Abstract
Aspergillus spp. are the most common phytopathogenic fungi able to produce various types of aflatoxins. Yeasts can produce volatile organic compounds (VOCs) that may be used as biocontrol agents against mycotoxigenic fungi. In this study, we aimed to evaluate antagonistic yeasts that are potentially capable of producing active VOCs against the aflatoxin-producing fungus, Aspergillus flavus A39. In total, 366 epiphytic and endophytic yeast strains isolated from leaves of rice, sugarcane, and corn in Thailand were screened for their potential. Only 49 yeast strains were able to produce antifungal volatile organic compounds (VOCs). Candida nivariensis DMKU-CE18 was the most effective yeast strain to inhibit the mycelium growth (64.9 ± 7.0% inhibition) and conidial germination (49.3 ± 3.3% inhibition) of A. flavus A39, and to reduce aflatoxin production (74.8 ± 6.5% reduction) in corn grains. The analysis results of headspace gas chromatography/mass spectrometry (GC/MS) revealed that the major VOC produced by this yeast strain was closest to 1-pentanol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytopathogenic fungi present one of the most significant problems in agriculture. They can cause damages and reduce the quality of agricultural products. Various fungal species can also produce mycotoxins (Bu'Lock 1980). Aspergillus, one of most important phytopathogenic fungi found in agricultural products, can produce various types of aflatoxins. A. flavus, A. parasiticus, A. nomius, and A. tamarii are commonly found in or on foods and feedstuff (Moss 1998). Contamination of agricultural products by aflatoxins leads to the annual destruction of an estimated 25% or more of the world’s food crops and feed (World Health Organization 2018). In Thailand, aflatoxins have been detected in food and feed products. The highest rate of contamination was found in peanuts (36% of all contaminated foods), followed by milk (20.7%), and poultry (17.5%) (Waenlor and Wiwanitkit 2003). Consumption of aflatoxin-contaminated food/feed products can pose a serious health threat to humans and livestock. It has been demonstrated that aflatoxins are carcinogenic agents in many animal species (Amaike and Keller 2011; Waliyar et al. 2015). The four main types of aflatoxins are B1, B2, G1, and G2 (Zain 2011). Aflatoxin B1 (AFB1) is most frequently found in agricultural crops, such as peanuts, corn, rice, soybeans, spices, etc. (Guchi 2015; Magan et al. 2003; Mannaa and Kim 2016; Waenlor and Wiwanitkit 2003). Moreover, the International Agency of Research on Cancer (IARC) categorized AFB1 into a group I carcinogen for humans (Min et al. 2011).
Yeasts are easily cultivated, fast growing, and readily found in a variety of substrates and conditions (Türker 2014). Several yeast strains have been identified as potential biocontrol agents against mycotoxigenic fungi. For example, Kluyveromyces thermotolerans strains were able to control A. carbonarius, A. niger, and ochratoxin A (OTA) in grapes (Ponsone et al. 2011). When used as a dip treatment during coffee processing, Saccharomyces cerevisiae reduced the incidence of ochratoxigenic mold, such as that of A. niger, A. ochraceus, and OTA, without affecting cup quality (Velmourougane et al. 2011). A supplement of 2% S. cerevisiae in the control diet containing AFB1 (200 ng g−1) was found to partly counteract some of AFB1’s toxic effects in growing chicks (Çelýk et al. 2003). The antagonistic characteristics of yeasts have been attributed to mechanisms such as competition for nutrients (Raspor et al. 2010); secretion of antifungal compounds, such as killer toxins (Petersson and Schnürer 1995); and production of hydrolytic enzymes (Comitini and Ciani 2010), siderophores (Ismail et al. 1985), and volatile organic compounds (VOCs) (Rezende et al. 2015). Therefore, yeast could be a promising antagonistic agent against mycotoxigenic fungi in post-harvest biocontrol.
In recent years, increased focus has been placed on VOCs produced by microorganisms as biological control agents. For instance, 3-methyl-1-butanol and 2-methyl-1-butanol produced by S. cerevisiae could inhibit the development of Phyllosticta citricarpa which causes citrus black spot (Toffano et al. 2017). The VOCs produced by Lachancea thermotolerans have revealed their potential to protect tomatoes inoculated with Fusarium oxysporum (Zeidan et al. 2018). In addition, Grzegorczyk et al. (2017) hypothesized that VOCs could be one of the main mechanisms of antagonistic Debaryomyces hansenii KI2a and Wickerhamomyces anomalus BS91 against Monilinia fructigena and M. fructicola which cause considerable economic losses in stone fruit crops.
