Abstract
A laboratory colony of the southern green stink bug, Nezara viridula L. (Heteroptera: Pentatomidae) was used to evaluate the effect of two strains of Beauveria bassiana (Balsamo-Crivelli) Vuillemin (Hypocreales: Cordycipitaceae) on its growth and reproductive rates. Four concentrations (106, 107, 108, and 109 conidia g−1) of native (NI8) and commercial (GHA) entomopathogenic fungi alongside a water control were used. Cumulative oviposition and survival of nine groups (ten couple per group per concentration) were used to calculate the demographic parameters of this insect. Two computations were done based on total offspring (fertile and infertile eggs) and eggs with developed embryo (fertile eggs). Net reproductive rates (Ro) on insect sprayed with NI8 calculated based on total offspring showed a dose-dependent effect (72.55, 85.50, 58.15, and 37.60 females per newborn female) compared with water control (87.65). In populations sprayed with GHA, only the highest concentration (109 conidia g−1) was lower than control (27.15). The calculated Ro values based on fertile eggs were much lower as it was expected with 60.75, 61.45, 45.45, and 32.05 females per newborn female from lowest to highest concentrations of NI8 and 21.50 for GHA highest concentration compared with water control (78.45). These results demonstrated that both native and commercial strains affected embryo development, decreasing growth and reproductive rates of N. viridula populations. Further field testing is needed to evaluate the potential for in-field control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The southern green stink bug, Nezara viridula L. (Heteroptera: Pentatomidae) is a destructive and highly polyphagous global pest that causes severe damage to several agricultural crops, including peanut, Arachis hypogaea L., soybean, Glycine max L (Fabales: Fabaceae), tomato, Solanum lycopersicum L. tobacco, Nicotiana tabacum L., potatoes, Solanum tuberosum L. (Solanales: Solanaceae), wheat, Triticum aestivum L., rice, Oryza sativa L., corn, Zea mays L. (Poales: Poaceae), cotton, Gossypium arboretum L. (Malvales: Malvaceae), and all cruciferous vegetables (Musolin 2012). Both nymphs and adults cause damage piercing and sucking part of the host plant tissues and developing fruits, which reduce photosynthesis and impedes plant growth (Esquivel et al. 2018). The potential for transmission of fungal and bacterial diseases directly to the plant’s vasculature system during feeding is heightened and often detrimental to yield, grain, and seed quality (Yukawa and Kiritani 1965; Esquivel and Medrano 2020). Combined, these factors continue to negatively impact the production of crop-derived food, feed, and fuel around worldwide.
Nezara viridula is widely established and distributed throughout tropic and subtropic areas (Esquivel et al. 2018) and still spreading to new locations (Esquivel and Medrano 2020). Worldwide control measures are based on chemical tactics contributing to insecticidal resistance and outbreak populations (Knight and Gurr 2007; Permadi et al. 2018). Infestations of N. viridula seasonally occur in the most valuable commodities including cotton and soybean across the Southern USA now require multiple insecticide applications during a growing season (Portilla et al. 2022a). Significant increases in insecticidal applications are costly for producers and may also have unintentional ecological impacts including mammalian toxicity and non-target effects on communities of beneficial insects (Esquivel et al. 2018). New approaches for managing the pest stink bug complex including N. viridula that are ecologically friendly are needed. The use of the entomopathogenic fungi such us Beauveria bassiana (Balsamo-Crivelli) Vuillemin (Hypocreales: Cordycipitaceae) has been used historically with successful integration in modern IPM in several integrated pest management programs where the climate conditions were favorable for fungal developments, with high susceptibility of the insect target, and a better conidium acquisition of the conidia from contact rather than direct spray. This is the case, for example, for the integrated management of the coffee berry borer, Hypothenemus hampei F. (Coleoptera: Curculionidae) in Colombia (Bustillo and Posada-Florez 1996) and Hawaii (Aristizabal et al. 2016). In these cases, the programs were developed after expending considerable efforts in screening candidate fungi, emphasizing virulence and spore production (Bustillo and Posada-Florez 1996). However, these variables will not be enough to develop a program for N. viridula, that is well known for its highly resistance to pathogenic fungi, high mobility, and frequently molting (Sosa-Gomez et al. 1997; Nada 2015; Gad and Nada 2020; Portilla et al. 2022a, b; Soliman et al. 2022).
