Abstract
Ducrosia anethifolia Boiss is an aromatic vegetable and medicinal plant of Apiaceae family. In this study, morphological and essential oil studies as well as ISSR analyses were employed to investigate genetic diversity in 120 Moshgak accessions of 24 Iranian populations. High variations were observed in morpho-physiological traits (morphological and essential oil contents) of the populations in 2 consecutive agronomic years. In both studied years, the highest leaf (1% and 1.2%) and seed (2.46% and 2.9%) essential oil contents were recorded for the Abarkuh population. For ISSR analysis, 15 primer combinations were employed that produced 120 polymorphic bands. Dendrogram and STRUCTURE software grouped the accessions into four clusters although such grouping did not fit the geographic regions perfectly. Among the populations, Abarkuh and Kerman exhibited the highest genetic distance. Based on analysis of molecular variance (AMOVA), only 4.32% of the total genetic diversity was observed among the populations, while 95.68% was detected within the populations. Moreover, the studied populations exhibited a low genetic differentiation (Gst = 0.13) but a high gene flow (Nm = 3.26). It may be concluded that the results of the study provide new insights regarding the genetic diversity of Moshgak germplasm that will be useful for its conservation management and breeding programs for oil- and yield-related traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ducrosia anethifolia Boiss belongs to the Apiaceae family locally known in Iran as Moshgak, Moshkbu, or Roshgak (Arbabi et al. 2018). The species grows not only in Iran but also in some Arabian countries (Shahabipour et al. 2013). In central Iran, the aerial parts of the plant are typically used as an aromatic vegetable to improve the smell of drinks and foods. In traditional medicine, the plant is used as a remedy for headache and backache while the seeds are used in an infusion for the treatment of colic and colds in children. Antibacterial, antimicrobial, and antianxiety properties of D. anethifolia have also been reported in the literature (Karami and Bohlooli 2017). One of the main components of medicinal plants with high economic value, including D. anethifolia, is their secondary metabolites (Arbabi et al. 2018). Moreover, the plants grown in wild habitats yield metabolites different from those cultivated in the field (Arabsalehi et al. 2018). Finally, essential oil and important aromatic constituents are isolated from the different medicinal plant parts (Tohidi et al. 2017).
Native germplasms are considered valuable genetic materials whose protection and sustainable applications are essential for breeding purposes (Hashemifar and Rahimmalek 2018). Nowadays, however, aromatic vegetable crops are endangered due to the devastation of natural habitats and excessive harvest of these plants by local people (Rahimmalek et al. 2009). In order to protect new vegetable crops, it is therefore necessary for selection and domestication of high-yield populations with desirable essential oil content to be used in breeding programs. Naturally, new insights may be gained regarding the variation of native plant species through genetic variation assessment of various populations based on morphological and phytochemical characteristics. One crucial step in this regard involves an evaluation of the germination efficiency of the plants in 2 agronomic years to measure their genetic × environment interaction effects (Arabsalehi et al. 2018), whereby the morphological characteristics and essential oil content of different populations affected by their genetics, environment, and genetic × environment are determined.
Analysis of genetic relationships at morphological, phytochemical, and molecular levels and the genetic diversity in crop populations are essential for their improvement to obtain products with health benefits (Fadaei Heidari et al. 2016; Liang et al. 2017). Molecular markers have been widely used to assess genetic variation, to categorize gene pools, and to define the genetic maps independent of age, physiological, and environmental conditions (Shojaiefar et al. 2015). Among the molecular tools, Inter Simple Sequence Repeats (ISSRs) have widely been used as dominant markers to track genetic relationships in plants since they offer advantages such as high polymorphism, high reproducibility, and easy handling (Gharibi et al. 2011; Verma et al. 2017; Muhaidat et al. 2018).
Many literature reports are available that focused on molecular variations as well as morphological and phytochemical characteristics in the aromatic plants of the Apiaceae family collected from natural habitats (Karami and Bohlooli 2017; Arbabi et al. 2018), including those on fennel (Maghsoudi Kelardashti et al. 2015), ajowan (Fadaei Heidari et al. 2016 and coriander. However, there is no comprehensive report available on morphological and phytochemical characters of D. anethifolia which has evaluated these characteristics in more than 1 agronomic year. Moreover, there is no report using ISSR molecular markers for detection of variations among and within studied populations. Finally, D. anethifolia is basically cross pollinated, resulting in a high degree of diversity both within and between populations to offer the opportunity for breeders to screen plants with desirable traits. It is therefore of utmost importance to cultivate this aromatic vegetable in 2 agronomic years in order to obtain essential data that can be used in its domestication process.
The aims of the present research were: (1) to assess different D. anethifolia populations in the field conditions in 2 agronomic years in order to obtain insight that can be exploited in the selection of high-yield populations with high essential oil contents; (2) to determine the genetic structure and variation among D. anethifolia populations using morphological and molecular markers and (3) to evaluate the level of inter and intra population variability and mechanisms.
