Abstract
This study shows the effectiveness of deliberately selecting for Coptera haywardi individuals to increase a population’s capacity to discriminate against parasitised hosts. In the ‘selected colony’ (F1–F4), females were selected based on their ability to discriminate parasitised fruit fly pupae, determined by their host searching, foraging and oviposition behaviour. Female parasitoids of successive generations of the selected colony (F1–F4) showed an increasing discriminatory ability, including reduced host searching and foraging time. The last selected generation, i.e. F4 showed an increase in fecundity compared to the standard colony. In F4 individuals from the selected colony, antennae length increased but the hind tibia size did not, compared to individuals from the control colony. Flight ability and survival remained unchanged across all generations. This selection process could be an effective method of recuperating the discriminatory capacity of a C. haywardi colony under mass rearing conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The colonization of an insect species inevitably goes through a “bottle neck” phase where, through successive generations, important attributes of individuals are reduced, or even lost (Wajnberg 2004; Parreño et al. 2014). The restoration of these desirable attributes becomes a challenge in many mass-reared colonies where the original genetic status and colonization route is unknown (Saul and McCombs 1993; Schütze et al. 2015). A viable option for maintaining important attributes is to select individuals with the desired traits in a practical and targeted way. There are several examples that have demonstrated the feasibility of such a technique for mass-reared insect colonies (Baeshen et al. 2014; Gilchrist and Meats 2014; Quintero-Fong et al. 2016; Tejada et al. 2017) and their natural enemies (Beukeboom et al. 2015; Coelho et al. 2016).
The solitary pupal endoparasitoid, Coptera haywardi (Oglobin) (Hymenoptera: Diapriidae) is specific to the tephritid genus Anastrepha and has a high capacity for discriminating parasitised hosts (Sivinski et al. 1998; Cancino et al. 2012). The close specificity of C. haywardi to pupae of the family Tephritidae, has been linked with its endoparasitic nature (Sivinski et al. 1998). The preference for Anastrepha species and the ability to discriminate parasitised hosts makes C. haywardi a desirable parasitoid for the concurrent augmentative release with the larval parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) to enhance biological control of pest Anastrepha populations in the field.
Thanks to mass rearing, natural enemies such as parasitoids are used in augmentative releases as a sustainable alternative to chemical control of pests (Mackauer 1976; van Lenteren 2012). When augmentative biological control involves releases of multiple species, an important requirement is that the parasitoids have a high capacity for host discrimination ensuring efficient host searching and thereby reducing conspecific and heterospecific competition in the field (Cancino et al. 2012; Evangelou et al. 2013; Qi-Fu and Tong-Xian 2017). These attributes are particularly important in tertiary or pupal parasitoids that attack towards the end of the host’s development period, in order that the parasitoid is able to successfully oviposit and pass on traits from parents to offspring (Scholz and Holler 1992; Ruschioni et al. 2015).
During the mass rearing process, the host searching ability of parasitoids is considered to be one of the main attributes that should be preserved (Mackauer et al. 1996; De Moraes et al. 2000). Host searching is an intrinsic character of a species. However, discrimination ability can decrease under artificial conditions of mass rearing (van Lenteren and Bigler 2010). Maintaining this capacity to discriminate between parasitised and non-parasitised hosts in a mass reared parasitoid colony is often reduced, or lost under mass rearing conditions where the aim is to maximise parasitoid production, while minimising cost. Recovering discrimination ability can be complex due to the interaction of different factors. For example, this decline may be the result of the use of artificial oviposition substrates or unparasitised hosts used in mass rearing to increase production efficiencies (Lewis et al. 2003). Qualities such as host specificity and the ability to discriminate hosts not previously parasitized are important components of parasitoid searching behavior. However, since they are adaptive attributes, they are sensitive to the selective process that occurs under colonization (van Baaren and Boivin 1998; Lebreton et al. 2008).
To recuperate attributes lost in mass rearing, a common technique is to re-introduce wild individuals (Boller and Chambers 1977; Sorensen et al. 2012). However due to the low percentage of C. haywardi parasitism in the field (Sivinski et al. 2000; Aguiar-Menezes et al. 2003; Hernández-Ortiz et al. 2006) and the difficulty of identifying parasitised pupa in the field (Ovruski 1995; Ovruski et al. 2000) this is not practical. Selection (Giunti et al. 2015; Bodino et al. 2016) is another option to optimize an attribute in mass reared colonies. Specialized lines can be used to strengthen the diminished attributes (Coelho et al. 2016) or to produce hybrids with selected attributes (Beukeboom et al. 2015).