Although there are many limitations to using B. bassiana to control N. viridula, a recent study demonstrated that N. viridula immature stages could easily acquire spores from treated surfaces and through direct spray (Portilla et al. 2022a). Portilla et al. (2022b) also demonstrated that high concentrations of native (NI8) (108 and 109 conidia g−1 and a commercial (GHA) (109 conidia g−1) strains of B. bassiana affected its fecundity. Although no significant differences in male survival were found among concentrations, they reported that females were much more susceptible than males. Moreover, several investigations have found effects of B. bassiana on insect populations dynamics including significant reduction in lifetime fecundity, egg hatchability and viability, and reduce fecundity of insect hosts (Noma and Strickler 2000; Fernandez-Ruvalcaba et al. 2010; Ugine 2012). Therefore, the objective of this study was to quantify the embryo development from the cumulative oviposition of N. viridula treated with different concentration of native (NI8) and commercial (GHA) strains of B. bassiana. Demographic parameters, in addition to growth and reproductive rates, were calculated based on total offspring and fertile eggs. This is the first study that estimate the reproductive rates of infected N. viridula populations with B. bassiana and compares the fecundity and fertility values to quantify its natural growth and reproduction after B. bassiana infection.
Materials and methods
Insects
A laboratory-reared N. viridula colony has been maintain at the United State Department of Agriculture, Agriculture Research Service (USDA-ARS), Southern Insect Management Research Unit (SIMRU) in Stoneville, MS, USA since 2018. This colony is regularly maintained following procedures outlined by Portilla and Reddy (2021), which was designed for medium-scale production of even-aged individuals. This study used a Fn generation of female and male adults 3–5 d old fed with an artificial diet (Portilla and Reddy 2021).
Bioassay procedure
The application of B. bassiana, and bioassay procedures were conducted following processes described in Portilla et al. (2022b). Briefly, technical spore powder (106, 107, 108, and 109 conidia g−1) of the native strain NI8 (collected, isolated, and genetically identified in 2005 (McGuire et al. 2006) and produced since then at USDA-ARS Southern Insect Management Research Unit) and the commercial strain GHA (Botani-Gard 22WP) were diluted to obtain concentrations of 5 × 104, 5 × 105, 7 × 106, 7 × 107 conidia ml−1. Aliquots of 6 ml of each spore suspension and water control were applied to groups of ten adult females and ten adult males. The suspensions of both strains were sprayed from the lowest to the highest concentrations using a spray tower modified for Burgerjon tower (Portilla et al. 2022a, b). Conidia mm−2 were quantified by counting spores dropped on disposable microscope cover slips of 2.2 cm2 (S1752) placed under the sprayer during treatment applications. After each application, the nozzles were rinsed once with 10 ml of 10% hypochlorite solution and twice with 10 ml of reverse-osmosis water. Spray nozzles were changed for each strain to avoid cross-contamination. Treated insects (females and males) were released into an insect rearing cage (30 × 30 cm) (1466 PB) (ten cages: ten couples per cage) and placed in environmental chambers (model I36VLC8) set at 27 ± 2C, 55 ± 10%RH, and a L:D 12:12 photoperiod (environmental conditions ideal for N. viridula rearing and reproduction). Insects were fed with artificial diet and kept for oviposition until all adults were dead. Diet was changed twice a week or when needed. The total eggs production of the nine groups (ten couples per group) sprayed with the four concentrations of native (NI8) and commercial (GHA) strains of B. bassiana including water control and their daily survival were used for growth and reproductive rates calculations.
Adult survival and egg collection
Eggs masses laid and pasted by the treated females to the wall of the oviposition cages (30 × 30 cm) were carefully removed every other day and total number of egg mases per cage, number of eggs per egg mass, number of eggs with developed embryo per egg mass, number of eggs with undeveloped embryo per egg mass, and number of eggs with no embryo per egg mass were recorded until all adults died. Adult mortality also was recorded every second day and female and male longevity was calculated. The Image Pro Plus 7.0.1 software was used for morphological differences for both sexes (males have claspers on the terminal abdominal segment), egg counting, and embryo recognition. From the total egg masses collected from each treated group, percentage of egg with undeveloped embryo and egg mass sizes were calculated. The morphological differences for both sexes were accessed to ensure that each treatment contain ten females and ten males.