Materials and Methods
Plant Material and Field Experiments
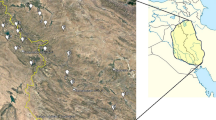
Plant material included 120 accessions belonging to 24 populations collected from different parts of Iran (Table 1, Fig. 1). Dr. Rahimmalek identified studied populations, and the voucher specimens were deposited in the herbarium of the Isfahan University of Technology. The experiments were performed at the Research Farm of Isfahan University of Technology located in Lavark Najaf Abad, Isfahan, Iran, at an altitude of 1630 m above sea level. The soil at the site was loamy clay (16% sand, 38% clay, and 46% silt) with pH 7.5 and an annual average rainfall of 122.8 mm and an annual average temperature of 9.1–23.4 °C. The seeds were planted in a Randomized Complete Block Design (RCBD) with three replicates. Each replication consisted of 10 plants for each population during the 2 agronomic years of 2019 and 2020. Each plot included two rows 2.5 m in length and 35 cm in width.
Sites of collection of Moshgak population from different geographical regions of Iran, used in this study. The colours used is based on geographical regions. The population gene bank number are shown in Table 1 (Color figure online)
Phenotypic Evaluation
The agro-morphological traits of plant height (PH) (cm), days to 50% flowering (DF), plant fresh weight (FW) (g/plant), plant dry weight (DW) (g/plant), number of umbels per plant (NUP), 1000-seed weight (SW) (g), seed yield per plant (SY) (g/plant), and harvest index (HI) (%) were measured using ten plants from each replicate and the values were reported as means.
Essential Oil Extraction
Young leaves and seeds from D. anethifolia populations grown in each agronomic year were accumulated in the morning and dried in the shade for 7 days in 25 °C. To extract essential oil, 30–50 g of each samples was subjected to 500 mL of distilled water and boiled for 5 h using Clevenger-type apparatus (Sabbaghi Rahimi and Rahimmalek 2019). The extracted essential oil was collected in a glass container. Finally, the essential oil content was presented as percentage based on the weight of the air-dried leaf and seed specimens.
Phenotypic Data Analysis
The normality of data were calculated using proc normality test of SAS software release 9.2 (SAS Institute, Cary, NC, USA) (SAS Institute 2001). The 2 years’ data were analyzed (ANOVA) using SAS software release 9.2 (SAS Institute, Cary, NC, USA) (SAS Institute 2001). The means of the populations were calculated based on the least significant difference (LSD) tests (p < 0.05) using SAS software release 9.2 (SAS Institute, Cary, NC, USA) (SAS Institute 2001). Phenotypic and genotypic coefficient of variances as well as the heritability were obtained using the following formulas:
where \(\sigma_{p}\) is phenotypic standard deviation, \(\sigma_{g}\) is genotypic standard deviation, and µ is the phenotypic mean. Heritability was estimated on a phenotypic mean basis averaged over replications and years using the following formula:
where \(\sigma_{g}^{2}\) is the genotype, \(\sigma_{gy}^{2}\) is the genotype × year interaction, and \(\sigma_{e}^{2}\) is the residual variance, while y and r represent the number of years and replications, respectively. The correlation coefficients among the traits were calculated using proc CORR of SAS software release 9.2 (SAS Institute, Cary, NC, USA) (SAS Institute 2001). Hierarchical cluster analysis (HCA) was performed based on the Ward’s method based on the Squared Euclidean dissimilarity to classify 24 Moshgak populations using Stat Graphics software ver. 17.2 (Statgraphics 2016). The cut-off value was determined using F-bill test using SAS software release 9.2 (SAS Institute, Cary, NC, USA) (SAS Institute 2001).
DNA Extraction
Fresh leaves were used to extract DNA based on the CTAB procedure with minor changes (Sarfaraz et al. 2021). Using both spectrophotometry and electrophoresis, the quality of DNA was evaluated based on OD 260/280 ratio using the following formula:
Finally, DNA was diluted to a concentration of 20 ng/μL.
ISSR Analysis
Among the ISSR primers screened, 15 generating clear and repeatable bands were chosen for analysis of Moshgaks (Table S1). The total volume of PCR reaction mixture was 15 μL containing 10 ng of DNA, 7 μL of the Master Mix Red (Ampliqon, Finland) including 10 pM from each primer, 0.25 mM dNTPs, 4 mM MgCl2, 1U Taq DNA polymerase, and 10× PCR buffer (Hashemifar and Rahimmalek 2018). PCR cycling was accomplished using the following programs: 2 min at 94 °C, followed by 40 cycles of 1 min at 94 °C, 1 min at appropriate annealing temperature for each primer, 2 min at 72 °C, and an extension of 10 min at 72 °C. PCR-amplified products were run for three hours on agarose gel (1.2%). Ethidium bromide was applied for visualizing the gel.
