Abstract
Glioblastoma (GBM) is the most malignant and challenging type of astrocytoma and also notoriously acknowledged as the most common primary brain tumor globally. Currently, chemotherapy is the most master therapy for tumor and is essential in clinical treatment for GBM. Nevertheless, the characterization of chemotherapy resistance seriously hinders clinical chemotherapy treatment. Accordingly, there are imperious demands for the exploitation of novel chemosensitizer to promote the efficacy of chemotherapy. Our current study was conducted to probe into the potential impacts of microRNA (miR)-640 on the chemosensitivity in GBM and the associated underlying mechanism. Initially, TargetScan software was utilized to predict the targeted genes of miR-640, and the target relationship between miR-640 and Bcl-2-modifying factor (BMF) was validated by double luciferase report assay. Additionally, to explore the role of miR-640/BMF in U251 cells, miR-640 inhibitor/BMF-siRNA was used. U251 cells were processed with 100 μM temozolomide (TMZ) and detected with CCK-8 kit. Eventually, RT-qPCR and Western blotting were used for evaluating Bcl-2, Bax mRNA, and protein expression level. Flow cytometry analysis was performed to measure cellular apoptosis. Initially, the results indicated that BMF was the target gene of miR-640. MiR-640 negatively regulated BMF expression in GBM cells. Besides, the findings revealed that miR-640 inhibition significantly inhibited U251 cell proliferation, promoted cell apoptosis, and increased the sensitivity of GBM cells to TMZ by targeting BMF. Moreover, BMF overexpression significantly suppressed U251 cell proliferation, induced cell apoptosis, and increased the sensitivity of GBM cells to TMZ. Inhibition of miR-640 expression enhances chemosensitivity of human GBM cells to TMZ by targeting BMF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is the most common and lethal primary brain tumor disease in this world that the incidence of disease was positively correlated with age (Au et al. 2022). Although advances have been accomplished in the clinical therapy in GBM and various of supplementary therapeutic methods have emerged with regulatory approvals in mountainous researches, the encouraging clinical manifestations are still absent. These patients diagnosed with GBM still suffered with the bitterness from disease, and despite accepted aggressive treatment, a dismal prognosis still present (Niu et al. 2021). Currently, the master clinical therapeutic approaches were chemotherapy, immunotherapy, and surgical treatment, which plays an essential role in the treatment of GBM (Laba and Ziolkowski 2021). In the early stage of GBM treatment, most of glioblastoma displayed prominent resistance to radiation and chemotherapy. However, the notorious characterization of chemotherapy resistance seriously hindered the clinical efficacy of chemotherapy. Accordingly, novel chemosensitizer needs to be developed for promoting the efficacy of chemotherapy.
MicroRNAs (miRNAs) currently emerged as the master players in cancer and numerous studies have indicated the important role of miRNAs in GBM (Nikaki et al. 2012; Tang et al. 2021; Yuan et al. 2017). Considering the emerging effects of miRNAs in tumor microenvironment and the close association between tumor microenvironment and clinical therapeutic resistance, many researches have been conducted to explore the importance of miRNAs in GBM as potential therapeutic targets and biomarkers (Regazzo et al. 2016). Emerging evidences have suggested the crucial role of miR-640 as the risk factor for various neoplastic diseases, which encompassing hepatocellular carcinoma, glioblastoma, and breast cancer (Tang et al. 2021; Luo et al. 2021; Zhai et al. 2019). Our previous study also indicated that miR-640 was prominently up-regulated in GBM tissue samples exerting tumor-promoting effects. However, the underlying mechanism and impacts of miR-640 in the clinical chemosensitivity displayed in GBM were still unveiled. Accordingly, in this study we explored the potential impacts of miR-640 on the chemosensitivity in GBM and the associated underlying mechanism.
BMF, also acknowledged as Bcl-2-modifying factor, is closely associated with cellular apoptosis and mainly involved in the regulation of autophagy and protein-containing complex assembly (Gramantieri et al. 2009; Contreras et al. 2013). Many researches have suggested its role in the course of cancer encompassing esophageal squamous cell carcinoma, colorectal cancer, and GBM (Fan et al. 2018). Although many researches have indicated that BMF is the target gene of many miRNAs, the role of miR-640-BMF axis in GBM remains unclear.