Embryo recognition, classification, and eggs mass sizes of N. viridula
Egg masses collected from each treated group of adults were stored in plastic solo-cups T-125 with a modified lid (three small openings added to the lids for ventilation, 3–4 mm in diameter) and maintained in environmental incubators (I36VLC8) at 27 ± 2 °C, 75 ± 10% RH, and a L:D 16:8 photoperiod for 4–6 days until fully embryo developed. After 4–6 days of egg storage, the embryo recognition tests were performed according to the description in Portilla and Reddy (2021) as follow: (1) eggs with no embryo—translucent appearance due to the absence of the embryo, (2) undeveloped embryo—opaque-yellow color egg, and (3) developed embryo—orange to reddish coloration that resides primarily in the eye-spots formation and other body parts of the developing embryo. The Image Pro Plus 7.0.1 software was used for egg recognition. Two classified group of oviposition were created for each treated population: (1) total offspring and (2) eggs with developed embryo. Therefore, to evaluate the actual effect of both strains and concentrations of the entomopathogenic fungi, each oviposition group was used separately to calculate each population’s demographic parameters, growth, and reproductive rates. This was performed assuming that, using the total offspring for life fertility table calculation, the values could be greater than the group with fertile eggs only.
Demographic parameters of treated N. viridula populations
Over 14,000 eggs were collected and individually classified. A total of 18 fertility life tables were calculated using codified Excel spreadsheets (Portilla et al. 2014). Calculation of egg-specific survival rate (lx) and age-specific fecundity (mx) was used to estimate the gross fecundity rate (Mx), net reproductive rate (Ro), doubling time (DT), mean generation time (T), finite rate of increase (λ), and intrinsic rate of increase (rm) which was obtained from the Lotka formula (Carey 1993; Krebs 2001). The adult life expectancy (ex) and reproductive values (Vx) were calculated as an additional column in the life and fertility table according to Carey (1993). The method of trial value r was used, incorporating a sex ratio of 50:50 (F:M) and developmental time (egg-adult) of 37 days according to Portilla and Reddy (2021). The experiment was repeated one time and each couple per treatment (ten couples per treatment) were considered replications.
Sublethal and lethal dose of N. viridula populations exposed to B. bassiana
From the survival column (lx) of the 18 fertility tables, mortality data from 15-, 20-, and 30-days post exposure (DPE) were used to estimate sublethal and lethal mortality of the total unsexed population. Estimations were done using spores mm−2.
Statistical analysis
Total number of eggs per collection, number of fertile eggs per collection, eggs with undeveloped embryos per collection, size of egg masses, and female and male longevity were analyzed using ANOVA in SAS Institute (2013) followed by Tukey’s test HSD. Slopes, sublethal (LC15, LC30), lethal concentrations (LC50), and resistance ratios (RR50) were calculated using the PROC PROBIT in SAS and using log10 of the concentrations. Mortality for each treated group was corrected for control effects using Abbott’s formula (Abbott 1925) and confidence intervals for RR50 were calculated using the formula from Robertson and Priesler (1992).
Results
Longevity of N. viridula exposed to different concentrations of B. bassiana
There were no statistically significant differences in male longevity among concentrations sprayed with NI8 (Table 1). However, high significant differences in longevity were found for females sprayed with the same strain (F = 15.19; df = 4, 9; p ≤ 0.0001). There were statistically significant differences in males and females sprayed with GHA strain with F = 19.63 and 15.67, respectively (df = 4, 9; p ≤ 0.0001). There was no dose-dependent between concentration and longevity, but in females treated with the highest concentrations (108 and 109 conidia g−1) of both NI8 and GHA lived shorter than the control, and only GHA highest concentration (109 conidia g−1) affected male longevity (Table 1).
