Abstract
The sap-sucking lace bug Gargaphia decoris Drake (Hemiptera: Tingidae) is a biological control agent released in New Zealand to control the invasive small tree or shrub Solanum mauritianum Scopoli (Solanaceae). The aim of this study was to investigate the effect of temperature, photoperiod and humidity on selected G. decoris life history traits. The selected traits were measured at six different temperatures (10, 15, 20, 25, 27.5 and 30 °C), three photoperiods (L:D 6:16, 14:10 and 16:8) and two relative humidity (RH) levels (50 and 70 ± 10%). Eggs and nymphs did not survive in the 10 °C and 30 °C treatments. The net reproductive rate (R0) resulted in population growth at all temperatures, but was highest at 25 °C. Egg hatch rate was highest at L:D 8:16, and nymphal and adult survival was better at L:D 16:8. Egg hatch was higher at 50 ± 10% RH and adult survival and population growth were higher at 70 ± 10% RH. These results will help to predict the persistence of established G. decoris populations and the potential for establishment at new release sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological control of weeds using insects, when successful, is one of the most cost-effective practices for the management of invasive species (Culliney 2005). However, the efficacy of biocontrol agents can be compromised by a variety of environmental factors, which may act together to reduce insect fitness (e.g. development, survival, fecundity, fertility and longevity). Temperature, photoperiod and humidity, for example, have each been shown to influence the establishment and success of biocontrol agents (Byrne et al. 2002; Cowie et al. 2016b; Bean et al. 2007). Therefore, knowledge on how environmental factors affect a biocontrol agent is a key to predicting success.
The sap-sucking lace bug Gargaphia decoris, native to parts of Argentina and southern Brazil, has a high potential to suppress the population of the invasive tree Solanum mauritianum Scopoli (Drake and Ruhoff 1965; Olckers and Borea 2009). This is because besides its host specificity, it bears a number of fitness traits that an effective biocontrol agent usually has (Olckers 2000). For example, G. decoris females are highly fecund. They can oviposit numerous eggs (up to 900 eggs), and are capable of having consecutive egg batches during their lifetime (Hayes 1996; Olckers 2000). This allows an overlap of generations on the same plant, thus increasing the chances of inflicting significant feeding damage to the host plant (Cowie et al. 2016a). This bug is a long-lived insect (up to 145 days) with short development times (30 days at 22 ± 3 °C), suggesting that it is highly capable of substantial population growth (Olckers 2000). South Africa was the first country to release G. decoris as a biocontrol agent, and after early reports of success, New Zealand introduced and released the insect in 2009 (EPA 2009; Olckers and Borea 2009; Witt 2007). Despite the reported establishment of populations and extensive damage on S. mauritianum, populations densities have strongly fluctuated with sporadic outbreaks (Klein 2011; Olckers and Borea 2009), and with a preference for semi-shaded areas in both South Africa (Patrick and Olckers 2011) and New Zealand (Fowler 2014). Initially, low temperatures were suggested to be the cause for the low densities of G. decoris found in the field (Hope and Olckers 2011), but later it was dismissed that low temperatures would affect G. decoris establishment by performing thermal assessments, and rather suggested that other causes might be involved (e.g. predation). However, thermal assessment studies were based on establishment observations in the field (Barker and Byrne 2005; as cited in Olckers 2011) and it is unknown at what extent environmental conditions influence G. decoris fitness. Here we apply the first systematic study into the effect of environmental conditions on fitness to explore whether environmental conditions are a likely predictor of success.
In the present study, we aimed to test how environmental conditions influenced G. decoris fitness and thus performance, and provide knowledge for prediction of its persistence and establishment in the field. We carried out a series of experiments to examine dynamics of various traits of G. decoris in response to different temperatures, photoperiods and humidity conditions.