This study was designed to probe into the underlying mechanism and effects of miR-640-BMF axis in the chemosensitivity of GBM, providing promising therapeutic approaches for the clinical resistance of GBM.
Materials and Methods
Cell Culture
U251 human glioma cell line was purchased from Punosai Life Technologies (Procell Life Science & Technology Co., Ltd.). U251 cells were cultivated in DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 mg/ml streptomycin, and 100 IU/ml penicillin (Thermo Fisher Scientific, Inc.) and cultured in at 37 °C in a 5% CO2 humidified atmosphere.
Cellular Transfection and Treatment
miR-640 inhibitor and inhibitor negative control (inhibitor control) were designed and purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). To obtain transfected cells, U251 cells were diluted into the cell counting of 2 × 105 cells/ml and cultured in 6-well plates. After 24 h of cellular culture, U251 cells were then transfected with miR-640 inhibitor or inhibitor control utilizing Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h.
To silence or up-regulate BMF in U251 cells, BMF-siRNA or BMF-plasmid obtained from Santa Cruz Biotechnology (USA) were transfected into U251 cells by utilizing Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h.
For TMZ treatment, U251 cells were treated with 100 μM TMZ for 48 h.
Dual-Luciferase Reporter Assay
For predicting the targeted genes of miR-640, the bioinformatic software StarBase was utilized (Li et al. 2014). To confirm the binding sites between miR-640 and BMF, dual-luciferase reporter assay was performed. The 3ʹUTR of BMF, which contains the miR-640 binding site or mutated target site, was synthesized by genomic PCR and cloned into pMIR vectors (Ambion, USA) to construct the reporter vector BMF wild-type (BMF-WT) or BMF mutated-type (BMF-MUT). The 293 T cells (5 × 104 cells per well; 24 well plates) were transfected with BMF-WT or BMF-MUT, and the 100 nM miR-640 mimic (Guangzhou RiboBio Co., Ltd., Guangzhou, China) or 100 nM mimic control (Guangzhou RiboBio Co., Ltd., Guangzhou, China) by Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the instructions. After 48 h of cellular transfection, these transfected cells were harvested and assessed for luciferase activity utilizing a dual-luciferase assay kit (Solarbio).
Western Blotting Analysis
After cellular transfection procedure, U251 cells were lysed and vortexed with cell lysis containing proteinase inhibitor (10 ×; Cell Signaling Technology, Inc.). Subsequently, a BCA kit was utilized for protein quantification and redissolve by 10% SDS–PAGE. After electrophoresis process, protein samples were transferred on PVDF membrane (EMD Millipore, MA, USA) and incubated with primary antibodies against GAPDH (cat. no. ab9485; dilution: 1:1000; Abcam), BMF (cat. no. ab9655; dilution: 1:1000; Abcam), Bcl-2 (ab196495; dilution: 1:2000; Abcam), and Bax (cat. no. #2772; dilution: 1:1000; Cell Signaling Technology, Inc.) at 4 ℃ overnight. After washing, the membranes were incubated with Anti-rabbit IgG, HRP-linked Antibody (cat. no. #7074; dilution: 1:2000; Cell Signaling Technology, Inc.) at 25 ℃ for 1 h. Eventually, ECL detection kit (Beyotime) was utilized for the visualization of protein bands.