Saprophytic yeasts are common on surfaces and tissues of plant leaves and fruits (Khunnamwong et al. 2018; Limtong and Nasanit 2017; Srisuk et al. 2019). As previously mentioned, some yeast strains effectively compete with post-harvest fungal pathogens. Saprophytic yeasts’ potential capability has also been shown when used in several applications to reduce aflatoxin contamination in food and agricultural products (Hua et al. 1999; Masoud and Kaltoft 2006). However, to the best of our knowledge, only a few articles have reported the use of antifungal VOCs produced by yeasts (Chen et al. 2018; Oro et al. 2018; Payne et al. 2000; Zeidan et al. 2018). In this study, we therefore aimed to evaluate the potential capability of saprophytic yeasts isolated from plant leaves to produce active VOCs against aflatoxin-producing fungi.
Materials and methods
Microorganisms
Three hundred and sixty-six yeast strains (165 epiphytic and 201 endophytic) were previously isolated from the surfaces and tissues of leaves of economic crops including rice, sugarcane, and corn in Thailand (Khunnamwong et al. 2018; Srisuk et al. 2019; Into et al. pers. communication). An aflatoxin-producing fungus, Aspergillus flavus A39 isolated from bael, was obtained from Dr Amara Chinaphuti, Department of Agriculture (DOA), Thailand. Other strains of A. flavus: CH016, CH033, CH271, CH307, and CH464, were obtained from Kasetsart University Research and Development Institute (KURDI). The yeast strains and fungi were maintained at 4 °C on yeast malt (YM) extract agar (0.3% malt extract, 0.3% yeast extract, 0.5% peptone, 1.0% dextrose, and 1.5% agar) and potato dextrose agar (PDA) (Titan Biotech LTD, India), respectively.
Screening of antagonistic yeast strains against aflatoxin-producing fungi
All yeast strains were tested for their potential against A. flavus A39 by performing the dual culture method (Rosa et al. 2010). Yeast culture (48 h old grown on PDA at 28 °C) was streaked on PDA, 2 cm from one dish edge. A 5 mm diameter disc of A. flavus A39 mycelium (seven days old grown on PDA at 28 °C) was placed 2 cm from the opposite edge of the dish. A control experiment was prepared by inoculation of the fungi without yeast. The dishes were sealed with parafilm and incubated at 28 °C in the dark for seven and 14 days. After incubation, the mycelium growth was measured. Each treatment was conducted in duplicate. Yeast strains that inhibited fungal mycelium growth were selected for further screening of antifungal VOC-producing yeasts.
Screening of VOC-producing yeasts against aflatoxin-producing fungi
To evaluate the production of antifungal VOCs by the selected yeasts, two-partition polystyrene Petri dishes were used (Rosa et al. 2010). Yeast culture (48 h old grown on PDA at 28 °C) was streaked onto PDA on one side of a Petri dish and incubated at 28 °C for 48 h. After incubation, a 5 mm diameter disc of A. flavus A39 mycelium (seven days old grown on PDA at 28 °C) was inoculated on the other side of the dish. The control experiment was performed without yeast inoculation. The dishes were sealed with parafilm and incubated at 28 °C in the dark for seven and 14 days. Two replications were conducted. The diameter of the fungal colony was measured and the fungal growth inhibition (%) was calculated compared to the control. The antifungal VOC-producing yeasts were then selected for further determination with other strains of A. flavus.
Efficacy of VOC-producing yeasts on fungal mycelium growth inhibition
The efficacy of VOC-producing yeasts against A. flavus A39 was determined in accordance with Farbo et al. (2018). Aliquots of 100 μl of yeast cell suspension [107 cells ml−1 prepared from yeast culture grown on yeast extract peptone dextrose (YPD) broth (1% yeast extract, 2% bacteriological peptone, 2% dextrose) at 28 °C, 150 rpm for 24 h] were evenly spread on PDA agar dishes and incubated at 28 °C for 48 h. The dish cover was then replaced by a PDA dish. Twenty microliters of fungal spore suspension (106 spores ml−1) prepared in Ringer’s solution [9% (w/v) NaCl, 0.2% (w/v) CaCl2, 0.2% (w/v) KCl, and 0.1% (w/v) Tween 20] were spotted onto the center of the PDA dish. The dishes were sealed with parafilm and incubated at 28 °C for seven days in the dark. Each treatment was conducted in triplicate. The control experiment was performed without yeast inoculation. The radial growth of fungal mycelium was measured and the fungal growth inhibition (%) was calculated compared to the control.
