Abstract
A population of the citrus nematode Tylenchulus semipenetrans Cobb (Tylenchida: Tylenchulidae) associated to a Pasteuria sp. (Bacillales: Bacillaceae) was studied in a naturally infested field. In a first population dynamics study, prevalence never exceed 50 % and showed a density-dependent relationship with the host at 2–3 months time lags, with a spring sharp increase. In a spatial sampling study, both organisms resulted uniformly distributed and matched the observed relationship. In top (10 cm) soil the adult females were lower than in deeper layers, whereas the nematodes with adhering endospores were higher. Anderson and May’s Model G applied showed that 50–400 endospores per cc of soil can sustain stable regulatory cycles. The nematode and bacterium populations were found in the field 12 years later with declining densities, below the calculated threshold needed to maintain the parasite infection. Data confirmed the bacterium potential in nematode regulation and showed that host numbers affect prevalence changes in time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tylenchulus semipenetrans Cobb (Tylenchida: Tylenchulidae) is a widespread pest of citrus severely affecting plants performance and yields in sub-optimal growth conditions. The nematode mainly attacks young feeder roots and its pathogenicity is enhanced by stress related to soil properties and/or water availability (Duncan and Noling 1987). Many biological antagonists have been reported for T. semipenetrans, including Pasteuria sp. (Bacillales: Bacillaceae) or nematophagous fungi, regulating its densities in mature trees (Gaspard and Mankau 1986; Fattah et al. 1989; Kaplan 1994; Sorribas et al. 2000).
The genus Pasteuria (Firmicutes: Bacillaceae) includes endospore-forming fastidious endoparasites with a narrow host specificity. In P. penetrans (Sayre & Starr), parasitic in root-knot nematodes (RKN), infection starts in soil with the attachment of dormant endospores to motile second stage juveniles (J2). The endospores eventually germinate penetrating the J2, yielding an infection synchronous with the host development. In optimal conditions P. penetrans completes its cycle in 3–4 weeks, sporulating in mature RKN females, once they established their feeding site in roots.
Further Pasteuria spp. show similar host adaptation and specificity, like P. nishizawae (Sayre, Wergin, Schmidt & Starr) sporulating in adult females of the cyst nematode Heterodera glycines (Ichinohe) (Noel et al. 2005). Other forms or putative species, however, complete their cycles sporulating in J2 and other motile stages, like a further Pasteuria sp. from the cereal cyst nematode H. avenae (Wollenweber) or other species from tylenchid or dorylaimid hosts (Davies et al. 1990; Ciancio et al. 1992; Ciancio 1995).
The Pasteuria sp. associated to T. semipenetrans has been reported from different citrus growing regions, worldwide. It sporulates in J2 and males (motile stages), yielding 320–400 small endospores (3.0–3.5 µm wide) per infected nematode, in less than three weeks (Fattah et al. 1989; Ciancio and Roccuzzo 1992; Kaplan 1994; Sorribas et al. 2008).
Pasteuria penetrans and related species infect juvenile nematodes and follow their life-cycle, sporulating in adult females. By this way they allow infected nematodes to feed on roots, a process that let the nematode induce a severe histologic damage. The Pasteuria spp. which kill the J2 and males, like the T. semipenetrans-associated bacterium, yield instead a more beneficial effect, because the infected nematodes do not reach roots and no damage is produced. However, due to host specificity and to maintain its generations, these endoparasites must rely on some unknown regulation mechanisms, allowing the perpetuation of the host population by some J2 that, reaching the roots, can reproduce. The J2-killing Pasteuria spp. may either remove only a surplus number of nematodes in soil (Stirling 2014), or depend on other regulation mechanisms allowing persistence of both host and parasite populations, through a genetic race of the “Red Queen” type.