Materials and methods
Insect and plant material
The G. decoris colony used in this study was derived from a glasshouse colony at Manaaki Whenua Landcare Research Ltd, Auckland, New Zealand (NZ). The colony had been maintained since then in a climate-controlled room at Massey University, Palmerston North, New Zealand. The insects were fed on S. mauritianum plants (15 cm from the top of the pot) with at least three fully matures leaves, grown in 0.75 l pots at 25 °C with a photoperiod of L:D 14:10 hours and 50 ± 10% RH during the duration of the experiment (2013–2017). To maintain a constant source of plant material, S. mauritianum seeds were sown and reared in a glasshouse at Massey University Plant Growth Unit, Palmerston North, New Zealand. Plants were maintained in a climate-controlled glasshouse at daily average minimum and maximum temperatures of 17.1 °C and 24.1 °C, respectively. Plants that reached a height of approximately 15 cm from the top of the pot were used for the experiments.
Temperature
Gargaphia decoris development from egg to adult was evaluated at six different temperatures: 10, 15, 20, 25, 27.5 and 30 ± 1 °C, with a photoperiod of L:D 14:10 and 50 ± 10% RH in environmental chambers (Sanyo MLR-350T; Japan) and climate-controlled rooms. Egg batches from different sets of G. decoris parents were collected from the colony by cutting off the part of the leaves where the egg batches were located. The leaf pieces with egg batches were then placed in plastic Petri dishes (8.5 cm diameter) lined with moist paper towel and incubated in climate-controlled rooms at one of the test temperatures. Each egg batch (approx. 30 eggs) was considered a sampling unit, with a total of ten egg batches per temperature. The eggs were checked daily (09:00–11:00) and the number of nymphs emerging each day was recorded until no nymphs emerged for more than two consecutive days.
To evaluate nymphal development, ten newly emerged nymphs from each of the ten egg batches were collected using a fine brush. Each group of ten nymphs were placed on a separate potted S. mauritianum plant (0.75 l pots). Thus we had ten S. mauritianum plants with ten nymphs each. The plants had at least two fully matured leaves and allocated to one of the tested temperatures for nymphal development. To prevent the lace bugs from wandering into the soil, each pot was fitted with a plastic lid which fitted perfectly along the edges of the pot. The plastic lid had a hole in the center which surrounded the plant stem, isolating the stem and leaves of the plant from the soil. To contain the insects, each potted plant was fully covered with an inverted plastic container. Fine metal mesh was placed at the top of container to allow ventilation. Nymphs on potted plants were observed daily and the duration and survival of each nymphal stage (instar) was recorded. Each nymphal stage was differentiated according to descriptions by Olckers (2000). Observations continued until the last surviving nymph emerged as an adult.
For each group of ten nymphs, the number of adult females (identified as F1), the life cycle duration (number of days it took to develop from egg to adult), and nymphal instar survival (percentage of surviving nymphs at each nymphal stage) were recorded at each temperature tested. For each temperature this experiment was replicated three times.
To compare adult reproduction and lifespan across temperatures, three lace bug pairs (F1 adults) were randomly selected from the adults that emerged from the prior experiment. To ensure that pairs were not siblings, adults were sourced from different egg batches. For each temperature there were 30 pairs tested. This procedure was replicated three times. Each F1 female was allowed to copulate and lay eggs during their lifetime and several reproductive parameters were recorded: (1) the number of females that oviposited successfully (i.e. produced egg batches that hatched), (2) the length of the pre-oviposition period (i.e. number of days counted from the day the female was placed with the adult male until the presence of an egg batch), (3) total number of eggs laid per female, (4) number of egg batches laid per female, (5) number of eggs per batch, (6) number of eggs in the first batch, and (7) proportion of eggs that hatched from the first batch.