RT-qPCR Analysis
To extract overall RNA in U251 cells, TRIzol reagent (Life Technologies, USA) was used in accordance with the instructions from manufactory. The total RNA was treated with DNase I (Thermo Fisher Scientific, Inc.) to digest the genomic DNA. Then, total RNA (2 μg) was reverse transcribed into cDNA utilizing PrimeScript™ RT Kit (Takara Biotechnology Co., Ltd.). Then PCR amplification was conducted using the SYBR PrimeScript RT-PCR Kit (TaKaRa) with the ABI 7500 Real-Time PCR System (Agilent Technologies, USA). Expression of target genes was calculated using the 2−ΔΔCt method. Primer sequences were listed as following:
miR-640 forward 5′-GTGACCCTGGGCAAGTTCCT-3′;
reverse 5′-CCCCAAGGCAACCGTAGAGG-3′;
U6 forward 5′-CTCGCTTCGGCAGCACATATACT-3′;
reverse 5′-ACGCTTCACGAATTTGCGTGTC-3′;
BMF forward 5′-CCACCAGCCAGGAAGACAAAG-3′;
reverse 5′-TGCTCCCCAATGGGCAAGACT-3′;
Bax forward 5′-TGCTACAGGGTTTCATCCAG-3′;
reverse 5′-ATCCACATCAGCAATCATCC-3′;
Bcl-2 forward 5′-TGGGATGCCTTTGTGGAAC-3′;
reverse 5′-CATATTTGTTTGGGGCAGGTC-3’;
GAPDH forward 5′-CGGAGTCAACGGATTTGGTCGTAT-3′;
reverse 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′.
Cellular Viability and Apoptosis Analysis
After cellular transfection, U251 cells were seeded onto 96-well plates and CCK-8 reagent (Sigma) was added into each well for 4 h incubation at 37 °C. Subsequently, 100 µl of DMSO was added and cells were lysed with RIPA for 10 min. Eventually, the microplate reader was utilized for measuring absorbance at 490 nm.
After cell transfection, BD flow cytometry (FCM) was utilized for U251 cells apoptosis analysis. In brief, the harvested transfected U251 cells were digested with 0.25% trypsin, then washed with PBS at 4 °C. Subsequently, 5 µl of fluorescein isothiocyanate labeled annexin V and 5 µl propidium iodide (PI; Catalog number 6592; Cell Signaling Technology, Inc.) were added into U251 cells and then cells were cultured in darkness at 4 °C for 30 min. Finally, to measure and calculate cellular apoptosis ratio, FlowJo software (Version 7.6.1; Tree Star Inc.) was conducted.
Statistical Analysis
SPSS 20.0 statistical software (IBM Corp.) was utilized for statistical analysis in this study and subsequently the data were expressed in terms of mean ± standard deviation (SD). Unpaired, two-tailed Student’s t tests or one-way ANOVA followed by Tukey’s test was performed for data analysis, and p < 0.05 indicated statistical significance.
Results
BMF Was Identified as the Target Gene of miR-640
Considering that our previous study has indicated the important role of miR-640 in GBM, we intend to identify the potential target genes of miR-640 and probe into the underling mechanism. To screen the target genes of miR-640, TargetScan software was utilized. As the predicted results indicated that, there were binding sites between miR-640 and BMF at certain region (Fig. 1A). Given that the emerging evidence that BMF plays an important role in cellular apoptosis and various cancer, BMF was selected for further exploration. Subsequently, double luciferase report assay was conducted to validate the association and binding region between miR-640 and BMF. As predicted, BMF was identified as a direct target of miR-640 (Fig. 1B).
MiR-640 Negatively Regulated the Expression of BMF in Glioblastoma Cells
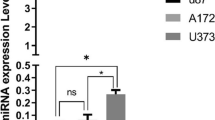
To explore the transcriptional regulatory associations between miR-640 and BMF in GBM, U251 cells were transfected, respectively, with inhibitor control, miR-640 inhibitor, control-siRNA, BMF-siRNA, miR-640 inhibitor+control-siRNA, and miR-640 inhibitor+BMF-siRNA for 48 h, and then RT-PCR analysis was conducted to measure the efficacy of transfection. Our results demonstrated that in comparison with inhibitor control group, miR-640 inhibitor vector transfection prominently suppressed the expression level of miR-640 in U251 cells (Fig. 2A). Additionally, transfection of BMF-siRNA significantly lowered the expression of BMF in U251 cells when compared with control-siRNA group. Subsequently, the results indicated that miR-640 inhibitor profoundly elevated the expression level of BMF in U251 cells and this effective was further inversed by BMF-siRNA transfection (Fig. 2C and D). Collectively, these evidences indicated the negatively regulatory association between miR-640 and BMF in U251 cells.