Studies on P. penetrans carried out in naturally infested fields, with artificially-infected hosts or using endospores produced in culturing media, and showed a high biocontrol potential of the bacterium (Oostendorp et al. 1991; Verdejo Lucas 1992; Ciancio and Quénéhervé 2000; Debiré et al. 2007; Mateille et al. 2009; Luc et al. 2010). The use and application of Pasteuria spp. as biocontrol agents require knowledge on their field ecology and biology, because their application is feasible today thanks to their culture in artificial conditions (Luc et al. 2010). The cultivation of Pasteuria spp. in fermentation units encourages further studies on these bacteria. RKNs have been the most common pests targeted in biocontrol assays with P. penetrans (Oostendorp et al. 1991; Davies and Redden 1997). Few data are available on other Pasteuria spp., in particular concerning field ecology and persistence in time of the bacteria and host nematode populations (Verdejo Lucas 1992; Ciancio 1995; Ciancio and Quénéhervé 2000; Sorribas et al. 2000; Mateille et al. 2009). Although the presence of a dose-response or density-dependent relationships were reported for these parasites (Ciancio and Quénéhervé 2000), many factors sustaining host regulation and biocontrol efficacy in nature remain to be determined (Atibalentja et al. 1998; Mateille et al. 2009).
The aim of the present work was to investigate the regulation of T. semipenetrans by the associated Pasteuria sp. through samplings repeated in time and space within a naturally infested field, to compare population dynamics of the host and the bacterial parasite. Long term observations on parasitism and population dynamics showed a density-dependent nematode regulation by the bacterium, providing data on endospore soil density and on changes of prevalence levels during time, as affected by roots and nematode abundance.
Materials and methods
Population dynamics study
Soil samples were collected in a citrus orchard at Racale (39°58′48.5″N; 18°4′3″E), in the province of Lecce (Italy), at regular monthly intervals from seven replicated citrus trees. The plants, grafted on sour orange, Citrus sinensis (L.) Osb. were naturally infested by a T. semipenetrans population associated to the specific small-size Pasteuria sp., thus far undescribed. Sampling was carried out at an average depth of 10–20 cm under each tree canopy, collecting three 0.5 l soil subsamples with a few grams of roots, from sites at 120° each other, at 40–60 cm from the trunk base.
Motile stages of T. semipenetrans were extracted using the Cobb’s sieving and decanting technique with 0.7 mm and 45–75 μm sieves. Mature females were extracted from a few grams of roots after 20 s maceration in a blender. Countings were performed at ×50 with a Leitz Orthoplan light microscope using a 1 ml Hawksley counting chamber. At each counting, approx. 45 specimens of T. semipenetrans J2 and males were randomly picked up with an eye lash from the suspension and examined at ×500 in light microscopy on a temporary water mount. The variables measured were the percent nematodes with the Pasteuria sp. endospores adhering to the cuticle, the number of adhering endospores per nematode, and the percent nematodes penetrated by germinating endospores and/or infected (showing the parasite vegetative stages or endospores internally). The latter percentage is indicated as prevalence. More than 800 T. semipenetrans females were also examined to check for the presence of Pasteuria sp. stages adhering to their cuticle or present within their bodies.
Vertical and spatial distribution studies
Vertical distribution of T. semipenetrans and Pasteuria sp. prevalence were studied in three replicated trees, collecting soil (250 cc) at 0–10, 10–20 and >20 cm depths from three sampling sites under each tree canopy, with a 8 cm diam. core sampler. The spatial spread of the host and parasite association was also studied sampling at each month further trees randomly chosen in the same field, by measuring the nematode density and parasite prevalence as previously described. A first one-time spatial sampling study was carried out in Nov. 1993 to provide data on the host-parasite relationship. One-time spatial samplings were used to demonstrate density-dependence between other fungal antagonists or Pasteuria sp. and host nematodes (Jaffee and McInnis 1991; Jaffee et al. 1992; Ciancio 1995). The basic assumption is that collecting a sufficient number of samples at one single moment, it can be possible to reconstruct the asynchronous and cyclic host–parasite relationships (if present). Each sample in fact accounts for a particular host–parasite combination in their phases space, which results in plotting density and prevalence data of each observation from a time data series. For the spatial sampling of T. semipenetrans, Taylor’s power law was applied to determine the number of replicated samples to collect, to get a 5 % standard error (SE) to mean ratio for motile stage densities (McSorley et al. 1985). For this purpose the time series means and variance were used, and 44 samples were collected. The same field was sampled again 12 years later, in January and July 2005, randomly collecting 44 samples from individual trees, to check long-term effects of Pasteuria sp. on the host population and possible biocontrol outcomes, the persistence of the cyclic relationships between host and parasite and the stability of the relationship, compared to the data from the previous samplings.
