Abstract
The population density of Eucallipterus tiliae (L.) (Hemiptera: Aphididae) required for initiating oviposition in Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) was established in Tilia cordata Mill. (Dicotyledoneae: Tiliaceae) stands sampled weekly throughout the vegetative seasons of 2017–2019. The number of aphids per leaf area and oviposition in H. axyridis females were determined at each sampling session. Seasonal changes in aphid abundance and the presence of ovipositing females in natural populations varied across years but in all years H. axyridis started oviposition as soon as the aphid density increased above 10.2 aphids per ten sweeps, which equals 1.04 aphids per 100 cm2 leaf area. These values exceed the threshold aphid density for starting oviposition in Coccinella septempunctata L. (Coleoptera: Coccinellidae), which is 0.40 aphids per 100 cm2 leaf area. This difference is in line with H. axyridis preference for trees, host plants with complex architecture and hard to search, and preference of C. septempunctata for spatially simple herbs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), the Harlequin ladybird, is a species native to east Palearctic and Oriental regions (Kovar 2007) recently spread into all continents except Antarctica (Camacho-Cervantes et al. 2017). In its native range (Kuznetsov 1975) as well as in recently colonized areas (Riddick 2017), this species is an efficient natural enemy of aphids and other insect pests (Hodek and Honek 2009). Following the unintended introductions H. axyridis became also an unwelcome competitor and predator of other members of aphidophagous guild (Brown et al. 2015; Kenis et al. 2017; Masetti et al. 2018; Zaviezo et al. 2019). Negative effects on communities of native aphidophagous species have caused an enormous interest in biology of H. axyridis (Roy et al. 2016). However, despite the vast amount of research work on H. ayridis biology, some aspects of species’ life cycle remain little studied. These include the factors of timing of species reproduction in the wild.
The oviposition activity of aphidophagous coccinellids is limited to a period (window) limited by two terms: It begins when the prey abundance reaches the level necessary for the females to start laying eggs and ends when the females are discouraged from further reproduction by an oviposition-deterring pheromone (Ruzicka 1997). Under laboratory conditions, the closing of the oviposition window has been studied in several coccinellid species (Oliver et al. 2006), including H. axyridis (Almohamad et al. 2010; Verheggen et al. 2017). Even in the wild oviposition likely terminates at the moment when the concentration of pheromone is high enough for the females to stop laying eggs. In contrast, the conditions necessary for the start of oviposition were studied less frequently (e.g., Honek 1978, 1980; Wright and Laing 1980). This is because coccinellids in the wild land soon after aphid arrival and begin to lay eggs quickly. Consequently, observing this ephemeral event is difficult. Knowledge of prey abundance required to start laying eggs for particular species of predators is necessary for assessing the timeliness and efficiency of biocontrol.
Laboratory studies have revealed that coccinellid species require a minimum amount of food to start egg maturation and oviposition (e.g., Ibrahim 1955; Ives 1981; Ferran et al. 1984; Stewart et. al. 1991; Agarwala et al. 2001). In the wild, the concurrent growth of aphid abundance and intensity of ladybird oviposition has been observed frequently (Hemptinne et al. 1993; Agarwala and Bardhanroy 1999; Schellhorn and Andow 2005). However, difficulties arise when we have to determine the threshold aphid population density necessary to start coccinellid oviposition. This is because the population density of both coccinellids and aphids is usually very low at that time (Wright and Laing 1980; Honek 1980). This difficulty especially applies to H. axyridis, which, in its native area in Japan, starts reproduction very soon after the beginning of the development of aphid populations, on herbs (Takahashi and Naito 1984; Takahashi 1987; Nakata 1995) as well as on trees (Hironori and Katsuhiro 1997). However, even in this case, the aphid abundance threshold has not been determined. Determining the threshold aphid abundance in Europe is even more difficult for several reasons: H. axyridis lives mainly on trees where the timing of the presence of aphids through the vegetative season varies from year to year. Therefore, it is not possible to set fixed sampling plans in advance. In lime trees, T. cordata Mill. (Dicotyledonae, Tiliaceae), the population peak of Eucallipterus tiliae (L.) (Hemiptera: Aphididae) may occur from spring to late summer. When the aphid abundance peaks in the spring, H. axyridis oviposition starts quickly after aphid arrival. When the aphid abundance peaks in late summer, H. axyridis adults stay on lime trees, long before aphid outbreak, feed on substitute prey, and start oviposition only after aphid arrival (Honek et al. 2019c). At the beginning of H. axyridis reproduction period, the number of batches laid is small and finding the batches scattered in the rich foliage of linden trees is impracticable. The onset of oviposition can be identified by sampling and detecting the presence of ovipositing females, which, after being caught, quickly deposit eggs (Honek et al. 2008).