Plants with F1 adults were monitored daily to record the adult’s lifespan and to check for egg batches. In a congeneric species G. solani, female investment in maternal care is associated with a significant reduction in fecundity (Tallamy and Denno 1982). Therefore, to avoid maternal care affecting the fecundity in G. decoris, only the first egg batch oviposited was selected for further analysis. Once an egg batch was observed, it was collected and placed in plastic Petri dish for incubation under similar environmental conditions as the parents. When F2 nymphs emerged, they were counted, placed on a new potted S. mauritianum plant, and followed until they reached adulthood. The F2 adults were sexed and their survival (percentage of F2 nymphs that moulted to adults) was recorded. During the experiment, plants were replaced with the new ones when at least 75% of the leaves were damaged.
Photoperiod and humidity
To evaluate the effect of photoperiod on G. decoris life history, the development from egg to adult was monitored at three different photoperiods L:D 8:16, 14:10 and 16:8 and at a constant temperature of 20 °C and 50 ± 10% RH. In a separate experiment, the effect of humidity was evaluated at 50 ± 10% and 70 ± 10% RH at 20 °C and L:D 16:8 photoperiod. The procedure and recorded variables were as described for the temperature experiment. There were three replicates for each photoperiod and humidity experiment.
Life table calculations
To examine the effect of temperature, photoperiod and humidity on G. decoris population growth, we calculated life table parameters using data collected from the above experiments. The intrinsic rate of natural increase (rm, females per females per day) was estimated by solving the Lotka–Euler equation, \(\sum e^{ - rmx} lxmx = \, 1\) as described by Wilson (2000). Other life table parameters: net reproductive rate [\({\text{R}}_{0} { = }\sum lxmx\) mean generation time [T = loge(R0)/rm, days], doubling time [DT = loge(2)/rm, days] and finite rate of increase [λ = er] were calculated for each female that laid eggs and their SE was computed using the jackknife method (Caswell 1989).
Statistical analysis
Gargaphia decoris development time, nymphal survival, fecundity, fertility and life table parameters were compared across different temperatures, photoperiods and humidity levels using Analysis of Variance (ANOVA) followed by Tukey’s HSD test for comparison of means at p < 0.05. These data were tested for normality using the Shapiro–Wilks test. If data violated the assumptions of normality after log-transformations were performed, data were analyzed using the non-parametric Kruskal–Wallis test and the Dunn’s multiple comparison test. Linear and quadratic regression for developmental rate (1/d), using temperature as a predictor variable (15 °C to 27.5 °C) were conducted. Following the assumption that development rate is proportional to temperature, the thermal constant for development (K) required for development was calculated as the reciprocal of the slope (1/slope) of a regression line fitted to the developmental data. All data was analyzed using R (R Development Core Team 2014). The estimation of the distribution of survival times was obtained by calculating the survival distribution function. The LIFETEST procedure (SAS 9.3) was used to compare survival curves using the non-parametric Wilcoxon test as reported in SAS Institute Inc. (2009).
Results
Effect of temperature on G. decoris development and population growth
Eggs kept at 10 °C failed to hatch, and nymphs that emerged at 30 °C died after one day. For the remaining treatments where egg and nymphal development did occur (i.e. 15–27.5 °C), the mean (± SE) developmental duration of G. decoris eggs, nymphs and total life cycle duration were all significantly affected by temperature treatment (slowest development at 15 °C and fastest at 25 °C) (Table 1). The influence of temperature on development rate was adequately described by linear and quadratic regression models (Table 2). The egg hatch, total nymphal development and life cycle better fit a quadratic regression (Fig. 1). However, the development rate of each developmental stage better fit a linear regression model (Table 2). Due to the gap in tested temperatures between 27.5 °C and 30 °C in this experiment, non-linear models such as Brière or Logan model (Logan et al. 1976; Briere et al. 1999) could not be used.
