when printing cells using inkjet technology. The working frequency of piezoelectric inkjet printers is 15–25 kHz, which is within the well-documented sonification damage to the cell membrane [41]. Although the heating element in thermal inkjet printers raises the local temperature to 300 °C [36], the ejected mammalian cells are only heated for 2 µs with a temperature raise of 4–10 °C above ambient and an average cell viability over 90 % [11]. In addition, the development, operation, and maintenance of thermal inkjet is usually more convenient than piezoelectric printing. Therefore, the majority successes in tissue bioprinting are based on thermal inkjet printing instead of piezoelectric inkjet printing. One limitation of inkjet bioprinting is the strict requirement of bioink viscosity. This issue has recently been minimized by using water based biomaterials or combination of various printing technologies. Water based bioink allows the printer to freely deliver cells from single to multiple cells by simply adjusting the bioink concentration and the digital patterns. Cells are usually well-protected in the aqueous environment during the printing process therefore it is assumed to be the safest strategy to deliver living systems.
Based on the discussion above, bioprinting based on thermal inkjet printing is so far the most appropriate approach for regenerative medicine and tissue engineering applications. Researchers keep developing this technology as an optimal approach for cell delivery and scaffold fabrication. Therefore, we will mainly focus on the advancement and applications using this bioprinting technology in this chapter.
2 Cell Printing
Although the term of bioprinting can be used on printing any biological systems, it usually involves living cell patterning in tissue engineering and regenerative medicine applications. Therefore, the capacity of printing living cells is critical to evaluate a bioprinting platform or system.
Although bioprinting based on thermal inkjet printing technology has many successful applications, there were concerns that the printing process may cause damages or cell death. The small printhead nozzle size is necessary for high printing resolution. Due to the thermal heat and mechanical stresses applied to the cells during printing, it is possible that the cells may be damaged or their phenotype may be altered [42]. Therefore, a comprehensive evaluation of cell viability, apoptosis, heat shock proteins production, cell membrane damages of the printed cells is desired to confirm the bioprinting safety. Using a modified Hewlett-Packard (HP) thermal inkjet printer, cell viability at various cell concentrations was between 85 and 95 %. No significant difference in apoptosis and heat shock protein expression was observed between printed and non-printed cells [11]. Quantitative cell seeding can be achieved by adjusting the cell concentration in bioink. The inkjet printing process does alter the cell membrane of printed cells . Fluorescent labeled dextran dye with molecular weight (MW) up to 40,000 can penetrate into the printed cells. No dye was found in the non-printed cells even with the lowest MW (3000) (Fig. 1). The cell membrane pore size was estimated as 105 Å according to the Stokes diameter of these dye molecules [11].
The pores developed during printing were transient and can be repaired by the cells in just a couple hours. The transient nature of the cell membrane pores as well as the self-repair mechanism can be utilized for targeted gene delivery during the printing process [11, 43].
3 Microvasculature Printing
Although the concept of tissue engineering was introduced more than two decades ago, the current tissue engineering strategies still cannot create fully vascularized tissue constructs. The current tissue engineering paradigm is that successfully engineered thick tissues must include vasculature. As biological approaches alone, such as VEGF or co-culture of vessel cells, have fallen short of their promises, one may look for an engineering approach to build microvasculature. Layer-by-layer approaches for customized fabrication of cell and scaffold constructs have shown great potential in building complex 3D structures [44]. With the advent of cell printing, one may be able to build precise human microvasculature with suitable bioink. Human microvascular endothelial cells (HMVECs) and fibrin scaffold were utilized as bioink for microvasculature construction [12].
A standard inkjet printer was modified to simultaneously deposit HMVECs and fibrin scaffold to form the microvasculature. The bioink and biopaper components for fibrin bioprinting were carefully evaluated for optimal condition of simultaneous deposition of cells and scaffold [12]. The printed microvasculature was incubated for 10–15 min after the printing to finalize the crosslinking and enhance the cell attachment.
After 3 weeks in culture, the printed HMVECs aligned themselves in the fibrin channel and proliferated to form a confluent lining. Confocal laser scanning images at the z-axis demonstrated tubular structure of the printed human microvasculature. The endothelial cells were forming a vessel-like structure in the printed fibrin channel [12]. This demonstrates the printed and proliferated endothelial cells possessed the crucial angiogenesis function. The simultaneous deposition of endothelial cells and fibrin using thermal inkjet printing technology can be used for human microvasculature fabrication (Fig. 2).