This study shows that selection for C. haywardi individuals capable of discerning parasitised Anastrepha pupae, increases a colony’s ability to successfully discriminate. Specifically, this study identified an effective means to increase the discrimination ability of a mass-reared C. haywardi colony, which will contribute towards an augmentative biological control program for the control of Anastrepha spp.
Materials and methods
Parasitoid source
A population of C. haywardi has been maintained for more than 150 generations in the Department of Biological Control of the Moscafrut Program located at Metapa, Chiapas, Mexico was utilised in this study. This C. haywardi strain was derived from a colony established in the Fruit Flies Project in INECOL, in Xalapa, Veracruz, México (Aluja et al. 2009). This population has been reared using pupae of Anastrepha ludens (Loew) as the host. In addition, A. ludens pupae previously parasitized during the larval stage by D. longicaudata have been maintained for over 450 generations and are cultured under the same conditions as C. haywardi.
Standard Coptera haywardi colony
The C. haywardi standard colony was kept under standard conditions of 22 ± 2 °C, RH and a of L:D 12:12 photoperiod. The parasitoid colonies were maintained in mesh-covered aluminum cages (30 × 30 × 100 cm). The host pupae were introduced to the cages on a thin layer of vermiculite in Petri dishes (14.5 cm diameter). The exposed pupae were left for 72 h and unparasitised pupae introduced every 24 h.
Selection for host discrimination by Coptera haywardi
All assays were conducted under standard conditions as described above, unless otherwise stated below. Assays were carried out inside a plexiglass cage (30 × 30 × 30 cm) covered with black cardboard to obtain a luminous intensity of 20 lux inside and facilitate the searching, foraging and oviposition behaviour of C. haywardi (darkness stimulates oviposition by C. haywardi) (Cancino et al. 2012). The A. ludens pupae exposed to C. haywardi were placed in cylindrical plastic containers (7 cm diameter; 4 cm height) with moistened vermiculite (2 g of vemiculite with 2 ml of water) and maintained at 26 °C for development. 29 days after parasitization, a day before adult eclosion, the pupae were separated from the vermiculite. Eclosed C. haywardi adults were placed in plexiglas cages and provided food (one ply paper soaked in honey) and a water soaked cotton wick which was replaced daily.
Females C. haywardi were selected for their ability to discriminate non-parasitised pupa based on their host searching, foraging and oviposition behaviour and used to produce four consecutive generations or the ‘selected colony’. To ‘train’, or allow the parasitoids to obtain experience in searching for hosts, i.e. learn, adult females aged 5–6 days were exposed to 100 A. ludens pupae for 24 h as previously described. At day 7, 50 randomly selected individual females with experience in host searching were evaluated separately as described below.
Two pupae, one unparasitised A. ludens pupa and the other an A. ludens pupa previously parasitized in the larval stage by D. longicaudata, were placed 10 cm apart on a Petri dish (14.5 cm diameter) and placed in the assay cage. An individual C. haywardi parasitoid female with prior learning experience was released inside the cage and observed for 20 min and a choice was recorded when the female foraged on and oviposited in a pupa. If a female did not make a choice within 20 min, it was marked as ‘no choice’. Host searching, foraging and oviposition time were recorded. Host searching time was considered the period elapsed since the release of the female until a choice was made, i.e. a parasitised or unparasitised host was selected for oviposition. Foraging time was considered the period from when a female selected a host, until insertion of the ovipositor. Oviposition time was the period in which the female held the ovipositor within the pupa.
Females C. haywardi that discriminated against previously parasitized pupae by ovipositing in the unparasitized pupa were subsequently selected and placed in a 30 × 30 × 30 cm plexiglass cage with water and food. Every 24 h, over a period of five days, 100 unparasitized A. ludens pupae were exposed to the females C. haywardi. Females who discriminated against parasitised hosts were selected for the subsequent generation. This process was repeated over four consecutive generations to produce the F1–F4 selected lines.