Development rates (day−1) for Gargaphia decoris developmental stages at four different constant temperatures: 15 °C, 20 °C, 25 °C, and 27 °C, as predicted by quadratic and linear regression models in a egg hatch, b total nymphal cycle, and c total life cycle. Open circles (n = 12) represent the experimental data (mean developmental rates), lines represent the fitted quadratic (dashed) and linear (solid) regressions
Effect of temperature on G. decoris survival
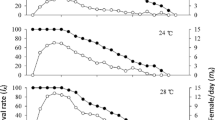
The lowest and highest total survival (from egg to adult) were observed at 15 and 25 °C (27.6 ± 4.1% and 39.8 ± 4.3%, respectively) although these differences were not statistically significant (data not shown). There were significant effects of gender and temperature on G. decoris longevity. On average, females lived 29–33% longer than males. This was significant at all temperatures but at 20 °C (Wilcoxon test: \(\chi_{1}^{2}\) = 9.48, p < 0.01 for 15 °C; \(\chi_{1}^{2}\) = 2.08, p = 0.149 for 20 °C; \(\chi_{1}^{2}\) = 17.83, p < 0.01 for 25 °C; \(\chi_{1}^{2}\) = 7.51, p < 0.01 for 27.5 °C) (Fig. 2). Adult survival was higher at lower temperatures (Wilcoxon test: \(\upchi_{3}^{2}\) = 18.50, p < 0.01 for females; \(\upchi_{3}^{2}\) = 13.70, p < 0.01 for males) (Fig. 2).
Effect of temperature on G. decoris fecundity and life table parameters
Temperature significantly affected some of the reproductive parameters of G. decoris. The total number of eggs was on average 94% higher at 20 °C and 25 °C compared to 15 °C (Table 3). A similar trend was observed for the number of eggs laid per female and eggs per batch. The pre-oviposition period increased as the temperature decreased (F3,8 = 5.16, p < 0.05). The number of eggs in the first batch was significantly higher at 20 °C. However, significant differences were found only between 15 °C and the rest of the temperatures tested. The number of egg batches laid per female (F3,8 = 2.07, p = 0.18), the number of females that oviposited successfully (F3,8 = 2.42, p = 0.14) and the percentage of eggs that hatched (F3,8 = 0.78, p = 0.53) were not significantly affected by temperature (Table 3).
Most of the life table parameters for G. decoris were affected by temperature. Net reproductive rate (R0) was highest at 25 °C and lowest at 15 °C, and was positive at all temperatures, denoting increasing populations. The intrinsic rate of natural increase (rm) and finite rate of increase (λ) were highest at 20–25 °C and significantly decreased at 15 °C. Doubling time (DT) and mean generation time (T) were lowest at 25 °C and highest at 15 °C (Table 4).
Effect of photoperiod on G. decoris life history traits
Photoperiod significantly affected only the mean percentage of egg hatch (F2,6 = 8.43, p < 0.05) and total nymphal survival (nymph to adult emergence) (F2,6 = 5.47, p < 0.05). The highest percentage of egg hatch and total survival were observed at L:D 16:8 photoperiod. Egg hatch and survival at L:D 14:10 were both lowest, but not significantly different to the L:D 8:16 treatment (Fig. 3).
Adult longevity was significantly affected by photoperiod. Females (\(\chi_{2}^{2}\) = 12.05, p < 0.01) and males (\(\chi_{2}^{2}\) = 7.66, p < 0.01) lived longer at L:D 16:8 photoperiod (♀75.79 ± 0.54 days, ♂76.26 ± 3.82 days), compared to the L:D 14:10 photoperiod (♀67.33 ± 17.31 days, ♂50.83 ± 13.89 days). No significant differences were observed in the longevity of females between L:D 8:16 and 16:8 photoperiods (p = 0.43) or in the longevity of males between L:D 8:16 and 14:10 photoperiods (p = 0.19) (data not shown).
Photoperiod significantly affected all measured G. decoris population growth parameters. The Ro was positive in all tested photoperiods, indicating population growth, with a faster rate at L:D 16:8 photoperiod (rm = 0.52 ± 0.001) and lower rate at L:D 14:10 photoperiod (rm = 0.15 ± 0.001). The time to produce one generation and to double it were shorter at L:D 16:8 photoperiod (T = 9.60 ± 0.02, DT = 1.33 ± 0.003 d) and longer at L:D 14:10 photoperiod (T = 25.20 ± 0.05, DT = 4.52 ± 0.03 d) (data not shown).