4 Muscle Printing
Biological microelectromechanical system (Bio-MEMS) devices conjugated with biological components are promising for the development of novel bioengineering microdevices, such as motors and actuators [45], heart pumps [46], and biosensors [47]. Muscle cells have been widely used in these applications by generating force activated by actin-myosin motors regulated by excitation-contraction coupling [48]. These muscle powered microdevices utilizing energy generated by biochemical reaction are promising to save energy, resources, and spaces [49]. C2C12 skeletal muscle cells possess the advantages of infinite proliferation and differentiation into multinucleated myotubes [50]. As a well established cell line, the overall properties of C2C12 cells cultured and differentiated in vitro have been tested to closely mimic the properties of skeletal muscle in vivo [51]. Although C2C12 cells have been widely used to incorporate with bio-microdevices for many applications, it is important that the muscle cells and microdevices are consistently conjugated to produce reliable and reproducible results. The traditional methodology for Bio-MEMS fabrication is to manually seed cells on or into the microdevices [52]. However, the randomly deposited cells through this approach were uneven and further affected the cell proliferation and differentiation. Therefore, it is critical to incorporate a precise cell seeding technology to develop the Bio-MEMS constructs with consistent cell arrangement.
Bioprinting was able to print and align C2C12 cells onto the tiny cantilevers at a resolution at 300 dpi (85 μm). In order to control the cell proliferation and differentiation with minimal variations, same amount of cells were printed to evenly cover each cantilever of the microdevices. The viability of printed C2C12 cells was 91.2 ± 2.6 % and the printed cells aligned closely with each other forming confluent myotubes on almost all the cantilevers. Conjugated myotube and cantilever constructs responded synchronously to the electric pulses of 2 V with 40 ms duration up to 5 Hz (Fig. 3). This showed the bioprinted microdevices possessed equal or even better physiological properties comparing to the conventionally fabricated constructs in term of the spontaneous responses to the stimulation with significantly less culture time. Moreover, the bioprinted myotubes can also be used for muscle exercise studies with electric stimulations at various frequencies, which demonstrates the versatility of this work.
5 Cartilage Printing
Cartilage defects resulting from osteoarthritis, aging, and joint injury are a major cause of joint pain and chronic disability [53]. Mature cartilage cannot heal spontaneously because of its avascular, aneural, and alymphatic nature. The most common clinical treatments for cartilage repair include microfracture, osteochondral transfer, and autologous chondrocyte implantation. All these invasive and complicated treatments are still not able to restore the long lasting healthy cartilage [54]. Although articular cartilage was predicted to be one of the first tissues to be successfully engineered [55], the current cartilage tissue engineering strategies still cannot fabricate new tissue that is indistinguishable from native cartilage with respect to the zonal organization, extracellular matrix (ECM) composition, and mechanical properties [56]. In addition, most current cartilage repair strategies involve removing healthy cartilage tissue around the lesion site to create artificial defects for further treatment [57]. This procedure in fact causes additional necrosis to the existing healthy cartilage and leads to ultimate cartilage degeneration and failure of implanted tissue [58].
Inkjet bioprinting is able to directly repair cartilage tissue with closely mimicked native cartilage anatomy to the lesion site without additional damage. The ideal implanted tissue is expected to integrate with existing native cartilage and to repair lesions of different sizes and thicknesses. The multifaceted nature of this challenge requires a technique adaptable to variable physical dimensions and properties for tissue repair; bioprinting technology, based on inkjet printing, provides the necessary capabilities.
A standard thermal inkjet printer was modified to precisely deposit human articular chondrocytes and poly(ethylene) glycol dimethacrylate (PEGDMA; MW, 3400) layer-by-layer into a cartilage defect within an osteochondral plug for cartilage repair (Fig. 4). For a representative defect of 4 mm diameter and cartilage thickness of 2 mm, a nominal 0.23 µL of bioink estimated to contain 1140 human chondrocytes (5 × 106 cells/mL) was printed and photopolymerized for each layer to repair the cartilage defect in a layer-by-layer assembly. The thickness of each printed layer was about 18 μm. Total firing time of printhead was 1.1 s and the whole printing process completed less than 2 min. Compared to manual zonal cartilage fabrication which requires at least 11 min for UV exposure [59], bioprinting reduced UV exposure to the cells by 80 %. The viability of human chondrocytes printed with simultaneous photopolymerization increased 40 % than that when exposed to the same UV light source continuously for 10 min in manual fabrication [60].