Egg mass sizes, total number of eggs, fertile eggs, and eggs with undeveloped and no embryo of N. viridula populations exposed to different concentrations of B. bassiana
There were statistically significant differences in the number of egg per egg mass among concentrations for NI8 (F = 2.14; df = 4, 133; p ≤ 0.0797) and GHA (F = 2.51; df = 4, 159; p ≤ 0.0442). Table 2 shows that the lowest concentration (106 conidia g−1) of GHA (62.17 ± 3.65 [SE] egg per mass) did not differ from the control (60.84 ± 4.47 eggs per mass) where both were larger than the other treatments. The smallest egg masses were obtained from couples sprayed with NI8 with the highest concentration (109 conidia g−1) (43.07 ± 5.39 egg per mass), followed by insects sprayed with the GHA 108 and 109 conidia g−1 with 46.08 ± 29.67 and 46.17 ± 29.67 eggs per mass, respectively. Similarly, Table 2 shows that although there were not statistically significant differences between couples sprayed with NI8 on the total number of eggs and number of fertile eggs per collection, there was a dose-dependent effect. Statistically significant differences among concentrations were observed for the total eggs and fertile eggs on couples treated with GHA, and eggs with undeveloped embryo on couple spray with NI8 and GHA strains (Table 2). The lowest number of eggs with undeveloped embryo was obtained in water control (11.50 ± 3.12) followed by 18.42 ± 5.55, 36.07 ± 8.68, 35.30 ± 8.76, and 31.86 ± 6.26 for NI8 and 36.75 ± 7.07, 28.22 ± 3.33, 33.37 ± 10.63, and 28.83 ± 2.86 for GHA from lowest to highest concentrations, respectively.
Total offspring, demographic parameters, and growth and reproductive rates of N. viridula treated with different concentration of B. bassiana
Nezara viridula sprayed with the native strain NI8
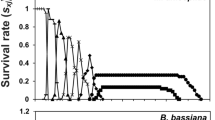
All demographic parameters were affected by NI8 applications at all concentrations using both calculations of total offspring and eggs with developed embryo (Table 3). The Mx, mx, Ro, rm, and λ values had a negative correlation with concentration. As expected, the demographic parameter values with the total offspring calculation were greater than that of egg with developed embryo calculation suggesting that the population treated with B. bassiana NI8 reduced N. viridula net reproductive rate (Ro) from 17% (72.55 female per new-born female) with the lowest concentration (106 conidia g−1) to 57% (37.60 females per new-born female) with the highest concertation (109 conidia g−1) compared to control (water) (87.65 females per new-born female). These values were even lower when the eggs with undeveloped or no embryo were removed from the calculation. Therefore, the true R0 value was reduced from 33% (60.75 females per new-born female) with the lowest concentration to 60% (32.05 females per new-born female) with the highest concentration compared with control (78.45 female per new-born female). No correlation was observed on T and DT when calculations were done with either total offspring or eggs with developed egg (Table 3), but adults sprayed with the highest concentration duplicate its population (11.26 d) almost three days slower than the control (8.94 d). The total population’s life expectancy (ex) of the total population (Fig. 1a) showed a high variability between control and the infected populations. At the same time, the calculation of the ex of the females (Fig. 1b) showed the susceptibility of females except for one of the lower concentrations (107 conidia g−1) that was higher than the control. The calculation of the gross fecundity (Mx) and reproductive value (Vx) are presented in Figs. 1cdef. Results demonstrated how the population of undeveloped and no embryo from the total offspring impacted N. viridula growth and reproductive rates as shown in Fig. 1c vs. Fig. 1d (Mx) and Fig. 1e vs. Fig. 1f (Vx).