In our study, we combined this method of detecting oviposition and the parallel determination of aphid abundance at the site of collection. A three-year study of the relationship between the occurrence of ovipositing females and aphid abundance made it possible to determine the threshold population density that the aphids had to reach for H. axyridis females to start laying eggs.
Material and Methods
Sampling
Harmonia axyridis and aphids were sampled in the western part of the Czech Republic at nine sites situated within a 5.0 × 1.5 km area (centred at 50.0860 °N, 14.2954 °E), at an altitude of 290–340 m. The sites selected as clusters of 5–25 lime trees (Tilia cordata) that were at least 200 m apart from each other. The beetles were swept at particular sites from the lower canopy, from heights up to 3 m (measured from the ground). Sampling was performed by the same person (AH) using a standard sweep net (35 cm in diameter, 140 cm handle) on sunny and calm days between 08h00 and 18h00. Each sampling session lasted 15–30 min depending on the number of trees. Sampling at each site was performed in 10-day (2017) or weekly (2018–2019) intervals, starting from foliage expansion in the spring until leaf shedding in the autumn. In total, there were 359 sampling sessions (defined as samplings at individual), with a mean of 81.0 ± 1.4 sweeps per session (range 40–190 sweeps; the number of sweeps taken at particular sites varied depending on the number of trees). Harmonia axyridis adults were sexed using the criteria of McCornack et al. (2007). Males were released, and females were collected for checking for oviposition. E. tiliae (L.) was the only aphid species found during the study. All the aphids swept during a sampling session were counted, and the number of aphids caught per unit effort (ten sweeps) was calculated. Pilot experiments revealed that aphid numbers were estimated with ~ 10% precision (Honek et al. 2018).
Calibrating aphid abundance
The relationship between aphid numbers in the swept material and the abundance of aphids (number of aphids per unit leaf area) was established in a short-term sampling study performed on the 3 and 4 September 2019, at a time when the aphid abundance at individual sites was very diverse. Aphids (E. tiliae) were sampled, at 14 sites, the lime tree groups growing within the above-mentioned area where H. axyridis was sampled and the recorder (AH) and method of sweeping were the same as above. At each site, 5 × 10 sweeps were made, and aphids were precisely counted in each of the ten sweep samples. Aphids were then counted on 250 randomly selected leaves, and 150 randomly selected leaves were cut and brought to the laboratory where their area was measured using a Li-3000 Portable Area Meter (Li-COR Biosciences, Lincoln, Nebraska, USA). Using these data, the aphid population density (number of aphids per 100 cm2 area) was calculated. The regression model of the ln aphid number per 100 cm2 leaf area vs. the ln aphid number per 10 sweeps was calculated (Fig. 1) and used to determine the threshold aphid population density necessary to start H. axyridis oviposition.