miR-640 negatively regulated the expression of BMF in glioblastoma cells. A Expression levels of miR-640 in U251 cells measured by RT-PCR analysis, B mRNA expression levels of BMF in U251 cells measured by RT-PCR analysis, and C, D mRNA and protein expression levels of BMF in U251 cells measured by RT-PCR analysis and western blot assay. All data are presented as mean ± SD. **p < 0.01, compared to the inhibitor control group; ##p < 0.01, compared to the control-siRNA group; &&, p < 0.01, compared to the miR-640 inhibitor+control-siRNA group
Suppression of miR-640 Inhibited the Cellular Proliferation and Induced Cellular Apoptosis in GBM Cells by Up-Regulating BMF Expression Level
Considering the important role of BMF in cellular proliferation and apoptosis, we thus further explored the potential role of miR-640-BMF axis in GBM cell proliferation and apoptosis. The results suggested that compared with inhibitor control group, miR-640 inhibitor transfection pronouncedly suppressed the proliferation activity (Fig. 3A) and induced cellular apoptosis of U251 cells (Fig. 3B and C). Additionally, the transfection of miR-640 inhibitor vector significantly elevated Bax and down-regulated Bcl-2 protein and mRNA expression levels in U251 cells (Fig. 3D–F). As expected, all these effects of miR-640 inhibitor on U251 cells were reversed by BMF-siRNA transfection.
Suppression of miR-640 inhibited the cellular proliferation and induced cellular apoptosis in GBM cells by up-regulating BMF expression level U251 cells were transfected with inhibitor control, miR-640 inhibitor, miR-640 inhibitor+control-siRNA, or miR-640 inhibitor+BMF-siRNA for 48 h. Then, cells proliferation activity was measured by CCK-8 kit (A), cell apoptosis was analyzed by flow cytometer (B, C), and the protein and mRNA levels of Bax and Bcl-2 were determined using western blot assay and RT-qPCR (D–F). All data are presented as mean ± SD. **p < 0.01, compared to the inhibitor control group; ##p < 0.01, compared to the miR-640 inhibitor+control-siRNA group
Inhibition of miR-640 Expression Promoted the Chemosensitivity to TMZ in GBM Cells by Up-Regulating the Expression of BMF
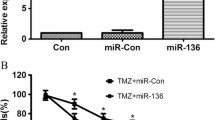
To probe into the potential role of miR-640 on the chemosensitivity of GBM cells to TMZ, U251 cells were co-transfected with inhibitor control, miR-640 inhibitor, miR-640 inhibitor+control-siRNA and miR-640 inhibitor+BMF-siRNA vector and processed with TMZ. Our results suggested that the cellular viability of TMZ+miR-640 inhibitor group was pronouncedly down-regulated and the cellular apoptosis level was significantly up-regulated in comparison with TMZ+inhibitor control group (Fig. 4A–C). Moreover, compared with TMZ+inhibitor group, the protein and mRNA level of Bax were prominently elevated in TMZ+miR-640 inhibitor group (Fig. 4D and E). Compared with TMZ+inhibitor group, the mRNA expression and protein level of Bcl-2 were significantly decreased by miR-640 inhibitor (Fig. 4D and F). Nevertheless, all these trends were reversed by BMF-siRNA transfection.
Inhibition of miR-640 promoted the chemosensitivity to TMZ in GBM cells by up-regulating the expression of BMF. U251 cells were transfected with inhibitor control, miR-640 inhibitor, miR-640 inhibitor+control-siRNA, or miR-640 inhibitor+BMF-siRNA in the presence of 100 μM of TMZ for 48 h. Then, cells proliferation activity was measured by CCK-8 kit (A), cell apoptosis was analyzed by flow cytometer (B, C), and the protein and mRNA levels of Bax and Bcl-2 were determined using western blot assay and RT-qPCR (D–F). All data are presented as mean ± SD. **p < 0.01, compared to the TMZ+inhibitor control group; ##p < 0.01, compared to the TMZ+miR-640 inhibitor+control-siRNA group
BMF Significantly Suppressed the Cellular Proliferation of U251 Cells and Enhanced the Chemosensitivity to TMZ
After co-transfected with control-plasmid or BMF-plasmid for 48 h, U251 cells were collected for RT-PCR and western blotting analysis to measure transfection efficiency. Subsequently, CCK-8 kit and flow cytometry were conducted to detect cellular viability and apoptosis. Western blotting and RT-PCR were, respectively, utilized for Bcl-2 and Bax protein and mRNA expression level measurements. Our results demonstrated that BMF-plasmid transfection significantly elevated the expression level of BMF in U251 cells compared with control-plasmid group (Fig. 5A and B). Additionally, BMF-plasmid significantly suppressed the cellular proliferation and induced cellular apoptosis in U251 cells (Fig. 5C–E). Western blotting and RT-qPCR results indicated that Bax protein and mRNA expression level were pronouncedly elevated and Bcl-2 protein and mRNA level were markedly decreased by BMF-plasmid transfection (Fig. 5F–H).