Molecular Data Analysis
The polymorphic DNA fragments for each gel were scored as present (1) or absent (0). The principal coordinate analysis (PCoA) was accomplished according to Rohlf (1998) using NTSYSpc 2.02e (Rohlf 1998). Polymorphism information content (PIC) was calculated based on Anderson et al. (1993) formula. Jaccard similarity index was used to determine Genetic similarity among all the populations (Jaccard 1908). The cluster analysis was done using UPGMA employing NTSYSpc 2.02e (Rohlf 1998). The Mantel test (Mantel 1967) was also performed using NTSYSpc 2.02e. This test was used to detect the correlation between each two dendrograms. The cut-off were calculated using proc cluster-ccc-plot of SAS software release 9.2 (SAS Institute, Cary, NC, USA) (SAS Institute 2001). Gene variation and analysis of molecular variance (AMOVA) were measured based on Arlequin (version 3.0) (Excoffier et al. 2005). The admixture of genotypes was evaluated with STRUCTURE software 2.0 (Pritchard and Wen 2003). It was accomplished using a burn-in of 5000 iterations with 50,000 Markov Chain Monte Carlo (MCMC) repetitions based on the admixture model with allele frequencies (K = 2–10 and iterations per K = 5). The online available program of Structure Harvester v6.0 was also applied to calculate Delta K as a prerequisite for the best fit value of K.
Results
Morphological Study
Analysis of variance (ANOVA) was performed to calculate the effects of year, population, and the interactions of studied characteristics of Moshgak populations. The results of ANOVA showed significant differences among the studied populations, years and Population × Year for most of the measured characteristics (Table S2). The 2019 agronomic year exhibited relatively high coefficients of variance (CV% ≥ 25) for both plant dry and fresh weights, number of umbels per plant, harvest index (HI) and seed yield per plant. In contrast, variation for 1000-seed weight, plant height and days to 50% flowering were relatively low in this year (Table 2). Among the studied characteristics, higher heritability levels (\({h}^{2}\) ≥ 70) were observed for fresh weight (98.43%), 1000-seed weight (97.22%), number of umbels (95.89%), seed yield (90.71%), harvest index (89.07%), dry weight (87.09%), and plant height (78.22%), while a low heritability (61.29%) was obtained for days to 50% flowering (Table 2).
For the 2nd year (2020), high phenotypic and genotypic coefficients of variation (CV% ≥ 25) were obtained for both plant fresh and dry weights, number of umbels per plant, seed yield per plant, and harvest index. However, moderately low phenotypic and genotypic coefficients (CV% < 25) were recorded for plant height, days to 50% flowering, and 1000-seed weight. Heritability was high (\({h}^{2}\) ≥ 70) for all the traits studied (Table 2).
The 2-year analysis revealed significant differences in studied populations for all the characteristics, while the effects of year were also significant for all the characteristics except for seed yield. The P × E (Population × Environment) interaction effects were also significant for all the characteristics except for plant height, days to 50% flowering, and leaf essential oil content. In 2019, Qazvin2 population revealed the highest (86.2 cm) and Abarkuh1 had the lowest (43.1 cm) plant height (Table 3). The highest days to 50% flowering was attributed to Hormozgan1 population while that of Bandarkhamir population was the lowest (Table 3). While Qazvin1 population possessed the highest plant fresh (368.2 g) and dry weights (177.3 g), the lowest plant fresh weight (75.2 g) was found for Abarkuh1 and the lowest plant dry weight was observed for Abarkuh2 (37.5 g) (Table 3). The number of umbels varied from 17 to 54 with an average of 33.86 (Table 2). The highest and lowest number of umbels were recorded for Lar and Qazvin1, respectively (Table 3). Moreover, the highest and lowest 1000-seed weights were obtained for Hormozgan1 (4.35 g) and Hormozgan3 (1.94 g) (Table 3). Seed yield in Abadeh was the highest (64.8 g) while the lowest values were recorded for Abarkuh2, Khatam, and Ettehad2. Regarding harvest index, the highest values were measured in Kerman (79.1%) and Hormozgan1 (78.7%), while the lowest ones belonged to Khatam and Qazvin1 populations (Table 3).
In 2020, Qazvin2 proved the population with highest plant height (63 cm) while Abarkuh2 was the shortest (28.83 cm) (Table 3). Bandarkhamir, Mahllat2, and Semirom2 had the earliest flowering times while Ettehad1 (189 days) was the slowest. The highest plant fresh (275.5 g) and dry weights (153.43 g) were observed in Qazvin1 but Abarkuh1 and Bardsir exhibited the lowest. The lowest and highest number of umbels were measured in Qazvin1 (42.3) and Hormozgan2 (86.7) (Table 3). Furthermore, the lowest and highest 1000-seed weights were recorded for Hormozgan1 (3.30 g) and Ferydun-Shahr 2 (1.41 g), respectively (Table 3). Finally, Kerman and Khatam had the highest (58.2 g) and lowest (17.2 g) seeds yield per plant, respectively (Table 3). The harvest index with an average of 0.63 varied from 14 to 79.1 (Table 2). The lowest and highest harvest index were measured in Qazvin1 (13.26) and Hormozgan1 and Lar (84.15) (Table 3).
Leaf and Seed Essential Oil Contents
In both studied years, relatively moderate to low genotypic and phenotypic coefficients (CV% ≤ 25) were obtained for seed essential oil content, while a relatively high leaf essential oil content (CV% ≥ 25) was recorded in 2020 (Table 2). Moreover, a high broad sense heritability (h2b > 80%) was observed for both leaf and seed essential oil contents in both studied years (Table 2). Maximum leaf essential oil percentages in both agronomic years (1% and 1.2%, respectively) were obtained for Abarkuh2 population but the lowest (0.152% and 0.25%, respectively) were observed in Ettehad1 (Fig. 2a). In 2019, seed essential oil percentage was observed to vary from 0.7 to 2.46% with an average value of 1.58% (Fig. 2b). However, in 2020 Abarkuh2 population recorded the highest (2.85%) while Ettehad1 (1.02%) showed the lowest seed essential oil content (Fig. 2b).