Growth and reproductive values of N. viridula populations sprayed with different concentrations of NI8 native strain of B. bassiana. a. Life expectancy calculated using the entire population (females-males) vs. b. Life expectancy calculated using females. c, e. Gross fecundity and the reproductive value calculated using the total offspring vs. d, f. calculated using fertile eggs with developed embryo only. Adults were fed with an artificial diet and fresh corn. Concentrations presented in spores ml−1
Nezara viridula sprayed with the commercial strain GHA
Compared to NI8, all demographic parameters were affected by GHA applications only with the highest concentration (109 conidia g−1) using both the total offspring calculation and the eggs with the developed embryo calculation (Table 4). The mx, Ro, rm, and λ values were affected with concentrations of 108 and 109 conidia g−1. Unexpectedly, the demographic parameters values of the couples sprayed with the lowest concentrations (106, 107 conidia g−1) were higher than the control. However, and similarly to NI8, the values calculated using the total offspring were greater than those of the egg with developed embryo calculation. The population treated with GHA 109 conidia g−1 reduced N. viridula net reproductive rate (Ro) to 69% (27.15 females per new-born female) that increased to 73% (21.5 females per new-born female) when removed from the calculation eggs with undeveloped and no embryo (Table 4). Both GHA calculations were higher than NI8 (57% and 60%, respectively) at the highest concentration. Like NI8, no correlation was observed on T and DT when calculations were done with either total offspring or eggs with developed eggs (Table 4). No difference in T and DT value was observed between GHA and NI8 in couples sprayed with the highest concentration: both groups duplicate their population three days slower (12.02 d) than the control (8.94 d). The life expectancy (ex) of the total population (Fig. 1a) and the ex of the females (Fig. 1b) showed similar trends among concentrations, suggesting that the commercial GHA will not kill N. viridula adults even with the highest concentration. However, and similarly to NI8, this commercial entomopathogenic fungi will affect reproduction and prevent embryo development which will reflect in its growth and reproductive rates mainly with the highest concentration as shown in Fig. 2c vs. Fig. 2d (Mx) and Fig. 2e vs. Fig. 2f (Vx).
Growth and reproductive values of N. viridula populations sprayed with different concentrations of GHA commercial strain of B. bassiana. a. Life expectancy calculated using the entire population (females-males) vs. b. Life expectancy calculated using females. c, e. Gross fecundity and the reproductive value calculated using the total offspring vs. d, f. calculated using fertile eggs with developed embryo only. Adults were fed with an artificial diet and fresh corn. Concentration presented in spores ml−1
Sublethal and lethal mortality of N. viridula to B. bassiana strains NI8 and GHA.
Although, no significant regression was obtained at any time of evaluation as LC15, and LC50 was determined by PROC PROBIT (Table 5), results show that the unsexed population of N. viridula was more susceptible to the native NI8 than the commercial GHA. Sublethal and lethal mortality reduced over the post-exposure period for both strains, dropping the LC50 from 10,443 conidia mm−2 (15 DPE) to 391 spores mm−2 (30 DPE) and from 106 conidia mm−2 (15 DPE) to 424 conidia mm−2 (30 DPE) for NI8 and GHA, respectively.
Discussion
Life and fertility tables constructions are typically used to determine the contribution to the future population that individual females will make (Carey 1993; Krebs 2001) varying depending on the population’s exposure factors. In the case of N. viridula, the growth and intrinsic reproductive rates under field conditions are unknown. However, a few studies previously calculated its demographic parameters under laboratory conditions using host plants and an artificial diet (Fortes et al. 2006; Gonzales and Ferrero 2008; Portilla et al. 2015; Rojas and Morales-Ramos 2023). The rm values (0.077–0.079) obtained from control (water) in our study using the rearing method from Portilla and Reddy (2021) differed from Portilla et al. (2015) (0.074) using an artificial diet but were similar to those found by Fortes et al. (2006) (0.076) who used green beans for N. viridula reproduction. Ro values, however, were much lower (87.65, 78.45) in our control than that in Portilla et al. (2015) (130.8) and Fortes et al. (2006) (132.7), but they were similar to the Ro value (117.5, 142.40) found in our infected population with the lower concentration of the commercial GHA. The rearing method using in this study was appropriate for N. viridula rearing and suitable to demonstrate the impact of B. bassiana on its reproduction. The interpretation of the fertility tables is the speed of a population increase, measured by λ and determined by rm and estimated for future populations (Krebs 2001). In our case the fertility tables calculated using total offspring should represent this insect’s true and ecological relevant growth and reproductive rates. However, it must be considered that a significant proportion of the total oviposition included eggs that do not survive to