Printed cartilage implant attached firmly with existing tissue and greater proteoglycan deposition was also observed at the interface of implant and native cartilage . Printed cartilage in 3D biopaper had elevated glycosaminoglycan (GAG) content comparing to that without biopaper. This study indicates the importance and feasibility of direct cartilage repair and bioprinting successfully controlled placement of individual cells, preserved cell viability, maintained chondrogenic phenotype, and demonstrated integration with host tissue.
6 Bone Printing
Although bone is well known for its self-healing capacities [61], the body cannot completely heal the bone defect without intervention when it is beyond the critical size [62, 63]. Large-scale bone loss resulting from tumor resections and high impact trauma is the major cause for bone repair and implantation in clinic. The availability and functionality of bone autografts and allografts are limited to restore the normal operations. The inert implants fail over time due to repetitive loading. Therefore, tissue engineered bone which can ideally be remodeled into new bone to restore, maintain or improve its functions is becoming increasingly attractive [64].
Thermal inkjet bioprinting has been developed as an enabling technology to simultaneously deposit cells, growth factors, and biomaterial scaffolds to the desired 2D and 3D locations [10–14, 17–21]. The ejected ink drops through the nozzles are smaller than 0.03 mm in diameter, which guarantees excellent printing resolution [34, 35]. Many inkjet printed scaffolds were natural hydrogels for the enhanced biocompatibility to the cells [12, 13, 65–67]. These scaffolds usually lacked mechanical strength due to the properties of material and crosslinking methods, limiting their applications to soft tissues. Previous work also showed bone grafts created using natural hydrogels such as fibrin or alginate [68–71]. Although the cells proliferated and differentiated well in these natural hydrogels, the compressive modulus of these scaffolds is less than 5 kPa even after 4 weeks in culture, which is not ideal for bone tissue engineering [69–71].
A 3D bioprinting platform with simultaneous photopolymerization using a synthetic polymeric hydrogel was recently developed. The compressive modulus of the printed PEGDMA using layer-by-layer assembly exceeds 500 kPa, which is 100 times more than the compressive modulus of the natural hydrogels [14, 21] and in the same order of magnitude as human musculoskeletal tissue [72]. In addition, PEG hydrogel has been demonstrated to maintain cell viability and promote ECM production [14, 21, 73, 74].
Bone marrow derived stem cells are capable to migrate to the skeletal sites, proliferate and differentiate at the local injured area. Isolated human mesenchymal stem cells (hMSCs) can maintain their osteogenic potential during monolayer cell expansion in vitro [75]. These cells are therefore commonly used to reconstruct skeletal tissues in orthopedic tissue engineering [76–78]. hMSCs isolated from bone marrow or adipose tissue can be induced for osteogenic differentiation and form bone tissue when stimulated by ceramic scaffold [79–81]. Bioactive glass (BG) and hydroxyapatite (HA) were also reported to promote bone tissue formation [70, 82].
In bone printing, the approaches mentioned above were integrated into a novel bioprinting setup, in which hMSCs and PEGDMA combined with BG or HA or both BG and HA nanoparticles were simultaneously printed to form the homogeneous bone constructs in a layer-by-layer approach. Biochemical analysis showed significantly higher total collagen production and alkaline phosphatase (ALP) activity in hMSCs printed within PEG-HA scaffold. The higher collagen production in PEG-HA scaffold was also observed in histology studies (Fig. 5), which was consistent with the previous work by Patel et al. that HA presence increased cell ALP activity and promoted osteogenesis [83]. Collectively, HA in PEG hydrogel maintained hMSCs viability, promoted hMSCs osteogenic differentiation and biosynthetic function.
This work demonstrates the feasibility of fabricating a neobone tissue by delivering hMSCs and osteogenic factors such as HA and BG nanoparticles in strong PEG scaffold for bone tissue engineering. Using layer-by-layer assembly, the deposited hMSCs were fixed at their initially deposited positions using simultaneous photopolymerization with reduced phototoxicity. HA in scaffold significantly stimulated hMSCs osteogenic differentiation as well as osteogenic ECM production with minimal cell toxicity. Combining with previous success in cartilage bioprinting [14], it is promising to construct osteochondral interface, which is one of the most important and difficult subjects in bone tissue engineering [84].