Detecting oviposition
At each sampling session, H. axyridis females were put singly into Eppendorf tubes (55 mm long, 16 mm diameter) with a perforated lid and an inserted piece of filter paper facilitating oviposition and climbing. The numbers of samples of females collected at particular sampling sessions were 80 in 2017, 92 in 2018 and 80 in 2019. Females were not caught in each sampling session. Therefore, the number of samples examined was lower than the total number of sampling sessions. The females were immediately transferred into a room with a natural photoperiod and maintained at a constant temperature of 25 °C. Due to variations in H. axyridis abundance and sex ratios, the sample size (the number of females per sample) varied largely (mean size 6.00 ± 0.36 females per sample; range, 1–26 females). The females were held in Eppendorf tubes for 48 h, and during this period the presence of batches and the number of deposited eggs were recorded in 2–6 h intervals. Most batches (> 90%) were deposited within 12 h after capture.
Data analysis
The seasonal variations in the presence/absence of H. axyridis oviposition and aphid abundance were examined separately in each vegetative season of 2017–2019. The presence of ovipositing females in a sample was classified alternatively as absent (no ovipositing female, class 0) or present (≥ 1 ovipositing females, class 1). Aphid abundance, D, in each sample (number of aphids per ten sweeps) was calculated as D = (10 N)/S, where N is the total number of aphids caught per sampling session and S is the number of sweeps made during this sampling session. The seasonal change in the density of aphids in each season was modelled using a semi-parametric regression within the generalised additive models (GAM) framework from the mgcv package in the R environment (Wood 2006) because the relationship was strongly non-linear. Thin-plate spline of the time predictor and Gaussian distribution of errors were used. To meet the assumption of homoscedasticity and normality of errors logarithmic transformations were applied to the densities prior to analysis.
The threshold aphid density for starting oviposition was calculated using the data on H. axyridis oviposition and aphid abundance established in samples from all sites and years. Since the frequency of ovipositing females at the start of oviposition period could have been low, the calculations were done using the data from samples where ≥ 4 females of H. axyridis were found. Samples consisting of 1–3 females were considered only if at least one female was ovipositing. In the calculations, the segments of the data from the sampling sessions immediately preceding and immediately following the start of oviposition (the first date in the season on which a female was found to oviposit) were included. For each year and site, three samples from class 0 from the period immediately preceding the start of oviposition and ≤ 3 of the earliest samples from class 1 following the start of oviposition were included (i.e., a maximum of six consecutive samples, or less when the number of class 1 samples was smaller). When no oviposition was detected in the given year and site, three consecutive samples from class 0 collected at the period when the aphid abundance was highest were included in the analysis. The outlier data caused by the accidental arrival of ovipositing females from neighbouring trees to lime trees with zero aphid abundance were excluded from analysis. The relationship between the presence/absence of H. axyridis oviposition and aphid abundance was studied using logistic regression within the generalised linear model framework (Pekár and Brabec 2016) with a binomial error structure and a logit link function. All analyses were performed in the R environment (R Core Team 2017).
In an earlier work (Honek 1980) we determined threshold aphid population density for starting oviposition in C. septempunctata L. (Coleoptera: Coccinellidae). This data was used to compare the difference between the threshold aphid density (set as corresponding to 50% presence of reproductive activity among the predators) necessary for starting oviposition in H. axyridis and the threshold aphid density necessary for starting oviposition of C. septempunctata. The difference was tested using Dixon’s Q test for outlier values (Anonymous 2019).
Results
Oviposition activity
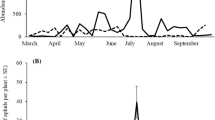
Seasonal distribution of H. axyridis oviposition and aphid abundance varied among sites and years (Fig. 2). In 2017, there was an overall low population density of aphids. The density had a significantly non-linear unimodal trend (GAM, effective degrees of freedom (EDF) = 7.1, P < 0.0001) with a peak early in the vegetative season (Fig. 2a). In 2018, there was an overall high population density with a significantly non-linear unimodal trend (GAM, EDF = 7.3, P < 0.0001) and a wide peak in the mid-season (Fig. 2b). In 2019, the aphid population density was lower with a significantly non-linear unimodal trend (GAM, EDF = 3.7, P = 0.004) and a peak that occurred very late in the season (Fig. 2c). Correspondingly, the timing of H. axyridis oviposition activity also differed. In 2017, oviposition was recorded in spring and only at some sites, and no oviposition was detected for the rest of the vegetative season (Fig. 2a). Altogether it was recorded in seven samplings. In 2018 oviposition occurred from early to mid season (on 33 samplings) (Fig. 2b). In 2019, H. axyridis oviposition occurred only in 13 samplings, from early to late season (Fig. 2c). Oviposition always began with occurrence of aphids and terminated soon after the peak of aphid abundance.