Survival, fecundity and flight ability
The C. haywardi F4 line that selected unparasitised pupae and the standard reared colony, were evaluated for survival, fecundity, and flight ability, following the standard procedures of an international protocol (FAO/IAEA/USDA 2014) adapted to this parasitoid species. To determine survival under stress, 15 males and 30 females newly emerged (< 12 h) C. haywardi of each line were placed in a Plexiglas cage (30 × 30 × 30 cm) without food and water. Daily mortality counts were recorded, until all parasitoids were dead. To assess fecundity, 35 days old, mated females C. haywardi from each line were placed in a Plexiglas cage (30 × 30 × 30 cm). Every 24 h for five consecutive days, 100 unparasitised A. ludens pupae were exposed to the parasitoids. After exposure, pupae were maintained under standard conditions and allowed to develop for 30 days. The number of offspring per C. haywardi female were recorded daily until emergence ceased. To determine flight ability, 100 pupae from each line were placed in the base of a black PVC tube (10 cm diameter and 10 cm high). The inside wall of the tube was coated with talc to prevent parasitoids from walking out. The number of adults that emerged and left the tube were counted as flying adults. The number of empty pupae, remaining in the tube on the fifth day after emergence was counted as total emerged adults. The percent of fliers among all emerged parasitoids was computed (adapted from FAO/IAEA/USDA 2014). Survival and fecundity experiments were replicated four times, flight ability experiments were replicated five times.
Body size and antenna length
A sample of ten randomly selected mated females C. haywardi from each the standard and the F4 colony were assessed for their size by measuring the length of the hind tibia which is a strong indicator of body size (Rosenheim and Rosen 1992), and the length of their antennae which is considered important for host searching (Quicke 2015; Wang et al. 2016), with a micrometer adapted to a stereoscope at 1.6 × (CARL ZEISS 2000 c).
Data analysis
To analyze discrimination, non-discrimination and no choice of individual female C. haywardi, a two-by-two contingency table was used. To avoid problems of dependence between generations, a Bonferroni adjustment was applied dividing the α = 0.05 value by five. Searching, foraging and oviposition time was analyzed using a one-way analysis of variance (ANOVA), with the data normalized using a Box-Cox transformation. Flight ability, fecundity and size of the hind tibia and antenna met the assumptions of normality and were analysed using a t test. Generational survival was analyzed using the non-parametric log-rank test. All statistical analysis were performed using JMP software (version 7.0.1, SAS Institute Inc.).
Results
Selection for host discrimination by Coptera haywardi
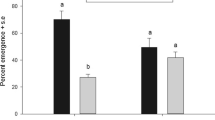
The ability to discriminate non-parasitised pupae from D. longicaudata parasitised pupae by females C. haywardi differed between the standard colony (63.83%), F1 (63.87%) and F2 (71.11%) and the F4 (76.59%) selected population (Table 1; Fig. 1). The percent F4 (23.41%) females that chose parasitized pupae was significantly lower in comparison with the standard colony (36.17%). There was no difference in the percent females that did not make a choice between the standard colony (30.88%) and the selected lines (F1–F4) (range from 22.07 to 23.78%) (Table 1). The searching and foraging time of C. haywardi females reduced as the selection process advanced. There was no difference in host searching time by the C. haywardi females of the standard colony and the first three selected generations but there was a significant difference between the F4 population and the standard colony (F4,112 = 10.96, P < 0.0001; Fig. 2a). Foraging time for female C. haywardi was reduced in all generations of the selected colony compared with the standard colony (F4,125 = 7.37, P < 0.0001; Fig. 2b). The oviposition time was similar for all generations of the selected line and the standard colony (F4,135 = 1.66, P = 0.16), ranging between 10 and 20 min.
Percentage of C. haywardi females (+ SE) that were able to discriminate from D. longicaudata parasitised hosts (i.e. A. ludens pupae), in a colony strain and four successive generations after undergoing a selection process to increase the capacity for discrimination. Columns with the same letter do not differ significantly using a contingency analyses with a Bonferroni correction (α/5 = 0.01)
Mean time (+ SE) spent searching (a) and foraging (b) for a host pupae (i.e. A. ludens) by C. haywardi females from a colony strain and four successive generations (F1–F4) developed through a selection process, where only females with the capacity to discriminate unparasitised pupae from those previously parasitized by D. longicaudata were selected. Means between columns with the same letter do not differ significantly (P > 0.05) using a Tukey test
Survival, fecundity and flight ability
Survival under stress was similar between the standard colony and F4 of the selected line (\(\chi_{1}^{2} = 0.03\), P = 0.85) (Fig. 3). The fecundity of C. haywardi females was higher in the F4 generation compared with the standard colony (t6 = 2.62, P = 0.03; Table 2). There was no difference in flight ability between the standard colony and the F4 selected line (t8 = 0.73, P = 0. 48; Table 2).