Effect of relative humidity on G. decoris life history traits
Relative humidity only affected the mean percentage of egg hatch (F2,6 = 8.21, p < 0.05). The percentage of egg hatch was significantly higher at 50 ± 10% RH (88.10 ± 0.34) than at 70 ± 10% RH (75.03 ± 4.57). Adult longevity was significantly affected by humidity. Adults lived longer at 70% RH (\(\chi^{ 2}_{ 1}\) = 10.74, p < 0.01), but females lived longer than males at both RH (\(\chi^{ 2}_{ 1}\) = 3.96, p < 0.05). Relative humidity affected all measured G. decoris population growth parameters. The R0 indicates that population growth took place at both 50 and 70% RH. The R0 was higher at 70% RH, the rm was higher at 50% RH. Therefore T and DT were both shorter at 50% RH (data not shown).
Discussion
We show that all tested environmental conditions (temperature, photoperiod and humidity) are likely to affect persistence and establishment of G. decoris. Immature stages are unlikely to develop at temperatures below 10 °C or above 30 °C. Although G. decoris was able to develop at 15 °C, nymphal development was very slow. This indicates that, for G. decoris to achieve a population increase necessary to inflict considerable damage to the host plant, temperatures should be higher than 15 °C. Currently, G. decoris is established in the North Island of New Zealand, specifically in the low-lying coastal areas of the Bay of Plenty, Auckland and Northland regions (Landcare Research, unpublished data), where mean annual temperatures range from 14 °C to 16 °C and summer temperatures easily achieve ≥ 20 °C but very seldom reach 30 °C (Chappell 2013a, b, c). The establishment of G. decoris in these areas has likely benefited from the more stable coastal climate, with less extreme fluctuations in temperature compared to inland regions which typically experience both hotter and colder temperatures that sometimes drop below 0 °C (Chappell 2013a, b, c).
Temperature is known to be the most restrictive environmental factor for insects (Bale et al. 2002). However, temperature effects on insects are intertwined with other environmental factors such as photoperiod and humidity (Simelane 2007), and such interactions between environmental factors may restrict the persistence and establishment of biocontrol agents (Byrne et al. 2002; Bean et al. 2007; Cowie et al. 2016b). For example, life table parameters reported in this study suggest that population growth of G. decoris is likely to be restricted to summer months at the Bay of Plenty, Auckland and Northland sites. The optimal month for population growth is likely to be December when there is a long photophase (\(\approx\) 15 h), RH of at least 70% and higher temperatures. After December, the photophase length is reduced to approximately 13 to 14 h (January–March) (LINZ, Land Information New Zealand 2018). We observed that a photoperiod of L:D 14:10, while enabling population growth, was less optimal than a photoperiod of L:D 16:8, despite sustained otherwise optimal temperatures and RH. The reduced egg hatch and survival of G. decoris at a 14 h photophase suggests that there are critical photoperiods that can trigger a change in the performance of this biocontrol agent. However, L:D 14:10 photoperiod conditions did not induce reproductive diapause, as has been reported for other insects when subjected to critical photoperiods (Beck 1980). We show that a short photophase (8 h) resulted in better performance and fitness of G. decoris than 14 h. A short photophase is known to induce diapause in some insects (Saunders 1987) and such impact often is in conjunction with lower temperatures (Taylor et al. 1995; Saunders 2014). Thus, in the present study we do not have evidence that photoperiod induces diapause in G. decoris, but it is possible that diapause may occur under conditions of short photophase and low temperature (< 20 °C).
Our data help to interpret a previous finding in South Africa and New Zealand that G. decoris shows a preference for shade conditions under field situations (Patrick and Olckers 2011; Fowler 2014). We show that G. decoris thrives under the tested environmental conditions present at those field study sites. Therefore, the reason for G. decoris seeking shade is likely to be some other untested environmental factors. For example, Cowie et al. (2016a) suggested that the preference for shade plants could be a result of observed damage to the photosynthetic machinery of the host plant under high light intensities, which ultimately could decrease plant host quality. Alternatively, biotic factors like predation could drive this preference, as proposed by Cowie et al. (2018).