Greenhouse test
An assay was performed to determine the effect of plant fertilization on the changes of the T. semipenetrans infestation and Pasteuria sp. prevalence, during time. Plants of sour orange at the fourth leaf stage were transplanted in soil from the first spatial sampling, used for the initial nematode and Pasteuria sp. inocula. The plants were maintained in 15 cm diam. plastic pots for ten months in a greenhouse at 22–26 °C. The treatments consisted in sequential fertilizer applications to soil scheduled one, two, seven and nine months after transplant, in six replicates with a 10–17–18 NPK powder (Orvital, Settimo Milanese, Italy). At each fertilization, a set of six plants was let unfertilized until the end of the trial, in order to provide five different treatments on a total of thirty plants, including plants never fertilized as controls.
Statistical analysis and modeling
The spatial and time series data were used for modelling the nematodes and Pasteuria relationship with Anderson and May’s Model G (AM-G). The model is based on parasite transmission and densities of healthy and infected hosts, and yields the density of the parasite propagules in soil (Anderson and May 1981; Jaffee et al. 1992). The AM-G model relies on three equations, accounting for the numbers of healthy (X) and infected hosts (Y), and the number of the parasite free propagules (W), with total host population H = X + Y. The AM-G model uses eight constants, that were derived from the parasite and host reported biology (for values and dimensions see Table 1).
Results
Population dynamics and vertical distribution studies
Motile nematode stages infected by Pasteuria sp. were found at each monthly sampling, but no female showed presence of vegetative stages or endospores of the parasite. The density of T. semipenetrans motile stages varied from 581 ± 187 (mean ± SE) to 13,986 ± 4121 nematodes per 100 cc of soil, with a stable trend from April until November, a minor winter increase and a dramatic ten-fold peak in late spring, in the following year (Fig. 1a). Prevalence varied from 0.8 ± 0.6 to 14.0 ± 4.3 %, with a peak showing an 11-fold increase from April until July (Fig. 1a). Pasteuria sp. prevalence showed delayed alternating trends similar (in scale) to those of the host population densities, with low fluctuations following at a 2–3 month time lags the density changes of T. semipenetrans. The numbers of parasitized nematodes were correlated with the previous densities recorded two months earlier (r = 0.859, P < 0.001, n = 13). Data showed a recursive cyclic trend when plotted on their phase space (Fig. 1b). Autocorrelation analysis of time series data showed significant coefficients (P < 0.05) for nematode densities at one and five months time lags (Fig. 1c).
Densities of T. semipenetrans and Pasteuria sp. parasitism in a naturally infested field at Racale (Italy). Time series (a) showing density changes of nematode motile stages (dots) and prevalence (squares). Same data plotted on the corresponding phase space (b), showing the counter-clockwise cycle in time (arrows). Autocorrelations (c) of density (squares) and prevalence (dots) data series (asterisks mark significant correlations at P < 0.05). Monthly density changes (d) of T. semipenetrans females per g of roots (dots) and roots recovered per samples (squares). Each observation is mean of seven replicates. Vertical bar shows max SE
The densities of adult females ranged from 294 ± 92 (Dec.) to 1208 ± 196 females per g of root (April), with alternated fluctuations (Fig. 1d). The total females were correlated with the densities of motile stages of the same (r = 0.710, P < 0.01, n = 15) and preceeding (r = 0.644, P < 0.02, n = 14) months. The percentages of T. semipenetrans with adhering Pasteuria sp. endospores were usually higher than prevalence and varied from 3.4 ± 1.8 % (Dec.) to 22.4 ± 4.7 % (July), with a final six-fold increase in late spring (Fig. 2). The mean number of endospores per infected nematode ranged from 1 to 3.5 during the winter period (Fig. 2). The maximum mean endospore number per nematode varied from 1.3 (April) to 7.2 (August). The monthly prevalence levels and the numbers of nematodes with adhering endospores were correlated (r = 0.754, P < 0.01, n = 15).