Body size and antenna length
The mean length of antenna increased progressively in the selective line for each generation (t18 = 2.14, P = 0.04; Table 2). The hind tibia length averaged 1.01 ± 0.02 mm, which was very consistent across both lines and generations (t18 = 0.22, P = 0.82).
Discussion
The selection of females based on their host searching, foraging and oviposition behaviour was an efficient process to recuperate the ability for host discrimination in mass reared C. haywardi populations. By the fourth generation of the selected line, we were able to show that key morphological and performance traits, important for host discrimination, were positively impacted.
In this study, several indicators were used to describe a complex behavior for the host searching of parasitoids which is influenced by several factors, including morphology and genetics (Vinson 1998; Henry et al. 2010). The components of searching behaviour have a high sensitivity to selection (Rolff and Kraaijeveld 2001; Rehman and Powel 2010). The changes in the parasitoid host searching behaviour can be contextualized under two scenarios. The first is that this is an intrinsic activity and that negative changes would imply a decrease in the population and therefore have a direct impact on the adaptation of the species (Boller 1972; Krivan and Sirat 1997; Hassell 2000). Alternatively, searching capability could vary in effectiveness between individuals or populations, but be maintained at a level ensuring the survival of a population (Bautista and Harris 1997; Rolff and Kraaijeveld 2001).
Competition in nature is one of the main challenges facing C. haywardi. Its high specificity requires females to search and find unparasitized pupae in an environment where there is a high likelihood that their hosts have already been attacked by a complex guild of larval parasitoids (López et al. 1999; Ovruski et al. 2000). This means that C. haywardi requires a high capacity for discrimination to maintain population levels. However, this capacity may be affected as a result of a strong trend to homozygous in laboratory strains, which are maintained in an environment where discrimination ability decreases, due to repeated exposure to non-parasitised hosts. Such a purge of the capacity of discrimination can be explained as a response of the genetic variation of this species (Forsman 2015). By selecting for individual C. haywardi females who are able to discriminate, we were able to restore the discrimination capability by the fourth laboratory generation. For C. haywardi, the capacity for discrimination is its most important attribute as a biological control agent of fruit flies. The reduction in the time spent searching for suitable hosts and an increase in fecundity of C. haywardi observed in the current study by the fourth selected generation, are common indicators of favourable lines with a greater capacity for discrimination (Messina and Karren 2003; Boivin 2010).
Host discrimination is very common within a species. However discrimination between species is less common, unless the relationship between the two different species is very close (Vet et al. 1984; Ardeh et al. 2005). C. haywardi are related to larval parasitoids of Anastrepha spp., and they compete for the same reproductive resource. C. haywardi must be able to discriminate and avoid parasitised hosts in order to successfully reproduce. For instance, first instar parasitoids of D. longicaudata within the host usually outcompete C. haywardi (López 2009; Cancino et al. 2012). The larval parasitoid can dominate the host (De Moraes and Mescher 2005; Harvey et al. 2009) and so coexistence requires a high rate of host discrimination by C. haywardi. As this parasitoid is typically reared and released into the field, to increase the level of biological control provided by the larval parasitoid D. longicaudata, the expectation is that this second parasitoid, with a high capacity for discrimination, will increase its parasitism rates and together obtain an increased level of control of pest fruit fly populations (Hoffmeister 2000; Cancino et al. 2012).
Parasitoids that are able to effectively discriminate hosts often report higher fecundity and faster host searching (Darrouzet et al. 2007; Lebreton et al. 2008), as we observed in the current study. An increase in wasp fecundity is an important attribute to improve fitness through phenotypic plasticity (Goibin 2010). The discrimination process can involve a cost where the female parasitoids must invest energetic resources to optimise their reproductive success by selecting non-parasitised pupae (Yamada 1988).