BMF significantly suppressed the cellular proliferation and induced cell apoptosis of U251 cells U251 cells were transfected with control-plasmid or BMF-plasmid for 48 h. Then, A, B mRNA and protein expression levels of BMF in U251 cells measured by RT-PCR analysis and western blot assay, C cells proliferation activity was measured by CCK-8 kit, cell apoptosis was analyzed by flow cytometer (D, E), and the protein and mRNA levels of Bax and Bcl-2 were determined using western blot assay and RT-qPCR (F–H). All data are presented as mean ± SD. **p < 0.01, compared to the control-plasmid group
To further explore the potential role of BMF in the chemosensitivity of GBM cells to TMZ, U251 cells were co-transfected control-plasmid or BMF-plasmid and then treated with 100 μM of TMZ. The results indicated that cellular viability of U251 cells in TMZ+BMF-plasmid group was prominently down-regulated and cellular apoptosis was significantly enhanced when compared with TMZ+control-plasmid group (Fig. 6A–C). Moreover, compared with TMZ+control-plasmid group, the Bax protein and mRNA expression level were pronouncedly elevated and Bcl-2 protein and mRNA level in U251 cells were significantly decreased (Fig. 6D–F) in TMZ+BMF-plasmid group. These results suggested that BMF could significantly suppress the cellular proliferation of U251 cells and enhanced the chemosensitivity of U251 cells to TMZ.
BMF promoted the chemosensitivity to TMZ in GBM cells U251 cells were transfected with control-plasmid or BMF-plasmid in the presence of 100 μM of TMZ for 48 h. Then, cells proliferation activity was measured by CCK-8 kit (A), cell apoptosis was analyzed by flow cytometer (B, C), and the protein and mRNA levels of Bax and Bcl-2 were determined using western blot assay and RT-qPCR (D–F). All data are presented as mean ± SD. **p < 0.01, compared to the TMZ+control-plasmid group
Discussion
In this current study, we demonstrated that miR-640 down-regulation significantly suppressed cellular proliferation and promoted cellular apoptosis by targeting BMF, which confirmed the stimulative effects of miR-640 on GBM. A current study indicated that miR-186-5p was closely associated with the chemosensitivity of GBM cells to TMZ (Xiong et al. 2019). This research demonstrated that inhibition of miR-640 expression could enhance the chemosensitivity of GBM cells to TMZ by up-regulating BMF expression.
Previous studies have illustrated the crucial role of miR-640 in various cancer and the biological processes. For example, miR-640 was reported to participate in breast cancer by targeting Wnt7b/β-catenin signaling pathway (Tang et al. 2021). Additionally, miR-640 has also been evidenced to be involved in angiogenesis process (Harel et al. 2020). Numerous researches have unveiled the underlying mechanism of miR-640 in several tumor-related diseases, the involved pathways were inflammatory signaling including NF-κB signaling pathway and HIF-1α signaling pathway, and WNT signaling pathways which are closely associated with tumor growth and progression (Masson et al. 2001; Kirikoshi et al. 2001; Hanada et al. 2009). Nevertheless, there are rare associated reports about the role of miR-640 in the development and tumor formation of GBM. Our previous study revealed that miR-640 promotes cell viability, proliferation, and adhesion in GBM cells (Luo et al. 2021). This study indicated that miR-640 down-regulation significantly suppressed GBM cell proliferation and induced cell apoptosis by targeting BMF.