Efficacy of VOC-producing yeasts on fungal conidial germination inhibition
Yeast strains were cultured in YPD broth at 28 °C, 150 rpm for 24 h. The slide culture technique was applied in this experiment. Briefly, a cotton pad was placed at the center of a Petri dish and a glass slide was placed on top of the cotton pad. Five ml of sterile distilled water were added to soak the cotton pad. A 5 mm square block of PDA was then placed in the center of the glass slide. Fungal spores (seven days old grown on PDA at 28 °C) were inoculated on four sides of the agar square with a teasing needle. A sterile cover slip was placed on the upper surface of the agar cube. The dish was then covered by a dish containing 48 h-old yeast culture and sealed with parafilm. The control experiment was performed without yeast inoculation. Three replications were conducted. The conidial germination was investigated after incubation at 28 °C for 21 h (Zhou et al. 2018). At least 100 conidia per treatment were observed at 100× magnification with a light microscope (Olympus CX21). The germinated spores were counted with the inhibition of conidial germination calculated and compared to the control, in accordance with Gong et al. (2015). In addition, the germ tube length was measured and the germination rating scale was recorded (germination rating scale: 1 = no germination; 2 = germ tube < 2× conidium size; 3 = germ tube 24× to 4× conidium size; 4 = germ tube > 4× conidium size) (Zhou et al. 2018).
Efficacy of VOC-producing yeasts on aflatoxin B1 reduction in corn grains
Ten g of corn grains were placed in one side of a two-partition Petri dish and inoculated with 1 ml of fungal conidial suspension (106 spores ml−1). Then, 5 ml of sterile distilled water were added to a cotton pad on the other side of the dish. The dish was covered by a dish containing 48 h-old yeast culture and sealed with parafilm. The dish was incubated at 28 °C for 14 days in the dark. The control treatment was carried out the same way but without yeast inoculation on the PDA dish. All treatments were conducted with three replications. For aflatoxin extraction, 10 g of corn grain sample were ground and then mixed with 50 ml of 70% methanol. The samples were shaken for 30 min at 300 rpm. The extract was filtered through a Whatman No. 4 filter paper. The filtrate was analyzed by ScreenEZ® Aflatoxin ELISA Test Kit (Siam Inter Quality, Thailand) (Chinaphuti et al. 2002).
Analysis of volatile organic compounds (VOCs)
An efficient yeast strain was cultured in a 20 ml headspace vial containing 7 ml of PDA and incubated at 28 °C in the dark for two days. The VOC compositions were then analyzed by headspace-gas chromatography/mass spectrometry (GC/MS). The GC/MS analysis was conducted, in accordance with Suwannarach et al. (2017), with slight modifications. The volatiles in the air space above the yeast culture were trapped using headspace for 45 min at 30 °C. The headspace was inserted into the splitless injection port of a gas chromatograph [Agilent 7890A for gas chromatography (GC) and 5975C MDS for mass spectrometry (MS), Agilent Technologies, USA] equipped with a DB-WAX capillary column (30 m × 0.25 mm, film thickness 0.25 µm) (Supelco, USA). The column temperature was programmed at 40 °C for 2 min and then to increase to 200 °C at 5 °C min−1. Helium was the carrier gas. Prior to trapping the volatiles, the headspace was cleaned at 250 °C for 57 min under the flow of helium gas. All mass spectra were compared with the data system library [National Institute of Standards and Technology (NIST08)]. Blank sample analysis (growth medium without yeast inoculation) was performed under the same conditions. Three replications were conducted.
Statistical analysis
Statistical analysis was performed using one-way ANOVA with IBM SPSS Statistics 20.0. Differences were considered significant when p ≤ 0.05.
Results
Antagonistic yeasts against A. flavus A39
The results of primary screening using the dual culture assay revealed that, of the total 366 yeast strains, 127 (39 epiphytic and 88 endophytic) could inhibit the mycelium growth of A. flavus A39. Only 49 yeast strains of 13 species (34 strains) belonging to Ascomycota and 14 species (15 strains) in Basidiomycota were able to produce antifungal VOCs against A. flavus A39 (see Supplementary Table S1). In addition, only Candida tropicalis DMKU-RE01 had the capability to inhibit the other five tested strains of A. flavus. Saitozyma flava DMKU-RE67 and W. anomalus DMKU-RP25 were able to inhibit four strains, while the other 11, 15, and 11 yeast strains inhibited three, two, and one strains of A. flavus, respectively. Moreover, nine yeast strains were unable to inhibit these A. flavus strains (see Supplementary Table S1).