In conclusion, the results of this study demonstrate that all environmental parameters tested have an effect on G. decoris fitness, and that environmental conditions are likely to be a major limiting factor for the establishment and persistence of this biocontrol agent. Therefore, there will be geographical constraints on where the biocontrol agent is likely to be useful, and these findings may also help explain the current establishment patterns in places like New Zealand. We present the life history of this insect at a level of detail not previously available, across a range of environmental conditions. Such information could be integrated into ecoclimatic models for helping to predict establishment, persistence, and seasonal population fluctuations at potential release sites. In addition, future climate scenarios could be explored to see how the real or potential insect distribution might change. It is suggested that a detailed life history, as presented here for G. decoris, should be performed as part of standard assessments of insect suitability, prior to introductions of biological control agents.
References
Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, Harrington R, Hartley S, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt AD, Whittaker JB (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Chang Biol 8:1–16
Barker AL, Byrne MJ (2005) Biotypes and biocontrol: physiological factors affecting the establishment of a South American lace bug Gargaphia decoris, a biological control agent of bugweed, Solanum mauritianum. In: Villet MH (ed). Proceedings of the 15th entomological congress. Entomological Society of Southern Africa. Pretoria, South Africa, p 8
Bean DW, Dudle TL, Keller JC (2007) Seasonal timing of diapause induction limits the effective range of Diorhabda elongata deserticola (Coleoptera: Chrysomelidae) as a biological control agent for Tamarisk (Tamarix spp.). Environ Entomol 36:15–25
Beck SD (1980) Insect photoperiodism, 2nd edn. Academic Press, New York, p 398
Briere JF, Pacros P, Le Roux AY, Pierre JS (1999) A novel rate model of temperature-dependent development for arthropods. Environ Entomol 28:22–29
Byrne MJ, Currin S, Hill MP (2002) The influence of climate on the establishment and success of the biological control agent Gratiana spadicea, released on Solanum sisymbriifolium in South Africa. Biol Control 24:128–134
Caswell H (1989) Matrix population models. Sinauer Assoc, Massachusetts
Chappell PR (2013a) The climate and weather of Bay of Plenty, NIWA Science and Technology Series, vol 62, p 40
Chappel PR (2013b) The climate and weather of Auckland, NIWA Science and Technology Series, vol 60, p 40
Chappel PR (2013c) The climate and weather of Northland. NIWA Science and Technology Series, vol 59, p 40
Cowie BW, Byrne MJ, Witkowski ETF, Venter N (2016a) Exacerbation of photosynthetic damage through increased heat-light stress resulting from Gargaphia decoris sap-feeding. Biol Control 94:82–89
Cowie BW, Venturi G, Witkowski ETF, Byrne MJ (2016b) Does climate constrain the spread of Anthonomus santacruzi, a biological control agent of Solanum mauritianum, in South Africa? Biol Control 101:1–7
Cowie BW, Venter N, Witkowski ETF, Byrne MJ, Olckers, T (2018) A review of Solanum mauritianum biocontrol: prospects, promise and problems: a way forward for South Africa and globally. BioControl 63:475–491
Culliney T (2005) Benefits of classical biological control for managing invasive plants. Crit Rev Plant Sci 24:131–150
Drake CJ, Ruhoff FA (1965) Lace bug genera of the world (Hemiptera:Tingidae). Proc USNM 112:1–105
EPA, Environmental Protection Authority Decision (2009) NORO8003 decision. https://epa.govt.nz/search/SearchForm?Search=Gargaphia+decoris
Fowler S (2014) Lace bug does best in shade. Weed biocontrol newsletter, Issue 69, Landcare Research, Manaaki Whenua, Lincoln