BMF has been prevalently accepted to be associated with cellular apoptosis by interacting with various intracellular proteins which including MCL1, BCL2, BCL2L1/BCL-Xl, BCL2A1, and BCL2L2/BCL-w (Gramantieri et al. 2009; Kang et al. 2014; Yee et al. 2016). BMF also has been identified as targeted gene of various miRNAs encompassing miR-874-3p, miR-125b, and miR-29b, which are involved in the process of osteoclastogenesis, ischemic injury, and cellular apoptosis of esophageal squamous cell carcinoma (Fan et al. 2018; Sul et al. 2019; Jiang et al. 2019). However, few studies about the potential role of BMF in GBM have been reported. In our study, we validated the crucial role of BMF in GBM performed on U251 cell line and identified BMF as a target gene of miR-640. The findings of current study indicated that BMF overexpression significantly suppressed U251 cell proliferation and induced cell apoptosis. Actually, there are nine protein isoforms of BMF, and this study did not study which isoform of BMF is. This was a limitation of this study.
In the clinical treatment of GBM, TMZ was the routine therapeutic medicine and the resistance of GBM to it was a common phenomenon (Karachi et al. 2018). Considering the highly refractory and highly poor clinical outcome refractory of TMZ treatment in GBM, various explorations have been conducted and numerous meaningful works have been put forward (Vehlow et al. 2019; Peng et al. 2016). Accordingly, unveiling the potential mechanism of the chemosensitivity to TMZ of GBM cells and supplementary therapy for it would be particularly important. In this current study, we initially explored the potential role of miR-640 and evidenced the miR-640-BMF axis in the chemosensitivity of GBM cells to TMZ. The data indicated that inhibition of miR-640 expression enhances chemosensitivity of human GBM cells to TMZ by up-regulating BMF expression.
In summary, our finding suggested that miR-640 may facilitate cellular proliferation and suppress cellular apoptosis in GBM by targeting BMF gene. Additionally, we established a miR-640-BMF axis and initially probed into its role in the chemosensitivity of GBM cells to TMZ.
Conclusions
Inhibition of miR-640 expression could enhance the chemosensitivity of GBM cells to TMZ by targeting BMF. MiR-640/BMF may be a potential target for GBM treatment.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Au TH, Willis C, Reblin M, Peters KB, Nghiemphu PL, Taylor JW et al (2022) Caregiver burden by treatment and clinical characteristics of patients with glioblastoma. Support Care Cancer 30(2):1365–1375
Contreras AU, Mebratu Y, Delgado M, Montano G, Hu CA, Ryter SW et al (2013) Deacetylation of p53 induces autophagy by suppressing Bmf expression. J Cell Biol 201(3):427–437
Fan YX, Bian XH, Qian PD, Chen ZZ, Wen J, Luo YH et al (2018) MicroRNA-125b inhibits cell proliferation and induces cell apoptosis in esophageal squamous cell carcinoma by targeting BMF. Oncol Rep 40(1):61–72
Gramantieri L, Fornari F, Ferracin M, Veronese A, Sabbioni S, Calin GA et al (2009) MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res 15(16):5073–5081
Hanada R, Leibbrandt A, Hanada T, Kitaoka S, Furuyashiki T, Fujihara H et al (2009) Central control of fever and female body temperature by RANKL/RANK. Nature 462(7272):505–509
Harel S, Sanchez-Gonzalez V, Echavarria R, Mayaki D, Hussain SN (2020) Roles of miR-640 and zinc finger protein 91 (ZFP91) in angiopoietin-1-induced in vitro angiogenesis. Cells. https://doi.org/10.3390/cells9071602
Jiang D, Sun X, Wang S, Man H (2019) Upregulation of miR-874-3p decreases cerebral ischemia/reperfusion injury by directly targeting BMF and BCL2L13. Biomed Pharmacother 117:108941
Kang Y, Nian H, Rajendran P, Kim E, Dashwood WM, Pinto JT et al (2014) HDAC8 and STAT3 repress BMF gene activity in colon cancer cells. Cell Death Dis 5:e1476