Efficacy of VOC-producing yeasts on fungal mycelium growth inhibition
Of the total 49 yeast strains, 46 were able to produce antifungal VOCs against the growth of fungal mycelium when using face-to-face plate assay. The fungal growth inhibition varied from 1.9 ± 2.6 to 64.9 ± 7.0%. The statistical results revealed that the efficacy of the VOCs produced by these yeasts was statistically clustered into ten groups (F48,98) = 9.362, p ≤ 0.001) (Table 1). The most effective strain against the growth of A. flavus A39 was Candida nivariensis DMKU-CE18 (64.9 ± 7.0% inhibition), followed by Naganishia liquefaciens DMKU-CE84 (38.9 ± 6.9% inhibition), Kwoniella heveanensis DMKU-CE82 (34.4 ± 3.0% inhibition), Hannaella sinensis DMKU-CP430 (34.4 ± 7.4% inhibition), and W. anomalus DMKU-RP25 (31.1 ± 21.0% inhibition) (Table 1).
Efficacy of VOC-producing yeasts on fungal conidial germination inhibition
The inhibition of conidial germination varied from 9.3 ± 13.6 to 49.3 ± 8.7% among the 46 yeast strains that could inhibit mycelial growth (Table 1). The most effective strain for reducing conidial germination was C. nivariensis DMKU-CE18 (49.33 ± 8.7% inhibition), followed by Saitozyma sp. DMKU-SE54 (45.0 ± 22.9% inhibition) and Meyerozyma caribbica DMKU-RP47 (44.0 ± 11.3% inhibition). Although, no significant difference was found in the inhibition of conidial germination by the VOCs produced by the tested strains (see scale 1 in Table 1) (F48,98 = 0.560, p > 0.05), C. nivariensis DMKU-CE18 seemed to be the most effective in reducing spore germination as it resulted in a low percentage of germinated spores in each germination rating scale.
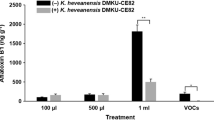
Reduction of aflatoxin in corn grains
Five potential yeast strains, based on the previous results, were selected for in vivo assay in corn grains (Fig. 1). The most effective strain in reducing AFB1 in corn grains was C. nivariensis DMKU-CE18 (74.8 ± 6.5% reduction), followed by K. heveanensis DMKU-CE82 (62.7 ± 10.5% reduction), N. liquefaciens DMKU-CE84 (60.2 ± 7.3% reduction), H. sinensis DMKU-CP430 (52.6 ± 22.4% reduction), and W. anomalus DMKU-RP25 (51.4 ± 10.3% reduction). In addition, all strains significantly reduced aflatoxin in corn grains when compared to the control experiment (F5,12 = 9.182, p ≤ 0.001). However, the efficacies of VOCs in reducing aflatoxin were not significantly different among these yeasts (p = 0.104).
Volatile organic compound (VOC) compositions
The most effective antifungal VOC-producing yeast strain, C. nivariensis DMKU-CE18, was selected for HS-GC/MS analysis. Three volatile compounds were detected from the yeast strain. The compound that was closely matched to 1-pentanol was the major volatile compound produced by C. nivariensis DMKU-CE18 (Table 2).
Discussion
Phytopathogenic fungi cause serious damages to and low quality in agricultural products. Fungal growth destroys pre- and post-harvest fruit, vegetables, and grains (Aidoo 1993; Spadaro and Gullino 2004; Wilson and Pusey 1985). Chemical fungicides have been widely used to control pathogenic fungi in agriculture. However, this strategy affects consumer health and raises environmental concerns. Antagonistic microorganisms have been successfully applied against fungal growth in food and agricultural products. This strategy generates benefits, not only in biocontrol efficacy, but also in safety for human beings (Gotor-Vila et al. 2017; Huang et al. 2012; Leelasuphakul et al. 2008).