7 The Future
Taken together, bioprinting based on thermal inkjet printing demonstrates great feasibility of printing living systems and the flexibility of printing various subjects from soft to hard tissues with minimal side effects. In fact, the benign effects to the printed cells can be used for many other attractive applications, such as gene transfection and targeted drug delivery. The bioprinting system is versatile for 2D and 3D tissue application as well as avascular and vascular tissue construction. One promising clinical application is to develop a hand-held printer or printhead with digital control for direct tissue repair. By using 3D reconstructions of scanned lesions, bioprinting is able to precisely deliver cells, growth factors, and biomaterial scaffolds to repair the lesion with various shape and thickness. One promising direction is to combine the bioprinting approaches based on various mechanisms to meet the different challenges. Ultimately, the successful application in microvasculature fabrication also revealed the bioprinting may be the only solution to engineer thick and complex tissues with fully functional vasculature and innervation.
References
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–6.
Auger FA, Gibot L, Lacroix D. The pivotal role of vascularization in tissue engineering. Annu Rev Biomed Eng. 2013;15:177–200.
Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23(7):821–3.
Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–6.
Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23(7):879–84.
Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001;7(1):23–33.
Kang HW, Park JH, Kang TY, Seol YJ, Cho DW. Unit cell-based computer-aided manufacturing system for tissue engineering. Biofabrication. 2012;4(1):015005.
Hu C, Uchida T, Tercero C, Ikeda S, Ooe K, Fukuda T, et al. Development of biodegradable scaffolds based on magnetically guided assembly of magnetic sugar particles. J Biotechnol. 2012;159(1–2):90–8. (Feb 14).
Nerem RM, Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225–43.
Cui X, Boland T, DʼLima DD, Lotz MK. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul. 2012;6(2):149–55.
Cui X, Dean D, Ruggeri ZM, Boland T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol Bioeng. 2010;106(6):963–9.
Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30(31):6221–7.
Cui X, Boland T. Simultaneous deposition of human microvascular endothelial cells and biomaterials for human microvasculature fabrication using inkjet printing. NIP24/digital Fabrication 2008: 24th International Conference on Digital Printing Technologies, Technical Program and Proceedings 2008;24:480–3.
Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18(11–12):1304–12.
Gao G, Yonezawa T, Hubbell K, Dai G, Cui X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol J. 2015. Jan 8 doi:10.1002/biot.201400635.
Gao G, Schilling AF, Yonezawa T, Wang J, Dai G, Cui X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J. 2014;9(10):1304–11.
Cui X, Breitenkamp K, Finn MG, Lotz M, Colwell CW Jr. Direct human cartilage repair using thermal inkjet printing technology. Osteoarthritis Cartilage. 2011;19:S47–S8.
Cui X, Gao G, Yonezawa T, Dai G. Human cartilage tissue fabrication using three-dimensional inkjet printing technology. J Vis Exp 2014;(88), e51294. doi:10.3791/51294.
Cui X, Gao G, Qiu Y. Accelerated myotube formation using bioprinting technology for biosensor applications. Biotechnol Lett. 2013;35(3):315–21.
Cui X, Hasegawa A, Lotz M, D’Lima D. Structured three-dimensional co-culture of mesenchymal stem cells with meniscus cells promotes meniscal phenotype without hypertrophy. Biotechnol Bioeng. 2012;109(9):2369–80.
Cui X, Breitenkamp K, Lotz M, D’Lima D. Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation. Biotechnol Bioeng. 2012;109(9):2357–68.
Cohen DL, Malone E, Lipson H, Bonassar LJ. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006;12(5):1325–35.
Iwami K, Noda T, Ishida K, Morishima K, Nakamura M, Umeda N. Bio rapid prototyping by extruding/aspirating/refilling thermoreversible hydrogel. Biofabrication. 2010;2(1):014108.
Shor L, Guceri S, Chang R, Gordon J, Kang Q, Hartsock L, et al. Precision extruding deposition (PED) fabrication of polycaprolactone (PCL) scaffolds for bone tissue engineering. Biofabrication. 2009;1(1):015003.