Correlation Analysis
Table 4 reports the correlation coefficients established among the characteristics in 2 years. In 2019, plant height showed positive correlation with plant fresh weight (r = 0.67, p < 0.01) and plant dry weight (r = 0.62, p < 0.01) Moreover, number of umbels per plant exhibited significantly positive correlations with seed yield (r = 0.73, p < 0.01) and harvest index (r = 0.63, p < 0.01) as did 1000-seed weight with seed yield per plant (r = 0.61, p < 0.01) and harvest index (r = 0.51, p < 0.05). Finally, harvest index established significant negative correlations with plant fresh weight (r = − 0.66, p < 0.01) and plant dry weight (r = − 0.66, p < 0.01) (Table 4).
In 2020, plant fresh weight revealed positive correlation with plant dry weight (r = 0.79, p < 0.01) and plant height (r = 0.52, p < 0.01). Moreover, it exhibited a negative correlation with harvest index (r = − 0.67, p < 0.01). Plant dry weight and Harvest index showed a significantly negative correlation (r = − 0.79, p < 0.01). The number of umbels per plant showed positive correlation with seed yield per plant (r = 0.70, p < 0.01) and 1000-seed weight (r = 0.41, p < 0.01) (Table 4). Furthermore, the same trait was also positively correlated with harvest index in 2019 but no such correlation was established in 2020 (Table 4). Finally, leaf and seed essential oils were positively correlated in both years.
Cluster Analysis of Morpho-Physiological Data
A dendrogram was constructed based on morpho-physiological traits. The dendrogram classified all the genotypes into four groups in either of the studied years (Fig. 3a, b). In 2019, Group 1 comprised 15 populations (namely, Bandarkhamir, Mahllat1, Lar2, Ettehad1, Hormozgan1, Abadeh, Qeshm, Mahllat2, Kerman, Bardsir, Semirom1, Bandarabbas, Ferydunshahr1, Hormozgan2, and Lar 1) while Group 2 consisted of the populations of Abarkuh1 and Abarkuh2, rich in leaf and seed essential oil contents. Group 3 consisted of the populations of Qazvin1, Qazvin2 and Khatam. Finally, the four populations of Hormozgan3, Ferydunshahr2, Semirom2, and Ettehad2 were assigned to the fourth group. The members of this group had the highest quantities of plant height, and plant fresh and dry weights (Fig. 3a).
Dendrogram generated from cluster analysis of 24 Moshgak population based on agro-morphological characters and essential oil content using Ward clustering method based on the Squared Euclidean dissimilarity calculated of 2019 (a) and 2020 (b). The dashed black line is cut-off value and each color means a group (Color figure online)
Group 1 in 2020 included 13 populations (namely, Bandarkhamir, Semirom2, Hormozgan2, Ferydunshahr2, Semirom1, Ettehad2, Hormozgan1, Abadeh, Mahllat2, Qeshm, Ferydunshahr1, Bandarabbas, and Kerman), while the second consisted of the populations of Ettehad1, Hormozgan3, Mahllat1, and Lar2. The Third one had the five populations with the shortest plant height as well as the highest leaf and seed essential oil percentages (namely, Bardsir, Qazvin2, Lar1, Abarkuh1, and Abarkuh2). Finally, the last group belonging to 2020 contained only the two populations of Qazvin1 and Khatam characterized by the lowest seed yield per plant, high fresh and dry weights, and the shortest plant height (Fig. 3b).
ISSR Amplification and Level of Polymorphism
ISSR analysis based on 15 primers revealed a range of 150–2000 bp in band size. High polymorphic average was obtained (96.37%) for studied primers. Number of polymorphic bands per primer ranged from 8 to 18 with an average value of 14.46 (Table S1). The average of PIC value as an index for extracting information from each marker was 0.48 (Table S1). The lowest value (0.32) was observed in P11[(CA)8RT)] while the highest (0.56) belonged to P7[(AG)8YT)] (Table S1). The amplified product was separated on agarose gel 1.2%, shown as an example in Fig. S1.
Cluster Analysis of Molecular Data
Cluster analysis was carried out to study relationships among the studied populations and their geographical origins (Fig. 4). The best coefficient for cluster construction was determined via the Mantel test (r = 0.83). The cluster analysis classified the populations into six clusters. The first one (I) comprised only the Northwestern populations while the second one (II) contained four subclusters from the Northwestern, Central, and Southern origins. Clusters III, IV, V, and VI included populations from most of the geographical origins examined. However, in most cases, the populations were not classified based on geographical patterns.