The results reported here suggest that increased antennae length favors the identification of unparasitised pupae by C. haywardi, leading A. ludens pupae previously parasitized by D. longicaudata to be rejected. The antennae has been considered as a basic functional structure used in searching behaviour (Lewis et al. 1990; Machtinger et al. 2015; Wang et al. 2016) and selection of hosts by pupal parasitoids (Ruschioni et al. 2015; Wang et al. 2016). Although there is limited published literature about the steps and indicators that guide the selection of a host by C. haywardi, it is likely that the antennae play an important role. The two published descriptions of the behaviour of searching and selection emphasise the antennae, which is rubbed on the cuticle of the pupa, to determine acceptance or rejection for oviposition (Fischer et al. 2004; McKay and Broce 2004). Similarly, in the current study we observed C. haywardi rubbing their antennae over the pupae before making a choice. Other sensors, including those in the abdomen and the ovipositor, are also thought to be important for discrimination of pupae by parasitoids (Goubault et al. 2011). Vinson (1998) concluded that there is strong selective pressure when host selection involves direct contact. The increase in the size of antennae is possibly a result of a combination a high level of genetic variation in C. haywardi and the strong selection pressure imposed at each generation. Where host-parasitoid relationships are involved, population changes can be relatively rapid (Hughes and Sokolowski 1996; Henry et al. 2010). Changes in survival and the ability to fly were not associated with an increase in discrimination. Both parameters have a close relationship with the quality of the host (Visser et al. 2010), but it is not apparent from this study that improved discrimination is at a cost to the parasitoids with regard to these performance attributes.
It is feasible to assume that the ability for C. haywardi to discriminate parasitised hosts was lost through homozygosis. Therefore, under mass rearing conditions, where the priority is to select for highly fecund females, the capacity to discriminate was predicted to decrease due to the pressures placed upon the strain. To maximise the ability of mass-reared parasitoids to discriminate parasitised hosts, there are several options that might be considered: (1) maintaining selected lines that have the highest level of discrimination and that can be mixed in the field with releases of other lines and wild parasitoids (Coelho et al. 2016), (2) hybridisation of lines; different trials have shown the high probability of increasing desirable attributes by combining strains (Gilchrist and Meats 2014), and (3) encourage discrimination by offering parasitised pupae to part of the colony each generation, or intermittently, i.e. every several generations. Further research is required to define and refine which option(s) will be the most effective, while minimizing costs of time and labour.
In augmentative biological programs using the pupal parasitoid C. haywardi to complement the activity of larval parasitoids, it is imperative to maintain the capacity for a high level of discrimination. The proposal to select individuals to increase host discrimination is a novel strategy for tephritid parasitoids that will have application in mass-rearing scenarios. However continual maintenance of the desired traits will be required to minimise the return to homozygosity. Further research to understand the mechanisms by which C. haywardi searches for, and accepts pupae will clarify aspects of the searching process, and inform better colony management. Genetic evaluation of the strains would also reveal a deeper insight into the capacity for host discrimination by C. haywardi.
References
Aguiar-Menezes E, Menezes E, Loiàcono MS (2003) First record of Coptera haywardi Loiàcono (Hymenoptera: Diapriidae) as a parasitoid of fruit-infesting Tephritidae (Diptera) in Brazil. Neotrop Entomol 32:355–358