Numerous microorganisms may be used as biocontrol agents against mycotoxigenic fungi. Several yeast strains have been identified as potential biocontrol agents. For instance, Hua et al. (1999) reported saprophytic yeasts, Pichia anomala WRL-076 and C. krusei WRL-038, isolated from the fruits of almond, pistachio, and walnut trees, were inhibitors to aflatoxin biosynthesis by A. flavus. In addition, Afsah-Hejri (2013) reported that five saprophytic yeasts comprising Pseudozyma fusiformata, Cryptococcus albidus, Rhodotorula fragaria, Cryptococcus hungaricus, and Rhodotorula hinula showed the highest level of biocontrol activities against A. flavus PTCC 5006. Their inhibitory effects on sporulation, colony expansion, biomass production, and prevention of AFB1 production were evaluated. P. fusiformata was the most effective yeast strain in the reduction of fungal spores (84.6%) and the inhibition of AFB1 production (89.1%).
In the current study, several yeast strains in Ascomycota and Basidiomycota were able to produce antifungal VOCs against A. flavus A39. The production of VOCs seemed to be strain-dependent not species-dependent. The most effective strain to inhibit the mycelium growth and conidial germination of A. flavus A39 and to reduce aflatoxin B1 production by this fungus in corn grains was C. nivariensis DMKU-CE18. This yeast strain was isolated from corn leaf tissue (Khunnamwong et al., 2018). A few previous studies on the ability of Candida spp. to produce antifungal VOCs have been reported. For example, C. intermedia C410 produced volatile compounds that effectively suppressed the conidial germination and mycelial growth of Botrytis cinerea. This could control Botrytis fruit rot in strawberries (Huang et al. 2011). Candida sake 41E and F36A produced VOCs which inhibited the growth of five pathogens of apples, comprising Penicillium expansum, B. cinerea, Alternaria alternata, A. tenuissima, and A. arborescens (Arrarte et al. 2017). To the best of our knowledge, the current study is the first to reveal that C. nivariensis could produce VOCs against the growth and conidial germination of A. flavus. It also reduced the production of aflatoxin when co-inoculated into the fungal-contaminated corn grains. However, C. nivariensis has been found as a pathogenic strain in humans (Alcoba-Flórez et al. 2005). This strain has been reported to have antifungal resistance including against azoles (Borman et al. 2008; Figueiredo-Carvalho et al. 2016; Fujita et al. 2007). Therefore, the utilization of VOCs from this strain by excluding yeast cells could be an alternative approach for safe treatment against the growth of A. flavus in agricultural products. In recent years, Parafati et al. (2017) reported the utilization of commercial hydrogel spheres as a support for VOC-producing yeasts including W. anomalus, Metschnikowia pulcherrima, Aureobasidium pullulans, and S. cerevisiae. These immobilized yeast cells could effectively produce VOCs against mold decay on strawberry and mandarin fruits caused by B. cinerea, Penicillium digitatum, and P. italicum.
The VOC profiles of C. nivariensis DMKU-CE18 comprised compounds closely matched to 1-propanol-2-methyl, 1-butanol-3-methyl-acetate, and 1-pentanol in the database. The one closest to 1-pentanol was the major volatile compound produced by this yeast strain. These compounds were also produced by an endophytic yeast, Nodulisporium sp., that was isolated from Myroxylon balsamum found in the upper Napo region of the Ecuadorian Amazon (Mends et al. 2012). This strain produced several VOCs, such as 1-butanol-3-methyl, 1-propanol-2-methyl, 1-pentanol, 1-hexanol, 1-heptanol, 1-octanol, and 1-nonanol along with phenylethyl alcohol, all of which were active against the pathogenic fungi Aspergillus fumigatus, Phytophthora cinnamomic, Rhizoctonia solani, and Sclerotinia sclerotiorum. Furthermore, S. cerevisiae produced 3-methyl-1-butanol and 2-methyl-1-butanol. A mixture of these VOCs showed antimicrobial activity against various phytopathogens in vitro (Rezende et al. 2015). Masoud et al. (2005) reported that volatile compounds produced by W. anomalus, Pichia kluyveri, and Hanseniaspora uvarum during coffee processing could inhibit the growth of A. ochraceus, thus leading to the prevention of OTA production. The most effective VOC compound on fungal growth inhibition was 2-phenyl ethyl acetate. Hua et al. (2014) revealed that 2-phenylethanol, produced by W. anomalus WRL-076 affected spore germination, growth, toxin production, and gene expression in A. flavus. To date, various new microbial products, including yeast-based products, have reached the commercial market as biocontrol agents (Janisiewicz and Korsten 2002). However, no yeast product has been registered on the commercial market as a biological control agent for mycotoxin-producing fungi.