Dendrogram of 120 studied Moshgak accessions based on ISSR markers according to the Unweighted Pair Group Mean Algorithm (UPGMA) with the Jaccards similarity index. The dashed red line is cut-off value. I: First group, II: Second group, III: Third group, IV: Fourth group, V: Fifth group, VI: Sixth group. Colors represent geographical areas (Color figure online)
PCoA Results
PCoA was also carried out to reveal the variations among the populations in more detail. While the results revealed 30.42% of the total variation for the first PCo (Fig. 5), they largely confirmed those of the dendrogram in that most of the populations were not grouped based on their geographical patterns.
Genetic Structure of Populations
The population genetic structure of 120 Moshgak accessions examined was investigated with respect to their geographical patterns (Fig. 6). The population level genetic diversity indices are reported in Table 5. The highest values of number of alleles (2), number of effective alleles (1.75), Shannon index (I) (0.60), genetic variation (0.41), and polymorphic loci were observed in the Southern and Central populations, while the lowest values were obtained in the Northern population from Qazvin (Table 5). AMOVA results showed significant variations (p < 0.001) (Table 6). Accordingly, 95.68% of the total variance was detected within the populations and the remaining 4.32% among the groups. The FST value of 0.043 obtained in this study demonstrated a low variance among the groups (Table 6).
Genetic structure of 120 Moshgak accessions as inferred by STRUCTURE software with 15 ISSR markers data set. Single vertical line represents an individual accession and different colors represent genetic stocks/gene pools. Segments of each vertical line show extent of admixture in an individual. The best fit value of K value was 4. A: Central, Southern, Southwestern, Northwestern, and Southeastern regions, B: accessions from all the regions, C: Southwestern, Northwest, Central, and Southern, D: Southern, Northwestern, and Central regions. X: Study populations, Y: Admixture degree of populations. (For interpretation of the references to colour in the text, the reader is referred to the web version of this article) (Color figure online)
Results of STRUCTURE analysis showed that the highest one was observed at K = 4 (Fig. S2). Figure 6 presents the clusters of accessions according to admixture model. Each column in this Figure represents an individual while the different colors demonstrate the gene sets and the sections with different colors in each column show the admixture in that genotype. The dark colored admixture signifies degrees of common ancestry of the accessions; hence, they might not be attributed to a specific population.
Based on the admixture model, the Moshgak accessions were divided into four clusters (color codes: red [A], green [B], blue [C], and yellow [D]) (Fig. 6). The first cluster, in Fig. 6, cluster A included the accessions belonging to the Central, Southern, Southwestern, Northwestern, and Southeastern regions. While cluster B included the accessions from all the regions, cluster C contained those from Southwestern, Northwest, Central, and Southern regions and cluster D included only those from Southern, Northwestern, and Central regions.
Discussion
The ANOVA conducted revealed significant differences in most of the studied characteristics in 2 studied years. High heritability of most of the studied characteristics was not unexpected since the studied characteristics were not only quantitatively inherited but also can be affected by the environment. This confirms the similar trends reported in fennel (Lal 2008) and D. anethifolia (Arbabi et al. 2018). The population × year interaction was also significant for all the traits studied except for plant height and days to 50% flowering, possibly due to different responses of Moshgak populations to the environments examined in different years. However, interaction of population × year had relatively low effects on such morphological characteristics as plant fresh weight and number of umbels (Table 3). For example, Abarkuh1 possessed the lowest plant fresh weight and Qazvin1 showed the lowest number of umbels per plant in both years. The results are in line with those previously reported similar trends for morphological characteristics (Shojaiefar et al. 2015; Arabsalehi et al. 2018). The intensity of P × E interaction in 2019 was high for seed yield per plant as evidenced by the highest value recorded for Abadeh population. This is while Kerman had the maximum value for this trait in 2020. Although higher interaction effects of G × E are reported to reduce selection efficiency in different environments (Yan and Hunt 1998), it is of utmost importance to plant breeders to discern particular genotypes adaptation to specific environments (Flores et al. 1998; Yan and Kang 2003).
A high variation was also obtained in essential oil percentage (EOs) in general, and in both seed and leaf essential oil contents in particular, in both studied years. This is in line with previous researches for EOs in Moshgak (Mostafavi et al. 2008; Sabbaghi Rahimi and Rahimmalek 2019). Similarly, findings of Shojaiefar et al. (2015) showed the high variation in the EOs of fennel in 2 years. It has been established that EOs is highly influenced by environmental factors and plant species (Tohidi et al. 2017; Sarfaraz et al. 2020).
The results of correlation analysis revealed no correlations between the studied traits and leaf or seed essential oil over the 2 agronomic years, while both leaves and seeds essential oil were found to be significantly correlated in both years.
The number of umbels per plant was found positively correlated with seed yield in the 2 studied years, indicating that improving this trait as a yield component might improve yield in Moshgak populations. Similar trend was also obtained in Iranian fennel (Bahmani et al. 2012). From a different perspective, number of umbels and harvest index (r = 0.63, p < 0.01) in 2019 revealed a significantly positive correlation while no such correlation was established between these traits in 2020. It may be noted that Arbabi et al. (2018) reported high and significant correlations among the morphological traits of Moshgak genotypes collected from different sites in southeastern parts of Iran.