contribute to the future population (eggs with undeveloped or no embryo) even for control. The rearing method used in this study (Portilla and Reddy 2021) reported a rate of 13.49% of not fertile eggs, which was close to the rate that was observed in our control (10.10%) (Table 2). For example, NI8 reduced N. viridula Ro from 17% (72.55 female per new-born female) with the lowest concentration (106 conidia g−1) to 57% (37.60 females per new-born female) with the highest concertation (109 conidia g−1) compared to control (water) (87.65 females per new-born female). These values were lower when the eggs with undeveloped or no embryo were removed from the calculation (10.10, 17.88, 31.67, 30.43, and 29.76% for control, 106, 107, 108, and 109 conidia g−1, respectively), reducing its Ro from 33% (60.75 females per new-born female) with the lowest concentration to 60% (32.05 females per new-born female) with the highest concentration compared with control (78.45 females per new-born female). In the case of GHA, the Ro for the lowest concentrations were unexpectedly higher than the control, but as shown in Table 4, Fig. 1c-f and Fig. 2c-f, the values of the actual growth and reproductive rates were much lower after removing 20.64, 24.97, 31.48, and 32.03% of not fertile eggs from the total oviposition of populations exposed from the lowest to the highest concentration of GHA, respectively, compared with the water control (10.10%). This is the first study that reports that B. bassiana affects embryo development on N. viridula, and no comparative study was available. However, there are reports that this entomopathogenic fungus affected the feeding and ovipositional behavior of Lygus hesperus knight (Hemiptera: Miridae) (Noma and Strickler 2000), decreased rate of reproduction of Lygus lineolaris Palisot de Beauvois (Hemiptera: Miridae) (Ugine 2012), and reduced oviposition and hatchability on Rhipicephalus microplus Canestrini (Acari: Ixodidae) (Fernandez-Ruvalcaba et al. 2010).
Studies on N. viridula and other Pentatomid’s mating behavior (Evans 1982; Panizzi and Mourao 1999; Portilla et al. 2015; Portilla and Reddy 2021) reported that fecundity depends on the quantity and quality of mating events. Portilla et al. (2015) found oviposition in N. viridula 2–3 days after every mating. Therefore, including males in ex calculation is fundamental for this insect to determine the true expected total lifetime fecundity at a given age of both sexes. This study corroborated many previous studies (Sosa-Gomez et al. 1997; Nada 2015; Gad and Nada 2020; Portilla et al. 2022a, b; Soliman et al. 2022) showing that N. viridula adults exhibit resistance to B. bassiana. The high variability of the life expectancy (ex) of N. viridula (total population) exposed to NI8 and GHA among concentrations including control (Fig. 1a and 2a) suggested that both strains did not significantly affect the population for this insect, which was verified in Table 5 where the concentration of 29.6-fold (10,443 spores mm−2) higher than the highest concentration of NI8 (356 spores mm−2) and 28.5 × 105-fold (100,000 conidia mm−2) higher than the highest concentration of GHA (352 conidia mm−2) required to kill 50% of unsexed population of N. viridula 15 days after exposure. Similar values were found by Portilla et al. (2022b) (1,9 × 103 conidia mm−2 by contact and 3.3 × 106 conidia mm−2 by direct spray) under laboratory conditions and Panizzi and Mourao (1999) (19.6 × 107 spore ml−1) under field conditions. However, it is essential to clarify, that females were to be found 106-fold (236 conidia mm−2) and 107-fold (326 conidia mm−2) more susceptible than males sprayed with NI8 and GHA, respectively (20 DAE). This behavior can be easily observed when the ex values were independently calculated for females (Fig. 1b and 2b). The female susceptibility based on ex trend showed a superior performance of NI8 compared to GHA.
In general, our results indicated that N. viridula female and male populations are highly resistant to B. bassiana and the concentration of both NI8 and GHA suggested in this study and in Portilla et al. (2022a) are considered impractical for its control. However, it is essential to consider that the real impact of this fungus is caused primarily by preventing embryo development. The life fertility tables constructed in this study indicated variation depending on the concentration of the B. bassiana strain even at sublethal doses (22 conidia mm−2). Conversely, the environmental conditions (27 ± 2C, 55 ± 10%RH, and a L:D 12:12 photoperiod) used in these experiments were optimal for N. viridula rearing and reproduction, not for B. bassiana development, meaning that these effects could be found in N. viridula populations under field conditions. Therefore, confirmations in-field studies are needed.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Aristizabal LF, Bustillo AE, Arthurs SP (2016) Integrated pest management of coffee berry borer: strategies from Latino America that could be useful for coffee farmers in Hawaii. Insects 7:6
Bustillo AE, Posada-Florez FJ (1996) El uso de entomopatogenos en el control de la broca del café in Colombia. Manejo Integr Plagas 42:1–13
Carey FG (1993) Applied demographic for biologist with special emphasis on insects. Oxford University Press, Oxford, UK.