In conclusion, our research results showed that various saprophytic yeast strains isolated from plant leaves produced antifungal VOCs against A. flavus A39. Our study is the first to report that VOCs produced by C. nivariensis could inhibit the mycelial growth and conidial germination of A. flavus and reduce aflatoxin production in contaminated corn grains. Therefore, the VOCs produced by this yeast have potential in agriculture as a biocontrol agent to manage aflatoxin contamination by A. flavus during grain storage. However, further research is needed on the appropriate methodology for using the VOCs produced by C. nivariensis.
Change history
07 February 2020
In the original article, in the last sentence of the subsection 'Efficacy of VOC-producing yeasts on fungal conidial germination inhibition' in Materials and Methods, it should have read "3 = germ tube 2x to 4x" instead of "3 = germ tube 24x to 4x".
References
Afsah-Hejri L (2013) Saprophytic yeasts: effective biocontrol agents against Aspergillus flavus. Int Food Res J 20:3403–3409
Aidoo K (1993) Post-harvest storage and preservation of tropical crops. Int Biodeterior Biodegrad 32(1–3):161–173
Alcoba-Flórez J, Méndez-Álvarez S, Cano J, Guarro J, Pérez-Roth E, del Pilar Arévalo M (2005) Phenotypic and molecular characterization of Candida nivariensis sp. nov., a possible new opportunistic fungus. J Clin Microbiol 43(8):4107–4111
Amaike S, Keller NP (2011) Aspergillus flavus. Annu Rev Phytopathol. 49:107–133
Arrarte E, Garmendia G, Rossini C, Wisniewski M, Vero S (2017) Volatile organic compounds produced by Antarctic strains of Candida sake play a role in the control of postharvest pathogens of apples. Biol Control 109:14–20
Borman AM, Petch R, Linton CJ, Palmer MD, Bridge PD, Johnson EM (2008) Candida nivariensis, an emerging pathogenic fungus with multidrug resistance to antifungal agents. J Clin Microbiol 46:933–938
Bu'Lock JD (1980) Mycotoxins as secondary metabolites. In: Steyn PS (ed) The biosynthesis of mycotoxins: a study in secondary metabolism. Academic Press, New York, pp 1–16
Çelýk K, Denlý M, Savas T (2003) Reduction of toxic effects of aflatoxin B1 by using baker yeast (Saccharomyces cerevisiae) in growing broiler chicks diets. R Bras Zootec 32(3):615–619
Chen P-H, Chen R-Y, Chou J-Y (2018) Screening and evaluation of yeast antagonists for biological control of Botrytis cinerea on strawberry fruits. Mycobiology 46(1):33–46
Chinaphuti A, Trikarunasawat C, Wongurai A, Kositcharoenkul S (2002) Production of in-house ELISA test kit for detection of aflatoxin in agricultural commodities and their validations. Kasetsart J (Nat Sci) 36:179–186
Comitini F, Ciani M (2010) The zymocidial activity of Tetrapisispora phaffii in the control of Hanseniaspora uvarum during the early stages of winemaking. Lett Appl Microbiol 50(1):50–56
Farbo MG, Urgeghe PP, Fiori S, Marcello A, Oggiano S, Balmas V, Hassan ZU, Jaoua S, Migheli Q (2018) Effect of yeast volatile organic compounds on ochratoxin A-producing Aspergillus carbonarius and A. ochraceus. Int J Food Microbiol 284:1–10
Figueiredo-Carvalho MH, Ramos Lde S, Barbedo LS, Chaves AL, Muramoto IA, Santos AL, Almeida-Paes R, Zancopé-Oliveira RM (2016) First description of Candida nivariensis in Brazil: antifungal susceptibility profile and potential virulence attributes. Mem Inst Oswaldo Cruz 111:51–58
Fujita S, Senda Y, Okusi T, Ota Y, Takada H, Yamada K, Kawano M (2007) Catheter-related fungemia due to fluconazole-resistant Candida nivariensis. J Clin Microbiol 45:3459–3461
Gong A-D, Li H-P, Shen L, Zhang J-B, Wu A-B, He W-J, Yuan Q-S, He J-D, Liao Y-C (2015) The Shewanella algae strain YM8 produces volatiles with strong inhibition activity against Aspergillus pathogens and aflatoxins. Front Microbiol 6:1091
Gotor-Vila A, Teixidó N, Di Francesco A, Usall J, Ugolini L, Torres R, Mari M (2017) Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol 64:219–225