Seasonal changes in the population density of Eucallipterus tiliae and the occurrence of oviposition in Harmonia axyridis in 2017 (a), 2018 (b) and 2019 (c). The black line is the fitted semi-parametric model, and the grey shaded areas are the 95% confidence bands. The rugs along the upper horizontal line show the occurrence of oviposition in H. axyridis
Threshold aphid abundance necessary for H. axyridis oviposition.
The transition from non-oviposition (presence of females not laying eggs) to oviposition (presence of active ovipositing females in the population) was associated with a significant increase in the aphid population density (GLM, χ21 = 15.3, P < 0.0001, Fig. 3). A 50% probability of the switch from non-oviposition to oviposition was estimated to be at 10.2 (CI95 = 8.6, 11.8) aphids per ten sweeps. This abundance corresponded to an aphid population density of 1.04 aphids per 100 cm2 leaf area (Fig. 1), which is the threshold aphid abundance required to achieve H. axyridis oviposition.
Relationship between the Eucallipterus tiliae (aphid) population density and the oviposition probability in Harmonia axyridis. The estimated logistic model (black line) and 95% confidence bands (grey shaded area) are shown. The marks along the upper horizontal line show aphid density available to H. axyridis samples which oviposited, the marks along the lower horizontal line show aphid density available to H. axyridis samples which did not oviposit
Discussion
The aphid abundance threshold necessary for the onset of oviposition in aphidophagous coccinellids is an essential mechanism in their breeding cycle. Establishing this threshold under natural conditions is methodologically difficult. Therefore, these results are still scarce, and obtaining new results is of great importance.
The threshold aphid density necessary to start H. axyridis oviposition (set as corresponding to 50% presence of reproductive activity among the predators) found in this study is low: 10.2 aphids per ten sweeps (i.e., 1.04 aphids per 100 cm2 leaf area). Nevertheless, these values are still higher than those observed earlier for C. septempunctata (Honek 1980). Using average data from four stands of beans (V. faba L.), sugar beets (B. vulgaris L., two stands), cereals (T. aestivum L., H. vulgare L., six stands) and alfalfa (M. sativa L., six stands) collected in 1978 and 1979, the aphid population density (leaf area per aphid) at the time of ovariole maturation was calculated at 240.0 ± 21.4 cm2 per aphid, which equals 0.43 ± 0.033 aphids per 100 cm2 leaf area. The threshold for C. septempunctata was significantly lower (Dixon’s Q test: n = 7, Q = 0.706, P < 0.01) than the threshold established in this study for H. axyridis.
There are two possible causes of the difference. The first is that we used a different method for establishing the ability to lay eggs, i.e., the dissection of C. septempunctata females and actual oviposition under standardised conditions in H. axyridis. The presence of mature eggs in ovaries does not automatically mean that they will be deposited in natural conditions. Some H. axyridis females may not oviposit within two days after capture even when they have mature eggs. Nevertheless, we believe that the risk of this type of bias in our study is low because we established the presence/absence of oviposition in a sample of H. axyridis females and, in each of the samples used to determine the threshold aphid abundance, there were ≥ 4 females. The second reason for the difference between the requirements of both species, in our opinion, is that there is a real difference between the aphid abundance threshold in both species. Coccinella septempunctata may actually start ovipositing at a lower aphid population density than H. axyridis. We assume that this difference is due to the different ecological requirements of both species. While invasive non-native populations of H. axyridis are typical inhabitants of trees in Europe (Roy et al. 2016), C. septempunctata is a typical inhabitant of herbaceous stands and crops (Honek et al. 2019b). Trees and herbs differ in their whole plant size and structure (spatial complexity of branching, richness and area of foliage), which is definitely greater in trees than in herbs and crops (Skuhrovec et al. 2016). Since body size and food consumption are nearly identical in both species (Honek et al. 2017), C. septempunctata colonising spatially simple herb and crop host plants may start ovipositing at a lower population density than H. axyridis populating spatially complicated tree host plants. This difference might thus reflect that searching and finding prey is easier in herbs and crops than in trees.