The populations studied in the 2 agronomic years were classified into three groups according to their morphological characteristics. Distinct groups were identified for each year in terms of their plant height, plant fresh and plant dry weights, and EOs. In both years, higher genotypic and phenotypic variations in all the morpho-physiological traits were detected except for days to 50% flowering. Finally, cluster 2 (Fig. 2a) consisting of the two populations of Abarkuh1 and Abarkuh2 exhibited the lowest plant fresh and dry weights, shortest plant height, and high EOs in the 2019. Based on the strong correlation of EOs with plant dry and fresh weights as well as that with plant height, it may be suggested that Moshgak breeders need to focus on these plant growth attributes in order to choose the best populations with the highest essential oil content.
The present study assessed the genetic diversity and structure among and within Moshgak genotypes using ISSR markers. The PIC was used to evaluate the validation of polymorphic bands in genetic variation among the populations. Previous studies had classified polymorphism content into three categories of high, medium, and low according to the PIC ranges PIC > 0.5, 0.5 > PIC > 0.25, and PIC < 0.25, respectively (Isshiki et al. 2008; Soleimani et al. 2012; Fadaei Heidari et al. 2016). However, all the primers in the present study revealed PIC values in the range of 0.5 > PIC > 0.25 with an average value of 0.48, implying the efficiency of the ISSR markers employed in developing acceptable polymorphism loci useful for evaluating genetic diversity in Moshgak populations. Similar PIC ranges had also been reported for D. anethifolia (Sabbaghi Rahimi and Rahimmalek 2019) and other Apiaceae species including Trachyspermum ammi (Fadaei Heidari et al. 2016) and Foeniculum vulgare (Maghsoudi Kelardashti et al. 2015).
Genetic diversity among populations as detected by molecular markers and morphological characteristics is an efficient and functional tool for further plant breeding purposes. Previous studies have reported relationships established among molecular characteristics, morphological characters, and geographic origin. Moreover, strong relationships have been reported between morphological traits and molecular markers in medicinal plants. Genetic diversity in Perovskia abrotanoides using ISSR markers has been assessed. Hashemifar and Rahimmalek (2018), for example, found an association between morphological and molecular data. In the present research, a weak correlation was obtained between molecular and morphological characteristics, confirming the similar findings reported in the literatures (Pirkhezri et al. 2010; Fadaei Heidari et al. 2016; Sabbaghi Rahimi and Rahimmalek 2019). This may indicate that morphological diversity is generally independent of ISSR diversity. Morphological characteristics mainly reveal polymorphisms in the coding regions, while molecular markers are the result of both non-coding and coding regions (Martinez et al. 2003).
It was hypothesized that cross-pollination, natural hybridization, and seed propagation might have contributed to the diversity observed. This finding is consistent with that observed in ajowan (Fadaei Heidari et al. 2016). Pollination system has been claimed as another possible reason for the low association between molecular and morphological evaluations (Gaffney et al. 2011). In most Apiaceae plants, a geitonogamous mode highly dependent on wind and insect is presented in their pollination systems (Ahmadi et al. 2014). Furthermore, environmental factors such as habitat fragmentation and plant biological traits as well as pollination system (i.e., degree of self- or cross-pollination) might also be involved in determining morphological traits to affect the weak relationship established between morphological and molecular markers (Soloukiet al. 2008; Ahmadi et al. 2014).
AMOVA analysis displayed that most of the genetic diversity could be attributed to within-population diversity, as confirmed by similar observations reported on other Apiaceae plants (Fadaei Heidari et al. 2016; Sabbaghi Rahimi and Rahimmalek 2019). The high genetic diversity within the groups could be explained by such varied factors as propagation, population size, breeding system, fertility, seed dispersal and pollinators (Sabbaghi Rahimi and Rahimmalek 2019). In Moshgak, the umbel and umbellate are round in shape, of moderate size with a strong odor that can attract pollinators. Furthermore, Moshgak is considered to be an aromatic plant whose pollination mostly occurs by wind and insects. Thus, its remarkable pollen distribution leads to a wide dispersal of alleles in the neighboring region, which improves its within-population genetic variation. Another interesting point worth noting is the high seed yield of Moshgak that might lead to its high seed dispersal by wind. Moreover, the plant height, moderate seed size, and high rate of seed falling are other factors that could contribute to its seed dispersal by wind and its allele distribution over a wide region, thereby giving rise to its enhanced within-population genetic diversity.
The low FST of 0.043 and the high gene flow (Nm = 3.26) showed that the genetic diversity of Moshgak was independent of geographical pattern. This is consistent with previous reports on other Apiaceae species such as Nigella sativa (Kapital et al. 2015).
Based on the ISSR results, a moderate genetic differentiation was observed among the populations (Gst = 0.13). Nei and Li (1979) divided the genetic differentiation coefficients into three classes of low (Gst < 0.05), moderate (0.15 > Gst > 0.05), and high (Gst > 0.15) (Maghsoudi Kelardashti et al. 2015). The present study detected a moderate differentiation and a relatively high gene flow among the populations. Similar data have also been obtained in other researches of Moshgak, including in those of Sabbaghi Rahimi and Rahimmalek (2019), which used SRAP markers. The high gene flow might be attributed to high seed dispersal, population size, and plant mating pattern (Sabbaghi Rahimi and Rahimmalek 2019).