Barron JA, Wu P, Ladouceur HD, Ringeisen BR. Biological laser printing: A novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed Microdevices. 2004;6(2):139–47.
Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010;6(7):2494–500.
Guillemot F, Souquet A, Catros S, Guillotin B. Laser-assisted cell printing: principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine (Lond). 2010;5(3):507–15.
Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30(12):2164–74.
Moon S, Hasan SK, Song YS, Xu F, Keles HO, Manzur F, et al. Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng Part C Methods 2010;16(1):157–66.
Odde DJ, Renn MJ. Laser-guided direct writing for applications in biotechnology. Trends Biotechnol. 1999;17(10):385–9.
Odde DJ, Renn MJ. Laser-guided direct writing of living cells. Biotechnol Bioeng. 2000;67(3):312–8.
Mohebi MM, Evans JRG. A drop-on-demand ink-jet printer for combinatorial libraries and functionally graded ceramics. J Comb Chem. 2002;4(4):267–74.
Beeson R. Thermal (TIJ) or Piezo? Who cares? IMI 7th Annual Ink Jet Printing Conference; 1998.
Hock SW, Johnson DA, Van Veen MA. Inventors; Print quality optimization for a color ink-jet printer by using a larger nozzle for the black ink only.US5521622. 1996.
Canfield B, Clayton H, Yeung KWW. Inventors; Method and apparatus for reducing the size of drops ejected from a thermal ink jet printhead.US5673069. 1997.
Hudson KR, Cowan PB, Gondek JS. Inventors; Ink drop volume variance compensation for inkjet printing.US6042211. 2000.
de Jong J, de Bruin G, Reinten H, van den Berg M, Wijshoff H, Versluis M, et al. Air entrapment in piezo-driven inkjet printheads. J Acoust Soc Am. 2006 ;120(3):1257–65.
Sirringhaus H, Kawase T, Friend RH, Shimoda T, Inbasekaran M, Wu W, et al. High-resolution inkjet printing of all-polymer transistor circuits. Science. 2000;290(5499):2123–6.
Okamoto T, Suzuki T, Yamamoto N. Microarray fabrication with covalent attachment of DNA using Bubble Jet technology. Nat Biotechnol. 2000;18(4):438–41.
Goldmann T, Gonzalez JS. DNA-printing: utilization of a standard inkjet printer for the transfer of nucleic acids to solid supports. J Biochem Biophys Methods. 2000;42(3):105–10.
Seetharam R, Sharma SK. Purification and analysis of recombinant proteins. New York: Marcel Dekker; 1991. p. 69.
Tirella A, Vozzi F, De MC, Vozzi G, Sandri T, Sassano D, et al. Substrate stiffness influences high resolution printing of living cells with an ink-jet system. J Biosci Bioeng. 2011;112(1):79–85.
Xu T, Rohozinski J, Zhao W, Moorefield EC, Atala A, Yoo JJ. Inkjet-mediated gene transfection into living cells combined with targeted delivery. Tissue Eng Part A. 2009;15(1):95–101.
Catros S, Guillemot F, Nandakumar A, Ziane S, Moroni L, Habibovic P, et al. Layer-by-layer tissue microfabrication supports cell proliferation in vitro and in vivo. Tissue Eng Part C Methods. 2012;18(1):62–70.
Xi J, Schmidt JJ, Montemagno CD. Self-assembled microdevices driven by muscle. Nat Mater. 2005;4(2):180–4.
Tanaka Y, Sato K, Shimizu T, Yamato M, Okano T, Kitamori T. A micro-spherical heart pump powered by cultured cardiomyocytes. Lab Chip. 2007;7(2):207–12.
Harms H, Wells MC, van dM Jr. Whole-cell living biosensors–are they ready for environmental application? Appl Microbiol Biotechnol. 2006;70(3):273–80.
Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205.
Asano T, Ishizua T, Yawo H. Optically controlled contraction of photosensitive skeletal muscle cells. Biotechnol Bioeng. 2012;109(1):199–204.
Yaffe D, Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7(3):159–66.
Miller JB. Myogenic programs of mouse muscle cell lines: expression of myosin heavy chain isoforms, MyoD1, and myogenin. J Cell Biol. 1990;111(3):1149–59.