Between both coccinellid species, a difference in the timing of oviposition under natural conditions was already found. In a particular stand, where both species developed together and the aphid population density gradually increased, C. septempunctata started oviposition earlier, that is, at a lower aphid population density, than H. axyridis (Hironori and Katsuhiro 1997; Honek et al. 2019a).
Comparison with the results of other authors is difficult due to differences in the methods used, aphid species and host plants included in the studies. Wright and Laing (1980) established for C. maculata (DeGeer) (Coleoptera: Coccinellidae) and H. tredecimpunctata (L.) (Coleoptera: Coccinellidae) that the threshold value is likely ≤ 1 aphid per young maize plant (approximately 10 cm high, leaf area not established), which is also a similar, rather low value as in our study. In other studies, a rapid onset of coccinellid oviposition was found as soon as the number of aphids began to increase rapidly (Hemptinne et al. 1993; Agarwala and Bardhanroy 1999; Schellhorn and Andow 2005). However, an estimate of the aphid threshold abundance cannot be made using data from these studies.
The results likely revealed variability among coccinellid species in the threshold density of the aphid population necessary for starting reproduction. This variability is an important phenomenon since it affects the timing of reproduction, which is vital for the life histories of the species (e.g., ability to compete with other aphidophagans, possibility to complete development before the onset of unfavourable conditions, etc.). The study of this variability should continue, taking into account other coccinellid species and all factors that might affect threshold prey densities necessary for reproduction.
References
Agarwala BK, Bardhanroy P (1999) Numerical response of ladybird beetles (Col., Coccinellidae) to aphid prey (Hom., Aphididae) in a field bean in north-east India. J Appl Entomol 123:401–405
Agarwala BK, Bardhanroy P, Yasuda H, Takizawa T (2001) Prey consumption and oviposition of the aphidophagous predator Menochilus sexmaculatus (Coleoptera: Coccinellidae) in relation to prey density and adult size. Env Entomol 30:1182–1187
Almohamad R, Verheggen FJ, Francis F, Haubruge E (2010) Intraguild interactions between the predatory hoverfly Episyrphus balteatus (Diptera: Syrphidae) and the Asian ladybird, Harmonia axyridis (Coleoptera: Coccinellidae): effect of larval tracks. Eur J Entomol 107:41–45
Anonymous (2019) Dixon’s Q test: definition, step by step examples + Q critical values tables. Accessed 20 Dec 2019 https://www.statisticshowto.datasciencecentral.com/dixons-q-test/.