Aluja M, Sivinski J, Ovruski S, Guillén L, López M, Cancino J, Torres-Anaya A, Gallegos-Chan G, Ruíz L (2009) Colonization and domestication of seven species of native New World hymenopterous larval-prepupal and pupal fruit fly (Diptera: Tephritidae) parasitoids. Biocontrol Sci Technol 19:49–79
Ardeh MJ, de Jong PW, van Lenteren JC (2005) Intra- and interspecific host discrimination in arrhenotokous and thelytokous Eretmocerus. Biol Control 33:74–80
Baeshen R, Ekechukwu NE, Toure M, Paton D, Coulibaly M, Traoré SF, Tripet F (2014) Differential effects of inbreeding and selection on male reproductive phenotype associated with the colonization and laboratory maintenance of Anopheles gambiae. Malaria J 13:9. https://doi.org/10.1186/1475-2875-13-19
Bautista RC, Harris EJ (1997) Effect of insectary varying on host preference and oviposition behavior of the fruit fly parasitoid Diachasmimorpha longicaudata. Entomol Exp Appl 83:213–218
Beukeboom LW, Koevoets TM, Morales HE, Ferber S, van de Zande L (2015) Hybrid incompatibilities are affected by dominance and dosage in the haplodiploid wasp Nasonia. Front Genet 6:140. https://doi.org/10.3389/fgene.2015.00140
Bodino N, Ferracini C, Tavella L (2016) Is host selection influenced by natal and adult experience in the parasitoid Necremmus tutae (Hymenoptera: Eulophidae). Anim Behav 112:221–228
Boivin G (2010) Phenotypic plasticity and fitness in egg parasitoids. Neotrop Entomol 39:457–463
Boller EF (1972) Behavioral aspects of mass-rearing of insects. Entomophaga 17:9–25
Boller EF, Chambers DL (1977) Quality aspects of mass-reared insects. In: Ridway RL, Vinson SB (eds) Biological control by augmentation of natural enemies. Enviromental Science Research, Springer, Boston, pp 219–235
Cancino J, Liedo P, Ruiz L, López G, Montoya P, Barrera JF, Sivinski J, Aluja M (2012) Discrimination by Coptera haywardi (Hymenoptera: Diapriidae) of hosts previously attacked by conspecifics or by the larval parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Biocontrol Sci Technol 22:899–914
Coelho A Jr, Rugman-Jones PF, Reigada C, Stouthamer R, Parra JRP (2016) Laboratory performance predicts the success of field releases in inbred lines of the egg parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). PLoS ONE 11(1):e0146153. https://doi.org/10.1371/journal.pone.0146153
Darrouzet E, Bignon L, Chevrier C (2007) Impact of mating status on egg-laying and superparasitism behaviour in a parasitoids wasp. Entomol Exp Appl 123:279–285
De Moraes CM, Mescher MC (2005) Intrinsic competition between larval parasitoids with different degrees of host specificity. Ecol Entomol 30:564–570
De Moraes CM, Lewis WJ, Tumlinson JH (2000) Examining plant-parasitoid interactions in tritrophic systems. An Soc Ent Bras 29:189–203
Evangelou VI, Bouga M, Emmanouel NG, Perdikis DCh, Papadoulis GT (2013) Discrimination of two natural biocontrol agents in the Mediterranean region based on mitocondrial DNA sequency data. Biochem Genet 51:825–840
FAO/IAEA/USDA (2014) Product quality control for sterile mass-reared and released Tephritid fruit flies, Version 6.0. International Atomic Energy Agency, Vienna, Austria
Fischer S, Samietz J, Dorn S (2004) Host location of a pupal parasitoid in tritrophic system compared to a model offering mechanosensory cues only. J Insect Behav 17:191–199
Forsman A (2015) Rethinking phenotypic plasticity and its consequences for individuals, population and species. Heredity 115:276–284
Gilchrist AS, Meats AW (2014) An evaluation of outcrossing to improve mass-reared strains of the Queensland fruit fly Bactrocera tryoni. Int J Trop Insect Sci 34:35–44
Giunti G, Canale A, Meesing RH, Donati E, Stefanini C, Michaud JP, Benelli G (2015) Parasitoid learning: current knowledge and implications for biological control. Biol Control 90:208–219
Goibin G (2010) Phenotypic plasticity and fitness in egg parasitoids. Neotrop Entomol 39:457–463
Goubault M, Corsetero AM, Paty C, Fourrier J, Dourlot S, Le Ralec A (2011) Abdominal sensory equipment involved in external host discrimination in a solitary parasitoid wasp. Micros Res Tech 74:1145–1153
Harvey JA, Gols R, Strand MR (2009) Intrinsic competition and its effects on the survival and development of three species of endoparasitoid wasps. Entomol Exp Appl 130:238–248