Fujita H, Shimizu K, Nagamori E. Novel method for measuring active tension generation by C2C12 myotube using UV-crosslinked collagen film. Biotechnol Bioeng. 2010;106(3):482–9.
Mow VC, Hayes WC. Basic orthopaedic biomechanics. 2 ed. Philadelphia: Lippincott Williams & Wilkins; 1997.
Rasanen P, Paavolainen P, Sintonen H, Koivisto AM, Marja B, Ryynanen OP, et al. Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop. 2007;78(1):108–15.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–95.
Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432–63.
Kalson NS, Gikas PD, Briggs TWR. Current strategies for knee cartilage repair. Int J Clin Pract. 2010;64(10):1444–52.
Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular-cartilage. J Bone Joint Surg-Am. 1993;75 A(4):532–53.
Kim TK, Sharma B, Williams CG, Ruffner MA, Malik A, McFarland EG, et al. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11(9):653–64.
Cui X, Breitenkamp K, Finn MG, Lotz MK, D'Lima DD. Direct human cartilage repair using 3D bioprinting technology. Tissue Eng Part A. 2012;18(11–12):1304–12.
Mourino V, Boccaccini AR. Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J R Soc Interface. 2010;7(43):209–27.
Jones AC, Arns CH, Sheppard AP, Hutmacher DW, Milthorpe BK, Knackstedt MA. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials. 2007;28(15):2491–504.
Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782–8.
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–31.
Deitch S, Kunkle C, Cui X, Boland T, Dean D. Collagen matrix alignment using inkjet printer technology. Mater Res Soc Symp Proc. 2008;1094:52–7.
Boland T, Xu T, Damon B, Cui X. Application of inkjet printing to tissue engineering. Biotechnol J. 2006;1(9):910–7.
Boland T, Cui X, Aho M, Baicu C, Zile M. Image based printing of structured biomaterials for realizing complex 3D cardiovascular constructs. J Imaging Sci Technol. 2006;2:86–8.
Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12(8):2385–96.
Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res A. 2008;86(1):1–12.
Khanarian NT, Jiang J, Wan LQ, Mow VC, Lu HH. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng Part A. 2011;18(5–6):533–45. Nov 8.
Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12(12):3497–508.
Hoenig E, Winkler T, Mielke G, Paetzold H, Schuettler D, Goepfert C, et al. High amplitude direct compressive strain enhances mechanical properties of scaffold-free tissue-engineered cartilage. Tissue Eng Part A. 2011;17(9–10):1401–11.
Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59(1):63–72.
Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51(2):164–71.
Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64(2):278–94.
Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7(6):259–64.
Triffitt JT. Osteogenic stem cells and orthopedic engineering: summary and update. J Biomed Mater Res. 2002;63(4):384–9.
Oreffo RO, Triffitt JT. Future potentials for using osteogenic stem cells and biomaterials in orthopedics. Bone 1999;25(2 Suppl):5S–9 S.
Leboy PS, Beresford JN, Devlin C, Owen ME. Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol. 1991;146(3):370–8.
Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11(3):312–24.
Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49(3):328–37.
Jiang J, Tang A, Ateshian GA, Guo XE, Hung CT, Lu HH. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng. 2010;38(6):2183–96.
Patel M, Patel KJ, Caccamese JF, Coletti DP, Sauk JJ, Fisher JP. Characterization of cyclic acetal hydroxyapatite nanocomposites for craniofacial tissue engineering. J Biomed Mater Res A. 2010;94(2):408–18.
Hunziker EB, Driesang IM. Functional barrier principle for growth-factor-based articular cartilage repair. Osteoarthritis Cartilage. 2003;11(5):320–7.
Acknowledgements
The author would like to acknowledge Guohao Dai, Arndt F. Schilling, M.G. Finn, Kurt Breitenkamp for constructive suggestions and technical support. This work was funded by the Fundamental Research Funds for the Central Universities (WUT: 2015IB004), NSF 1011796, New York Capital Region Research Alliance grant, and Stemorgan Therapeutics R&D support (TERM002). The authors indicate no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cui, X. (2015). Three-Dimensional Bioprinting in Regenerative Medicine. In: Turksen, K. (eds) Bioprinting in Regenerative Medicine. Stem Cell Biology and Regenerative Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-21386-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-21386-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21385-9
Online ISBN: 978-3-319-21386-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)