Grzegorczyk M, Żarowska B, Restuccia C, Cirvilleri G (2017) Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol 61:93–101
Guchi E (2015) Implication of aflatoxin contamination in agricultural products. Am J Food Nutr 3(1):12–20
Hua S-ST, Baker JL, Flores-Espiritu M (1999) Interactions of saprophytic yeasts with a nor mutant of Aspergillus flavus. Appl Environ Microbiol 65(6):2738–2740
Hua S-ST, Beck JJ, Sarreal SBL, Gee W (2014) The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res 30(2):71–78
Huang R, Li G, Zhang J, Yang L, Che H, Jiang D, Huang H (2011) Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 101(7):859–869
Huang R, Che H, Zhang J, Yang L, Jiang D, Li G (2012) Evaluation of Sporidiobolus pararoseus strain YCXT3 as biocontrol agent of Botrytis cinerea on post-harvest strawberry fruits. Biol Control 62(1):53–63
Ismail A, Bedell GW, Lupan DM (1985) Siderophore production by the pathogenic yeast Candida albicans. Biochem Biophys Res Commun 130(2):885–891
Janisiewicz W, Korsten L (2002) Biological control of postharvest diseases of fruits. Annu Rev Phytopathol 40:411–441
Khunnamwong P, Jindamorakot S, Limtong S (2018) Endophytic yeast diversity in leaf tissue of rice, corn and sugarcane cultivated in Thailand assessed by a culture-dependent approach. Fungal Biol. 122(8):785–799
Leelasuphakul W, Hemmanee P, Chuenchitt S (2008) Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillium digitatum Sacc.) of citrus fruit. Postharvest Biol Technol 48(1):113–121
Limtong S, Nasanit R (2017) Phylloplane yeasts in tropical climates. In: Buzzini P, Lachance MA, Yurkov A (eds) Yeasts in natural ecosystems: diversity. Springer, Cham, pp 199–223
Magan N, Hope R, Cairns V, Aldred D (2003) Post-harvest fungal ecology: impact of fungal growth and mycotoxin accumulation in stored grain. In: Xu X, Bailey JA, Cooke BM (eds) Epidemiology of mycotoxin producing fungi. Springer, Dordrecht, pp 723–730
Mannaa M, Kim KD (2016) Microbe-mediated control of mycotoxigenic grain fungi in stored rice with focus on aflatoxin biodegradation and biosynthesis inhibition. Mycobiology 44(2):67–78
Masoud W, Kaltoft CH (2006) The effects of yeasts involved in the fermentation of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus. Int J Food Microbiol 106(2):229–234
Masoud W, Poll L, Jakobsen M (2005) Influence of volatile compounds produced by yeasts predominant during processing of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus. Yeast 22(14):1133–1142
Mends MT, Yu E, Strobel GA, Riyaz S, Booth E, Geary B, Sears J, Taatjes C, Hadi M (2012) An endophytic Nodulisporium sp. producing volatile organic compounds having bioactivity and fuel potential. J Pet Environ Biotechnol 3(3):117
Min W-K, Kweon D-H, Park K, Park Y-C, Seo J-H (2011) Characterisation of monoclonal antibody against aflatoxin B1 produced in hybridoma 2C12 and its single-chain variable fragment expressed in recombinant Escherichia coli. Food Chem 126(3):1316–1323
Moss M (1998) Recent studies of mycotoxins. J Appl Microbiol 84:62S–76S
Oro L, Feliziani E, Ciani M, Romanazzi G, Comitini F (2018) Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int J Food Microbiol 265:18–22
Parafati L, Vitale A, Restuccia C, Cirvilleri G (2017) Performance evaluation of volatile organic compounds by antagonistic yeasts immobilized on hydrogel spheres against gray, green and blue postharvest decays. Food Microbiol 63:191–198
Payne C, Bruce A, Staines H (2000) Yeast and bacteria as biological control agents against fungal discolouration of Pinus sylvestris blocks in laboratory-based tests and the role of antifungal volatiles. Holzforschung 54(6):563–569
Petersson S, Schnürer J (1995) Biocontrol of mold growth in high-moisture wheat stored under airtight conditions by Pichia anomala, Pichia guilliermondii and Saccharomyces cerevisiae. Appl Environ Microbiol 61(3):1027–1032
Ponsone ML, Chiotta ML, Combina M, Dalcero A, Chulze S (2011) Biocontrol as a strategy to reduce the impact of ochratoxin A and Aspergillus section Nigri in grapes. Int J Food Microbiol 151(1):70–77