Hassell MP (2000) Host-parasitoid population dynamics. J Anim Ecol 69:543–566
Henry LM, May N, Acheampong S, Gillespie DR, Roitberg BD (2010) Host-adapted parasitoids in biological control: Does source matter? Ecol Appl 20:242–250
Hernández-Ortiz V, Delfin-González H, Esalante A, Manrique-Saide P (2006) Hymenopteran parasitoids of Anastrepha fruit flies (Diptera: Tephritidae) reared from different hosts in Yucatán, México. Fla Entomol 89:508–515
Hoffmeister TS (2000) Marking decisions and host discrimination in a parasitoid attacking concealed host. Can J Zool 78:1494–1499
Hughes K, Sokolowski MB (1996) Natural selection in the laboratory for a change in resistance by Drosophila melanogaster to the parasitoid wasp Asobara tabida. J Insect Behav 9:477–491
Krivan V, Sirat E (1997) Searching for food and hosts: the influence of parasitoid behavior on host parasitoid dynamics. Theor Popul Biol 51:201–209
Lebreton S, Labarussias M, Chevrier C, Darrouzet E (2008) Discrimination of the age of conspecific eggs by an ovipositing ectoparasitoid wasp. Entomol Exp Appl 130:28–34
Lewis WJ, Martin WR Jr (1990) Semiochemicals for use with parasitoids: status and future. J Chem Ecol 16:3067–3089
Lewis WJ, Vet LEM, Tumlinson JH, van Lenteren JC, Papaj DR (2003) Variations in natural enemy foraging behaviour: essential element of a sound biological control theory. In: van Lenteren JC (ed) Quality control and production of biological control agents: theory and testing procedures. CABI Publishing, Wallingford, pp 41–58
López G (2009) Capacidad de discriminación del parasitoide de pupas de moscas de la fruta Coptera haywardi (Hymenoptera: Diapriidae) en hospederos parasitados. Tesis Ingeniero Agrónomo Tropical UNACH, Huehuetán, p 51
López M, Aluja M, Sivinski J (1999) Hymenopterous larval-pupal and pupal parasitoids of Anastrepha flies (Diptera: Tephritidae) in Mexico. Biol Control 15:119–129
Machtinger ET, Geden CJ, Teal PE, Leppla NC (2015) Comparison of host-seeking behavior of the filth fly pupal parasitoid, Sapalangia cameroni and Muscidifurax raptor (Hymenoptera: Pteromalidae). Environ Entomol 44:330–337
Mackaueer M, Michaud JP, Völk W (1996) Host choice by Aphidiid parasitoids (Hymenoptera: Aphidiidae): host recognition, host quality, and host value. Can Entomol 128:959–980
Mackauer M (1976) Genetic problems in the production of biological control agents. Annu Rev Entomol 21:369–385
McKay T, Broce AB (2004) Discrimination of self-parasitized hosts by the pupal parasitoid Muscidifurax zaraptor (Hymenoptera: Pteromalidae). Ann Entomol Soc Am 97:592–594
Messina FJ, Karren ME (2003) Adaptation to a novel host modifies host discrimination by seed beetle Callosobruchus maculatus. Anim Behav 65:501–507
Ovruski S (1995) Pupal and larval-pupal parasitoids (Hymenoptera) obtained from Anastrepha spp. and Ceratitis capitata (Dip.: Tephritidae) pupae collected in four localities of Tucumán Province Argentina. Entomophaga 40:367–370
Ovruski S, Aluja M, Sivinski J, Wharton R (2000) Hymenopteran parasitoids on fruit-infesting Tephritidae (Diptera) in Latin America and the Southern United States: Diversity, distribution, taxonomic states and their use in fruit fly biological control. Int Pest Manag Rev 5:81–107
Parreño M, Scannapieco AC, Renis MI, Juri M, Vera MT, Segura DF, Cladera JL, Lanzavecchia SB (2014) Dynamics of genetic variability in Anastrepha fraterculus (Diptera: Tephritidae) during adaptation to laboratory varing conditions. BMC Genet 15(Suppl 2):S14
Qi-Fu L, Tong-Xian L (2017) Interspecific host discrimination and intrinsic competition between Aphelinus asichis and Apidus gifuensis in Myzus persicae. Entomol Exp Appl 163:265–271
Quicke DL (2015) The braconid and ichneuomid parasitoid wasps: Biology, systematics, evolution and ecology. Wiley, West Sussex
Quintero-Fong L, Toledo J, Ruiz L, Rendon P (2016) Selection by mating competitiveness improves the performance of Anastrepha ludens males of the genetic sexing strain Tapachula-7. B Entomol Res 106:624–632
Rehman A, Powell W (2010) Host selection behavior of aphid parasitoids (Aphidiidae: Hymenoptera). J Plant Breed Crop Sci 2:299–311