Brown PMJ, Ingels B, Wheatley A, Rhule EL, De Clercq P, van Leeuwen T, Thomas A (2015) Intraguild predation by Harmonia axyridis (Coleoptera: Coccinellidae) on native insects in Europe: molecular detection from field samples. Entomol Sci 18:130–133
Camacho-Cervantes M, Ortega-Iturriaga A, del-Val E, (2017) From effective biocontrol agent to successful invader: the harlequin ladybird (Harmonia axyridis) as an example of good ideas that could go wrong. PeerJ 5:e3296
Ferran A, Cruz de Boelpaepe MO, Schanderl H, Larroque MM (1984) Les aptitudes trophiques et reproductrices des femelles de Semiadalia undecimnotata (Col.: Coccinellidae). Entomophaga 29:157–170
Hemptinne JL, Dixon AFG, Doucet JL, Petersen JE (1993) Optimal foraging by hoverflies (Diptera, Syrphidae) and ladybirds (Coleoptera, Coccinellidae)—Mechanisms. Eur J Entomol 90:451–455
Hironori Y, Katsuhiro S (1997) Cannibalism and interspecific predation in two predatory ladybirds in relation to prey abundance in the field. Entomophaga 42:153–163
Hodek I, Honek A (2009) Scale insects, mealybugs, whiteflies and psyllids (Hemiptera, Sternorrhyncha) as prey of ladybirds. Biol Control 51:232–243
Honek A (1978) Trophic regulation of postdiapause ovariole maturation in Coccinella septempunctata (Col.: Coccinellidae). Entomophaga 23:213–216
Honek A (1980) Population density of aphids at the time of settling and ovariole maturation in Coccinella septempunctata (Col., Coccinellidae). Entomophaga 25:427–430
Honek A, Dixon AFG, Martinkova Z (2008) Body size and the temporal sequence in the reproductive activityof two species of aphidophagous coccinellids exploiting the same resource. Eur J Entomol 105:421–425
Honek A, Martinkova Z, Ceryngier P (2019a) Different parasitization parameters of pupae of native (Coccinella septempunctata) and invasive (Harmonia axyridis) coccinellid species. Bull Insectol 72:77–83
Honek A, Martinkova Z, Roy HE, Dixon AFG, Skuhrovec J, Pekar S, Brabec M (2019b) Differences in the phenology of Harmonia axyridis (Coleoptera: Coccinellidae) and native coccinellids in Central Europe. Env Entomol 48:80–87
Honek A, Martinkova Z, Strobach J (2018) Effect of aphid abundance and urbanization on the abundance of Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 115:703–707
Honek A, Brabec M, Martinkova Z, Dixon AFG, Pekár S, Skuhrovec J (2019c) Factors determining local and seasonal variation in abundance of Harmonia axyridis (Coleoptera: Coccinellidae) in Central Europe. Eur J Entomol 116:93–103
Honek A, Martinkova Z, Evans EW, Skuhrovec J (2017) Estimating prey consumption in natural populations of Harmonia axyridis (Coleoptera: Coccinellidae) using production of feces. J Econ Entomol 110:2406–2012
Ibrahim MM (1955) Studies on Coccinella undecimpunctata aegyptiaca Reiche. 2. Biology and life-history. Bull Soc Entomol Égypte 39:395–423
Ives PM (1981) Feeding and egg production of two species of coccinellids in the laboratory. Can Entomol 113:999–1005
Kenis M, Adriaens T, Brown PMJ, Katsanis A, San Martin G, Branquart E, Maes D, Eschen R, Zindel R, van Vlaenderen J, Babendreier D, Roy HE, Hautier L, Poland R (2017) Assessing the ecological risk posed by a recently established invasive alien predator: Harmonia axyridis as a case study. BioControl 62:373–384
Kovar I (2007) Coccinellidae. In: Löbl I, Smetana A (Eds) Catalogue of palaearctic Coleoptera. Volume 4. Apollo Books, Stenstrup
Kuznetsov VN (1975) Fauna and ecology of coccinellids (Coleoptera, Coccinellidae) in the primorye territory. Trudy Biologo-Pochvennogo Instituta, Novaya Seria 28:3–24
Masetti A, Magagnoli S, Lami F, Lanzoni A, Burgio G (2018) Long term changes in the communities of native ladybirds in Northern Italy: impact of the invasive species Harmonia axyridis (Pallas). BioControl 63:665–675
McCornack BP, Koch RL, Ragsdale DW (2007) A simple method for in-field sex determination of the multicolored Asian lady beetle Harmonia axyridis. J Insect Sci 7:10