Raspor P, Miklič-Milek D, Avbelj M, Čadež N (2010) Biocontrol of grey mould disease on grape caused by Botrytis cinerea with autochthonous wine yeasts. Food Technol Biotechnol 48(3):336–343
Rezende DC, Fialho MB, Brand SC, Blumer S, Pascholati SF (2015) Antimicrobial activity of volatile organic compounds and their effect on lipid peroxidation and electrolyte loss in Colletotrichum gloeosporioides and Colletotrichum acutatum mycelia. Afr J Microbiol Res 9(23):1527–1535
Rosa MM, Tauk-Tornisielo SM, Rampazzo PE, Ceccato-Antonini SR (2010) Evaluation of the biological control by the yeast Torulaspora globosa against Colletotrichum sublineolum in sorghum. World J Microbiol Biotechnol 26(8):1491–1502
Spadaro D, Gullino ML (2004) State of the art and future prospects of the biological control of postharvest fruit diseases. Int J Food Microbiol 91(2):185–194
Srisuk N, Nutaratat P, Surussawadee J, Limtong S (2019) Yeast communities in sugarcane phylloplane. Microbiology 88(3):353–369
Suwannarach N, Kaewyana C, Yodmeeklin A, Kumla J, Matsui K, Lumyong S (2017) Evaluation of Muscodor cinnamomi as an egg biofumigant for the reduction of microorganisms on eggshell surfaces and its effect on egg quality. Int J Food Microbiol 244:52–61
Toffano L, Fialho MB, Pascholati SF (2017) Potential of fumigation of orange fruits with volatile organic compounds produced by Saccharomyces cerevisiae to control citrus black spot disease at postharvest. Biol Control 108:77–82
Türker M (2014) Yeast biotechnology: diversity and applications. In: 27th VH yeast conference: advances in science and industrial production of baker’s yeast, Istanbul, 14–15 April 2014, pp 1–26
Velmourougane K, Bhat R, Gopinandhan T, Panneerselvam P (2011) Management of Aspergillus ochraceus and ochratoxin-A contamination in coffee during on-farm processing through commercial yeast inoculation. Biol Control 57(3):215–221
Waenlor W, Wiwanitkit V (2003) Aflatoxin contamination of food and food products in Thailand: an overview. Southeast Asian J Trop Med Public Health 34:184–190
Waliyar F, Umeh V, Traore A, Osiru M, Ntare B, Diarra B, Kodio O, Kumar KVK, Sudini H (2015) Prevalence and distribution of aflatoxin contamination in groundnut (Arachis hypogaea L.) in Mali, West Africa. Crop Prot 70:1–7
Wilson CL, Pusey P (1985) Potential for biological control of postharvest plant diseases. Plant Dis 69(5):375–378
World Health Organization (2018) Aflatoxin. In: Food safety digest. DIALOG. https://www.who.int/foodsafety/FSDigest_Aflatoxins_EN.pdf?fbclid=IwAR23wb_6iGDd-MFJVJKRXTM11qEK7_n9BSw4QZr89yTocEpUlxqpVoNt5T8. Cited 31 July 2018
Zain ME (2011) Impact of mycotoxins on humans and animals. J Saudi Chem Soc 15(2):129–144
Zeidan R, Ul-Hassan Z, Al-Thani R, Balmas V, Jaoua S (2018) Application of low-fermenting yeast Lachancea thermotolerans for the control of toxigenic fungi Aspergillus parasiticus, Penicillium verrucosum and Fusarium graminearum and their mycotoxins. Toxins 10(6):242
Zhou Y, Li W, Zeng J, Shao Y (2018) Mechanisms of action of the yeast Debaryomyces nepalensis for control of the pathogen Colletotrichum gloeosporioides in mango fruit. Biol Control 123:111–119
Acknowledgements
This work was supported by the Thailand Research Fund (TRF) through the TRF Research-Team Promotion Grant (RTA 6080004). The authors would like to thank Dr Amara Chinaphuti, Department of Agriculture (DOA), Thailand, for providing Aspergillus flavus A39.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Jane Debode
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jaibangyang, S., Nasanit, R. & Limtong, S. Biological control of aflatoxin-producing Aspergillus flavus by volatile organic compound-producing antagonistic yeasts. BioControl 65, 377–386 (2020). https://doi.org/10.1007/s10526-020-09996-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-020-09996-9