Karachi A, Dastmalchi F, Mitchell DA, Rahman M (2018) Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol 20(12):1566–1572
Kirikoshi H, Sekihara H, Katoh M (2001) Molecular cloning and characterization of WNT14B, a novel member of the WNT gene family. Int J Oncol 19(5):947–952
Laba AE, Ziolkowski P (2021) Trends in glioblastoma treatment research: an analysis of clinical trials and literature. Neurol Neurochir Pol 55(3):269–280
Li JH, Liu S, Zhou H, Qu LH, Yang JH (2014) starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucl Acids Res. https://doi.org/10.1093/nar/gkt1248
Luo C, Lu Z, Chen Y, Chen X, Liu N, Chen J et al (2021) MicroRNA-640 promotes cell proliferation and adhesion in glioblastoma by targeting Slit guidance ligand 1. Oncol Lett 21(2):161
Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J 20(18):5197–5206
Nikaki A, Piperi C, Papavassiliou AG (2012) Role of microRNAs in gliomagenesis: targeting miRNAs in glioblastoma multiforme therapy. Expert Opin Investig Drugs 21(10):1475–1488
Niu X, Wang C, Zhou X, Yang Y, Liu Y, Zhang Y et al (2021) Pineal region glioblastomas: clinical characteristics, treatment, and survival outcome. World Neurosurg 146:e799–e810
Peng CH, Chen ZX, Wang S, Wang HW, Qiu WJ, Zhao L et al (2016) The error-prone DNA polymerase kappa promotes temozolomide resistance in glioblastoma through rad17-dependent activation of ATR-Chk1 signaling. Cancer Res 76(8):2340–2345
Regazzo G, Terrenato I, Spagnuolo M, Carosi M, Cognetti G, Cicchillitti L et al (2016) A restricted signature of serum miRNAs distinguishes glioblastoma from lower grade gliomas. J Exp Clin Cancer Res 35(1):124
Sul OJ, Rajasekaran M, Park HJ, Suh JH, Choi HS (2019) MicroRNA-29b enhances osteoclast survival by targeting BCL-2-modifying factor after lipopolysaccharide stimulation. Oxid Med Cell Longev 2019:6018180
Tang C, Wang X, Ji C, Zheng W, Yu Y, Deng X et al (2021) The role of miR-640: a potential suppressor in breast cancer via Wnt7b/beta-catenin signaling pathway. Front Oncol 11:645682
Vehlow A, Klapproth E, Jin S, Hannen R, Hauswald M, Bartsch JW et al (2019) Interaction of discoidin domain receptor 1 with a 14-3-3-beclin-1-akt1 complex modulates glioblastoma therapy sensitivity. Cell Rep. https://doi.org/10.1016/j.celrep.2019.02.096
Xiong Y, Chen R, Wang L, Wang S, Tu Y, Zhu L et al (2019) Downregulation of miR186 promotes the proliferation and drug resistance of glioblastoma cells by targeting twist1. Mol Med Rep 19(6):5301–5308
Yee YH, Chong SJ, Pervaiz S (2016) The anti-oxidant and pro-oxidant dichotomy of Bcl-2. Biol Chem 397(7):585–593
Yuan GQ, Wei NL, Mu LY, Wang XQ, Zhang YN, Zhou WN et al (2017) A 4-miRNAs signature predicts survival in glioblastoma multiforme patients. Cancer Biomark 20(4):443–452
Zhai Z, Fu Q, Liu C, Zhang X, Jia P, Xia P et al (2019) Emerging roles of hsa-circ-0046600 targeting the miR-640/HIF-1alpha signalling pathway in the progression of HCC. Onco Targets Ther 12:9291–9302
Funding
This study was supported by the Scientific Research Project of Wuhan Municipal Health Commission (Grant No. WX21C05).
Author information
Authors and Affiliations
Contributions
SJ and CL contributed to the study design, data collection, statistical analysis, data interpretation, and manuscript preparation. YC, JC, ST, and QZ contributed to data collection and statistical analysis. CH and SD contributed to data collection, statistical analysis, and manuscript preparation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, S., Luo, C., Chen, Y. et al. MicroRNA-640 Inhibition Enhances the Chemosensitivity of Human Glioblastoma Cells to Temozolomide by Targeting Bcl2 Modifying Factor. Biochem Genet 61, 538–550 (2023). https://doi.org/10.1007/s10528-022-10264-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-022-10264-x