Nakata T (1995) Population fluctuations of aphids and their natural enemies on potato in Hokkaido, Japan. Appl Entomol Zool 30:129–138
Oliver TH, Timms JEL, Taylor A, Leather SR (2006) Oviposition responses to patch quality in the larch ladybird Aphidecta obliterata (Coleoptera: Coccinellidae): effects of aphid density, and con- and heterospecific tracks. Bull Entomol Res 96:25–34
Pekár S, Brabec M (2016) Modern analysis of biological data. Generalised linear models in R. Masaryk University Press, Brno
R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna URL https://www.r-project.org/. Accessed 15 Mar 2020
Riddick EW (2017) Spotlight on the positive effects of the ladybird Harmonia axyridis on agriculture. BioControl 62:319–330
Roy HE, Brown PMJ, Adriaens T, Berkvens N, Borges I, Clusella Trullas S, Comont R, De Clercq P, Eschen R, Estoup A, Evans EW, Facon B, Gardiner MM, Gil A, Grez AA, Guillemaud T, Haelewaters D, Herz A, Honek A, Howe AG, Hui C, Hutchinson WD, Kenis M, Koch RL, Kulfan J, Lawson Handley L, Lombaert E, Loomans A, Losey J, Lukashuk AO, Maes D, Magro A, Murray KM, San Martin G, Martinkova Z, Minnaar IA, Nedved O, Orlova-Bienkowskaja MJ, Osawa N, Rabitsch W, Ravn HP, Rondoni G, Rorke SL, Ryndevich SK, Saethre MG, Sloggett JJ, Soares AO, Stals R, Tinsley MC, Vandereycken A, van Wielink P, Viglasova S, Zach P, Zakharov IA, Zaviezo T, Zhao Z (2016) The harlequin ladybird, Harmonia axyridis: global perspectives on invasion history and ecology. Biol Invas 18:997–1044
Ruzicka Z (1997) Recognition of oviposition-deterring allomones by aphidophagous predators (Neuroptera: Chrysopidae, Coleoptera: Coccinellidae). Eur J Entomol 94:431–434
Schellhorn NA, Andow DA (2005) Response of coccinellids to their aphid prey at different spatial scales. Popul Ecol 47:71–76
Skuhrovec J, Martinkova Z, Lukas J, Dixon AFG, Saska .(2016). Role of plant stature in determining habitat preferences of coccinellid species. Ecology of Aphidophaga. Retrieved from https://aphidophaga.de/fileadmin/Datein/Conference_Guide_online.pdf
Stewart LA, Hemptinne JL, Dixon AFG (1991) Reproductive tactics of ladybird beetles: relationships between egg size, ovariole number and development size. Funct Ecol 5:380–385
Takahashi K (1987) Differences in oviposition initiation and sites of lady beetles, Coccinella septempunctata brucki Mulsant and Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) in the field. Japan J Appl Entomol Zool 31:253–254
Takahashi K, Naito A (1984) Seasonal occurrence of aphids and their predators (Col. Coccinellidae) in alfalfa fields. Bull Natl Grassl Res Inst 29:62–66
Verheggen FJ, Vogel H, Vilcinskas A (2017) Behavioral and immunological features promoting the invasive performance of the Harlequin ladybird Harmonia axyridis. Front Ecol Evol 5:156
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall/CRC, Boca Raton
Wright EJ, Laing JE (1980) Numerical response of coccinellids to aphids in corn in southern Ontario. Can Entomol 112:977–988
Zaviezo T, Soares AO, Grez AA (2019) Interspecific exploitative competition between Harmonia axyridis and other coccinellids is stronger than intraspecific competition. Biol Control 131:62–68
Acknowledgement
We thank Ms Helena Uhlířová and Jana Kohoutová for their excellent technical assistance. Alois Honek and Zdenka Martinkova were supported by grant number QK1910281 from the Ministry of Agriculture of the Czech Republic and grant number 17-06763S of the Czech Science Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has a conflict of interest.
Research involving human participants and/or animals
Research involved no human or animal participants.
Additional information
Handling Editor: Eric Riddick
Rights and permissions
About this article
Cite this article
Honek, A., Martinkova, Z. & Pekar, S. Threshold aphid population density for starting oviposition in Harmonia axyridis. BioControl 65, 425–432 (2020). https://doi.org/10.1007/s10526-020-10019-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-020-10019-w