Esquivel JF, Medrano IG (2020) Retention of Pantoea agglomerans SC1R across stadia of the southern green stink bug, Nezara viridula (L.)(Hemiptera: Pentatomidae). PLoS ONE 15(12):e0242988
Esquivel JF, Musolin D, Jones W, Rabitsch WG, Jeremy T, Schwertner CF (2018) Nezara viridula (L). In: McPherson RM (ed) Invasive stink bugs and related species (Pentatomidae); biology, higher systematics, semiochemistry and management, 1st edn. CRC Press, Boca Raton, Fl, pp 351–423
Evans EW (1982) Consequences of body size for fecundity in the predatory stink bug Podisus maculiventris (Hemiptera: Pentatomidae). Ann Entomol Soc Am 75:418–420
Fernandez-Ruvalcaba F, Berlanga-Padilla AM, Cruz-Vazquez C, Hernandez-Velazquez VM (2010) Evaluacion de cepas de Beauveria bassiana y Metarhizium anisopliae sobre la inhibicion de oviposicion, eclosion y potencial reproductivo en una cepa triple resistente de garapata Rhipicephalus (Boophilus) microplus (Canestrini) (Acari: Ixodidae). Entomotropica 25:109–115
Fortes P, Magro SR, Panazzi AR, Parra JP (2006) Development of a dry artificial diet for Nezara viridula (L.) and Euschistus heros (Fabricious) (Heteroptera: Pentatomidae). Neotrop Entomol 35:567–572
Gad AA, Nada MS (2020) Effect of entomopathogenic fungi Beauveria bassiana on the cellular immunity and biochemistry of green bug Nezara viridula L. J Biopestic 13:135–144
Gonzales JOW, Ferrero AA (2008) Table of life and fecundity by Nezara viridula var. Smaragdula (Hemiptera: Pentatomidae) fed on Phaseolus vulgaris L. (Babaceae) fruits. Idesia (Chile) 26:9–13
Kapongo JP, Shipp L, Kevan P, Broadnemt B (2008) Optimal concentration of Beauveria bassiana vectored by bumble bees in relation to pest and bee mortality in greenhouse tomato and sweet pepper. BioControl 53:797–812
Knight KM, Gurr GM (2007) Review of Nezara viridula (L.) management strategies and potential for IPM in field crops with emphasis on Australia. J Crop Prot 26:1–10
Krebs CJ (2001) Ecology: the experimental analysis of distribution and abundance, 5th edn. Wesley Longman, San Francisco, Ca., USA, p 695
Lotka AL (1907) Studies on the mode of growth of material aggregates. Am J Sci 24:199–216
Musolin D (2012) Surviving winter: diapause syndrome in the southern green stink bug Nezara viridula in the laboratory, in the field, and under climate changes conditions. Physiological Entomol 37:309–322
Nada SM (2015) Response of green stinkbug Nezara viridula (L), to the activity of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae. J Plant pro Pathol 6:1633–1644
Noma T, Strickler K (2000) Effects of Beauveria bassiana on Lygus hesperus (Hemiptera: Miridae) feeding and ovipositing. Environ Entomol 29:394–402
McGuire M, Leland J, Dara S, Park YH, Ulloa M (2006) Effect of different isolates of Beauveria bassiana on field populations of Lygus hesperus. Bio Control 38:390–396
Marin P, Posada-Forez FJ, Gonzales MT, Bustillo AE (2000) Calidad biologica de formulaciones de Beauveria bassiana usadas en el control de la broca del café Hypothenemus hampei (Ferrari). Rev Colomb Entomol 26:17–23
Panizzi AR, Mourao PM (1999) Mating, ovipositional rhythm, and fecundity of Nezara viridula (L.) (Heteroptera: Pentatomidae) fed on privet, Ligustrum lucidum Thunb., and on soybean, Glycine max (L.) merrill fruits. Ann Soc Entomol Brazil 28:35–40
Permadi MA, Lubis RA, Harahap MQH, Siregar US (2018) Virulence of entomopathogenic fungi isolates against green ladybug Nezara viridula L. (Hemiptera: Pentatomidae) eggs. J Agrohita 2:52–60