Hayes L (1996) The biological control of weeds book: a New Zealand guide. ISBN 0-478-09306-3. Published by Manaaki Whenua, Landcare Research, p 150. http://www.landcareresearch.co.nz/publications/books/biocontrol-of-weeds-book
Hope KJ, Olckers T (2011) Gargaphia decoris (Hemiptera:Tingidae) from two South American provenances are equally safe for release against the invasive tree, Solanum mauritianum (Solanaceae). Afr Entomol 19:106–112
Klein H (2011) A catalogue of the insects, mites and pathogens that have been used or rejected, or are under consideration, for the biological control of invasive alien plants in South Africa. Afr Entomol 19:515–549
LINZ, Land Information New Zealand (2018) Sunrise and sunset tables July 2018–June 2019. Data retrieved from United States Naval Observatory (USNO). https://www.linz.govt.nz/sea/nautical-information/astronomical-information
Logan JA, Wollkind DJ, Hoyt SC, Tanigoshi LK (1976) An analytical model for description of temperature dependent rate phenomena in arthropods. Environ Entomol 5:1133–1140
Olckers T (2000) Biology, host specificity and risk assessment of Gargaphia decoris, the first agent to be released in South Africa for the biological control of the invasive tree Solanum mauritianum. BioControl 45:373–388
Olckers T (2011) Biological control of Solanum mauritianum Scop. (Solanaceae) in South Africa: will perseverance pay off? Afr Entomol 19:416–426
Olckers T, Borea CK (2009) Assessing the risks of releasing a sap-sucking lacebug, Gargaphia decoris, against the invasive Solanum mauritianum in New Zealand. BioControl 54:143–154
Patrick K, Olckers T (2011) Influence of shade on the persistence of Gargaphia decoris (Tingidae), a biological control agent of Solanum mauritianum (Solanaceae) in South Africa. Afr Entomol 22:891–895
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
SAS Institute Inc (2009) SAS/STAT® 9.2. User’s guide, 2nd edn. SAS Institute Inc, Cary. http://www.r-project.org/
Saunders DS (1987) Maternal influence on the incidence and duration of larval diapause in Calliphora vicina. Physiol Entomol 12:331–338
Saunders DS (2014) Insect photoperiodism: effect of temperature on the induction of insect diapause and diverse roles for the circadian system in the photoperiodic response. J Entomol Sci 17:25–40
Simelane DO (2007) Influence of temperature, photoperiod and humidity on oviposition and egg hatch of the root-feeding flea bettle Longitarsus bethae (Chrysomelidae:Alticinae), a natural enemy of the weed Lantana camara (Verbenacea). Bull Entomol Res 97:111–116
Tallamy DW, Denno RF (1982) Life history trade-offs in Gargaphia solani (Hemiptera:Tingidae): the cost of reproduction. Ecology 63:616–620
Taylor PS, Shields EJ, Tauber MJ, Tauber CA (1995) Induction of reproductive diapause in Empoasca fabae (Homoptera:Cicadellidae) and its implications regarding southward migration. Environ Entomol 24:1086–1095
Wilson EO (2000) Sociobiology: the new synthesis. Harvard University Press, Massachusetts, p 697
Witt ABR (2007) Bugweed under attack. Plant Prot News 72:11
Acknowledgements
We thank Landcare Research based in Auckland, New Zealand for providing insects and plant stock for purposes of this study and the staff of the Institute of Agriculture and Environment and Plant Growth Unit, Massey University, for providing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
No humans and/or animals were used in this study that required and informed consent or submission to animal welfare committee for evaluation.
Additional information
Handling Editor: S. Raghu
Rights and permissions
About this article
Cite this article
Falla, C., Najar-Rodriguez, A., Minor, M. et al. Effects of temperature, photoperiod and humidity on the life history of Gargaphia decoris. BioControl 64, 633–643 (2019). https://doi.org/10.1007/s10526-019-09969-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-019-09969-7