Based on the results obtained from STRUCTURE, a high admixture was obtained in most of the studied genotypes (Fig. 6). These results are consistent with those reported on Moshgak (Sabbaghi Rahimi and Rahimmalek 2019). Pollen distribution might serve as a crucial factor for the admixture in the Apiaceae (Maghsoudi Kelardashti et al. 2015). Another probable reason for this admixture is high level of seed shattering in D. anetifolia. High seed shattering have also been observed in other Apiaceae plants with moderate to large seed size such as Ferula assa-foetida (Hassanabadi et al. 2019). The size of seeds and their wings also play a crucial role in shattering and scattering by wind (Sabbaghi Rahimi and Rahimmalek 2019) and consequently cause a high admixture ratio in these genera. Overall, a knowledge of the wild populations might lead to new insights useful for genetic studies aimed at protecting the natural population diversity, while the information thus gained could also be beneficial in the selection of populations with favorable characteristics.
Conclusion
The results revealed significant variations in morpho-physiological traits and molecular markers in the Moshgak populations. The highest genetic diversity was obtained within the populations. Moreover, a high admixture of accessions was observed among the populations. Essential oil content elevated in most of the studied population in the 2nd year. Finally, according to the genetic distances, EOs, and the morphological characteristics, Abarkuh and Kerman populations might be recommended as appropriate populations for further breeding purposes of Moshgak.
Abbreviations
- ISSR:
-
Inter Simple Sequence Repeats
- CV:
-
Coefficient of variances
- ANOVA:
-
Analysis of variance
- PCoA:
-
Principal coordinate analysis
References
Ahmadi H, Rahimmalek M, Zeinali H (2014) Assessment of the genetic variation of chamomile (Matricaria chamomilla L.) populations using phytochemical, morphological and ISSR markers. Biochem Syst Ecol 54:190–197
Anderson J, Churchill G, Autrique J, Tanksley S, Sorrells M (1993) Optimizing parental selection for genetic linkage maps. Genome 36:181–186
Arabsalehi F, Rahimmalek M, Ehtemam MH (2018) Phytochemical and morphological variation of Stachys lavandulifolia Vahl. populations as affected by genotype × year interaction. Ind Crops Prod 112:342–352
Arbabi M, Naghdi Badi H, Labbafi M, Mehrafarin A, Saboki E (2018) Morphophysiological and phytochemical variability in some wild populations of Ducrosia anethifolia Boiss. from Iran. Chem Biodivers 15:301–323
Bahmani K, Izadi-Darbandi A, SadatNoori SA, Jafari A, Moradi N (2012) Determination of interrelationships among phenotypic traits of Iranian fennel (Foeniculum vulgare Mill.) using correlation, stepwise regression and path analyses. J Essent Oil Bear Plant 15:424–444
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform 1:1–47
Fadaei Heidari E, Rahimmalek M, Mohammadi S, Ehtemam MH (2016) Genetic structure and diversity of ajowan (Trachyspermum ammi) populations based on molecular, morphological markers, and volatile oil content. Ind Crop Prod 92:186–196
Flores F, Moreno MT, Cubero JI (1998) A comparison of univariate and multivariate methods to analyze G × E interaction. Field Crop Res 56:271–286
Gaffney A, Allen G, Brown P (2011) Insect visitation to flowering hybrid carrot seed crops. N Z J Crop Hortic Sci 39:79–93
Gharibi S, Rahimmalek M, Mirlohi A, Majidi MM, Tabatabaei BES (2011) Assessment of genetic diversity in Achillea millefolium subsp. millefolium and Achillea millefolium subsp. elbursensis using morphological and ISSR markers. J Med Plant Res 5:2906–2916
Hashemifar Z, Rahimmalek M (2018) Genetic structure and variation in Perovskia abrotanoides and P. atriplicifolia as revealed by molecular and morphological markers. Sci Hortic 230:169–177
Hassanabadi M, Ebrahimi M, Farajpour M, Dejahang A (2019) Variation in essential oil components among Iranian Ferula assa-foetida L. accessions. Ind Crop Prod 140:598–604
Isshiki Sh, Iwata N, Khan MMR (2008) ISSR variations in eggplant (Solanum melongena L.) and related Solanum species. Sci Hortic 117:186–190
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Bull Soc Vaud Sci Nat 44:223–270
Kapital B, Feyissa T, Petros Y, Mohammed S et al (2015) Molecular diversity study of black cumin (Nigella sativa L.) from Ethiopia as revealed by inter simple sequence repeat (ISSR) markers. Afr J Biotechnol 14:1543–1551
Karami A, Bohlooli A (2017) Essential oil chemical diversity of Ducrosia anethifolia (DC.) Boiss. accessions from Iran. J Essent Oil Bear Plant 5:1342–1348
Lal RK (2008) Stability and genotypes × environment interactions in fennel. J Herbs Spices Med Plants 13:47–54
Liang S, Jie CH, Kai X, Wencai Y (2017) Origin of the domesticated horticultural species and molecular bases of fruit shape and size changes during the domestication, taking tomato as an example. Hortic Plant J 3:125–132