Rolff J, Kraaijeveld AR (2001) Host preference and survival in selected lines of a Drosophila parasitoid, Asobara tabida. J Evol Biol 14:742–745
Rosenheim JA, Rosen D (1992) Influence of egg load and host size on host-feeding behavior of the parasitoid Aphytis lingnanensis. Ecol Entomol 17:263–272
Ruschioni S, van Loon JJA, Smid HM, van Lenteren JC (2015) Insects can count: Sensory basis of host discrimination in parasitoid wasp revealed. PLoS ONE 10(10):e0138045
Saul PH, McCombs SD (1993) Genetics and ecology of colonization and mass rearing of Hawaiian fruit flies (Diptera: Tephritidae) for use in sterile insect control programs. P Hawaii Entomol Soc 32:21–37
Scholz D, Höller C (1992) Competition for hosts between two hyperparasitoids of aphids, Dendrocerus laticeps and Dendrocerus carpenteri (Hymenoptera: Megaspilidae): The benefit of interspecific host discrimination. J Insect Behav 5:289–300
Schutze MK, Dammalage T, Jessup A, Vreysen MJB, Wornoayporn V, Clarke AR (2015) Effects of laboratory colonization on Bactrocera dorsalis (Diptera: Tephritidae) mating behaviour: “what a difference a year makes”. ZooKeys 540:369–383
Sivinski J, Vulinec K, Menezes E, Aluja M (1998) The bionomics of Coptera haywardi (Oglobin) (Hymenoptera:Diapridae) and the other pupal parasitoids of tephritid fruit flies (Diptera). Biol Control 11:193–202
Sivinski J, Piñero J, Aluja M (2000) The distribution of parasitoids (Hymenoptera) of Anastrepha fruit flies (Diptera: Tephritidae) along an altitudinal gradient in Veracruz, México. Biol Control 18:258–269
Sørense JG, Addison MF, Terblanche JS (2012) Mass-rearing of insects for pest management and advances from evolutionary physiology. Crop Prot 38:87–94
Tejada MT, Arredondo-Gordillo JA, Orozco-Dávila D, Quintero-Fong L, Díaz-Fleisher F (2017) Directional selection to improve the sterile insect technique: survival and sexual performance of dissecation resistant Anastrepha ludens strains. Evol Appl 10:1020–1030
van Baaren J, Boivin G (1998) Learning affects host discrimination behavior in parasitoid wasp. Behav Ecol Sociobiol 42:9–16
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl 57:1–20
van Lenteren JC, Bigler F (2010) Quality control of mass-reared egg parasitoids. In: Consôli FL, Parra JRP, Zucchi RA (eds) Egg parasitoids in Agroecosystems with emphasis on Trichogramma. Springer, Netherlands, pp 315–340
Vet LEM, Meyer M, Bakker K, van Alphen JJM (1984) Intra- and interspecific host discrimination in Asobara (Hymenoptera) larval endoparasitoid of Drosophilidae: comparison between closely related and less closely related species. Anim Behav 32:871–874
Vinson SB (1998) The general host selection behavior of parasitoid Hymenoptera and comparison of initial strategies by a larvaphagous and oophagous species. Biol Control 11:79–96
Visser B, Le Lann C, den Blanken FJ, Harvey JA, van Alphen JJM, Ellers J (2010) Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. P Natl Acad Sci USA 107:8677–8682. https://doi.org/10.1073/pnas.1001744107
Wajnberg E (2004) Measuring genetic variation in natural enemies used for biological control: Why and how? Ehler LE. Mateille T, Genetics, evolution and biological control, CABI Publishing, Wallingford, UK, Sforza R, pp 19–37
Wang D, Lü L, He Y, Shi Q (2016) Mate choice and host discrimination behavior of the parasitoid Trichogramma chilonis. B Entomol Res 106:530–537
Yamada Y (1988) Optimal use of patches by parasitoids with a limited fecundity. Res Popul Ecol 30:235–249
Acknowledgements
We wish to thank the Biological Control Department staff of the Moscafrut Program, Javier Valle for statistical advice and Cesar Perez for his assistance in the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Stefano Colazza
Rights and permissions
About this article
Cite this article
Cancino, J., Pérez, B., Johnson, A.C. et al. Parasitoids are choosy: increase in the capacity to discriminate parasitised tephritid pupae by Coptera haywardi. BioControl 64, 357–366 (2019). https://doi.org/10.1007/s10526-019-09941-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-019-09941-5