Portilla M, Ramos-Morales J, Rojas G, Blanco C (2014) Life table as tools of evaluation and quality control for arthropods mass production. In: Morales Ramos J (ed) Mass production of beneficial organisms. Academic Press, New York, pp 248–275
Portilla M, Reddy GVP (2021) Development of a method for rearing Nezara viridula (Heteroptera: Pentatomidae) on a semi-solid artificial diet. J Ins Sci 12:12
Portilla M, Snodgrass G, Street D, Luttrell R (2015) Demographic parameters of Nezara viridula (Heteroptera: Pentatomidae) reared on two diets developed for Lygus spp. J Ins Sci 15:165
Portilla M, Zhang M, Glover JP, Reddy GVP, Johnson C (2022a) Lethal concentration, and sporulation by contact and direct spray of the entomopathogenic fungus Beauveria bassiana on different stages of Nezara viridula (Heteroptera: Pentatomidae). J Fungi 8:1164
Portilla M, Reddy GVP, Tertuliano M (2022b) Effect of two strains of Beauveria bassiana on the fecundity of Nezara viridula L. (Heteroptera: Pentatomidae). Microbiol Res 13:514–522
Robertson JL, Priesler HK (1992) Pesticide bioassay with arthropods. CRC Press, Boca Raton, Fl. USA
Rojas MG, Morales-Ramos JA (2023) Effects of taurines as dietary supplement on the biological and demographic parameters of Nezara viridula (Heteroptera: Pentatomidae). J Ins Sci 23:6
SAS Institute (2013) SAS/STAT user’s manual, version 9, 4th ed. SAS Institute, Cary, NC, USA.
Soliman AM, Nada MS, Gad AA (2022) Evaluation the effects of the entomopathogenic fungus Beauveria bassiana (Ascomycota: Hypocrales) on some histological and physiological parameters for the green bug Nezara viridula (L.) (Hemiptera: Pentatomidae). Alexandria Sci Ex J 43:229–238
Sosa-Gomez DR, Boucias DG, Nation JL (1997) Attachment of Metarhizium anisopliae to the southern green stink bug Nezara viridula cuticle and fungistatic effect of cuticular lipids and aldehydes. J Invertebr Pathol 69:31–39
Ugine T (2012) The effect of temperature and exposure to Beauveria bassiana on tarnished plant bug Lygus lineolaris (Heteroptera: Meridae) population dynamics, and the broader implication of treating insects with entomopathogenic fungi over the range of temperatures. Biol Control 59:373–383
Yukawa J, Kiritani K (1965) Polymorphism in the southern green stink bug. Pacific Ins 7:639–642
Acknowledgements
The work is supported by USDA-ARS Research Project# 6066-22000-090-00D-Insect Control and Resistance Management in Corn, Cotton, Sorghum, Soybean, and Sweet Potato, and Alternative Approaches to Tarnished Plant Bug Control in the Southern United States. The authors thank Tabatha Nelson and Kelnisha Westbrook – ARS-USDA-SIMRU for maintaining the Nezara viridula egg production, recognition, and classification. Thanks to Julianna Jojoa, LA summer student—Pollinator Health in Southern Crop Ecosystem Research Unit, for her valuable help entering data.
Funding
This work is supported by USDA-ARS Research Project # 6066–22000 = 090-00D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institution review board statement
Not applicable.
Informed consent statement
Not applicable.
Additional information
Handling Editor: Linda Muskat.
Rights and permissions
About this article
Cite this article
Portilla, M., Tertuliano, M., Parys, K. et al. Effects of Beauveria bassiana on the growth and reproductive rates of Nezara viridula. BioControl 69, 413–425 (2024). https://doi.org/10.1007/s10526-024-10258-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-024-10258-1