Maghsoudi Kelardashti M, Rahimmalek M, Talebi M (2015) Genetic diversity in Iranian fennel (Foeniculum vulgare Mill.) populations based on sequence related amplified polymorphism (SRAP) markers. J Agric Sci Technol 17:1789–1803
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Martinez L, Cavagnaro P, Masuelli R (2003) Evaluation of diversity among Argentine grapevine (Vitis vinifera L.) varieties using morphological data and AFLP markers. Electron J Biotechnol 6:37–45
Mostafavi A, Afzali D, Mirtadzadini SM (2008) Chemical composition of the essential oil of Ducrosia anethifolia (DC.) Boiss. from Kerman Province in Iran. J Essent Oil Res 20:509–512
Muhaidat R, Brake MH, Al Zoubi M et al (2018) Integrating morphological characters, molecular markers, and distribution patterns to assess the identity of Blepharis species from Jordan. Bot Stud 59:18
Nei M, Li H (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Pirkhezri M, Hassani ME, Hadian J (2010) Genetic diversity in different populations of Matricaria chamomilla L. growing in southwest of Iran, based on morphological and RAPD Markers. J Med Plant Res 4:1–13
Pritchard JK, Wen W (2003) Documentation for STRUCTURE software: version 2. Available from http://pritch.bsd.uchicago.edu
Rahimmalek M, Bahreininejad B, Khorrami M, Sayed Tabatabaaei BE (2009) Genetic variability and geographic differentiation in Thymus daenensis an endangered medicinal plant, as revealed by inter simple sequence repeat (ISSR). Biochem Genet 47:831–842
Rohlf F (1998) NTSYS-pc version 2 0. Numerical taxonomy and multivariate analysis system. Exeter Software Setauket, New York
Sabbaghi Rahimi B, Rahimmalek M (2019) Genetic structure and variation of Moshgak (Ducrosia anethifolia Boiss.) populations based on morphological and molecular markers. Sci Hortic 257:668–676
Sarfaraz D, Rahimmalek M, Saeidi G, Sabzalian MR (2020) Genetic relations among and within wild and cultivated Thymus species based on morphological and molecular markers. 3 Biotech 10:289
Sarfaraz D, Rahimmalek M, Saeidi G (2021) Polyphenolic and molecular variation in Thymus species using HPLC and SRAP analyses. Sci Rep 11:5019
SAS Institute (2001) User’s guide. Release 9.2. SAS Institute, Cary, pp 225–293
Shahabipour S, Firuzi O, Asadollahi M, Faghihmirzaei E, Javidnia K (2013) Essential oil composition and cytotoxic activity of Ducrosia anethifolia and Ducrosia flabellifolia from Iran. J Essent Oil Res 25:160–163
Shojaiefar S, Mirlohi A, Sabzalian MR, Yaghini H (2015) Seed yield and essential oil content of fennel influenced by genetic variation and genotype × year interaction. Ind Crops Prod 71:97–105
Soleimani MH, Talebi M, Sayed Tabatabaei BE (2012) Use of SRAP markers to assess genetic diversity and population structure of wild cultivated and ornamental pomegranates (Punica granatum L.) in different regions of Iran. Plant Syst Evol 298:1141–1149
Solouki M, Mehdikhani H, Zeinali H, Emamjomeh A (2008) Study of genetic diversity in Chamomile (Matricaria chamomilla) based on morphological traits and molecular markers. Sci Hortic 117:281–287
Statgraphics (2016) Statgraphics. Version 17.2.1. Stat Point Inc., Warrenton
Tohidi B, Rahimmalek M, Arzani A (2017) Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem 220:153–161
Verma KS, ul Haq S, Kachhwaha S, Kothari SL (2017) RAPD and ISSR marker assessment of genetic diversity in Citrullus colocynthis (L.) Schrad: a unique source of germplasm highly adapted to drought and high-temperature stress. 3 Biotech 7:288
Yan W, Hunt LA (1998) Genotype by environment interaction and crop yield. Plant Breed Rev 16:135–178
Yan W, Kang MS (2003) GGE biplot analysis: a graphical tool for breeders, geneticists, and agronomists. CRC Press, Boca Raton, p 280
Acknowledgements
We would like to thank Mr. Amir Barzegar for germplasm support and Isfahan University of Technology for financial support.
Funding
The article was supported by Isfahan University of Technology and there are no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10528_2022_10237_MOESM1_ESM.docx
Supplementary file1 (DOCX 87 kb)—Figure S1. ISSR marker profiles of Moshgak accessions generated by P13 ISSR primer. M: 50 bp ladder.
10528_2022_10237_MOESM2_ESM.docx
Supplementary file2 (DOCX 24 kb)—Figure S2. Estimation of Delta K as a prerequisite for the best fit value of K obtained with STRUCTURE analysis.
Rights and permissions
About this article
Cite this article
Arabsalehi, F., Rahimmalek, M. & Sabzalian, M.R. Morpho-Physiological and Molecular Characterization Reveal Low Genetic Variation for Conservation of Endangered Iranian Moshgak (Ducrosia anethifolia Boiss). Biochem Genet 60, 2587–2610 (2022). https://doi.org/10.1007/s10528-022-10237-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-022-10237-0