Time changes of the T. semipenetrans motile stages with adhering Pasteuria sp. endospores (%, dots), and mean number of spores per nematode (squares) as per Fig. 1. Each observation is the mean of seven replicates. Vertical bar shows max SE
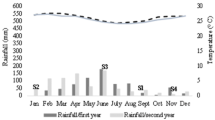
The mean monthly temperatures and rainfall data for the field area and first sampling period are shown in Fig. 3. When considering the time lag, the monthly rainfall values were correlated to the total amounts of roots per sample observed after two months (r = 0.626, P < 0.02, n = 15) and inversely correlated to the densities of females (r = −0.564, P < 0.05, n = 15).
Sampling depths did not significantly affect the range of densities for T. semipenetrans motile stages and Pasteuria sp. prevalence or the numbers of adhering endospores. However, female numbers at 10–20 and >20 cm depth were significantly higher (P < 0.05) than in top soil, whereas the nematodes with adhering endospores decreased from 10.9 ± 4.2 % (top soil) to 2.9 ± 1.1 % (>20 cm deep soil).
Spatial distribution study and modelling
The densities of T. semipenetrans and prevalence by Pasteuria sp. were measured on 44 samples randomly scattered in the citrus field. The mean number of sedentary forms was 922 ± 117 (62–3716) females per g of root, the mean density of motile stages was 4370 ± 539 (250–15,400) nematodes per 100 cc of soil. Pasteuria sp. prevalence was 12.1 ± 0.8 % (2.4–27.8), and the percentage of nematodes with adhering endospores was 11.0 ± 9.2 % (0–41.6). The densities of motile stages and females were correlated (r = 0.573; P < 0.001, n = 44).
An insight into the Pasteuria sp. nematode regulation cycle, including the estimation of the endospore numbers in soil, was provided by the model AM-G. When soil nematode density and prevalence data from the first spatial sampling were plotted on their phase spaces, the observations were included within an area defined by the AM-G cycle produced by the constant and initial density values applied (Table 1, Fig. 4a). The AM-G model showed an estimated number of Pasteuria sp. free propagules in soil ranging in the order of 0.5–4.0 × 104 endospores per 100 cc of soil (Fig. 4b). The AM-G model also allowed computation of the transmission coefficient β = vλ/µ, whose value (2.3375 × 10−5) was used to determine the threshold host density Ht = (α+β+γ)/β (Anderson and May 1981), corresponding to 1720 nematodes per 100 cc of soil. This density is the minimal number of nematodes required to maintain the Pasteuria sp. infection in the host population. The model also allowed identification of the equilibrium densities, at which host and parasite densities remain unchanged in time, corresponding to 3593 nematodes per 100 cc of soil and 1.56 × 104 propagules per 100 cc of soil (Fig. 4b).
Relationship of T. semipenetrans densities (motile stages) with Pasteuria sp. prevalence in a naturally infested field, measured with spatial samplings. (a) First (1993) sampling data, with the AM-G model cycle prevalence values calculated using the constants and initial values shown in Table 1 (arrows show the cycle direction). Densities of Pasteuria sp. endospores and host nematodes in soil (b), calculated by the same model. The calculated points, produced on a hourly time scale, form a continuous cycle which allows the identification of the equilibrium point (squares) and the host density threshold Ht (vertical line). Second spatial sampling data for nematode densities vs. prevalence (c) and nematodes with endospores (d), in the same field, 12 years later, in January (dots) and July (squares) 2005
The nematode population and the associated Pasteuria sp. were still present 12 years after the first sampling (Fig. 4c, d). In January 2005 the data from the replicated spatial sampling showed a low prevalence (3.1 ± 4.3 %, 0–15), as was the fraction of nematodes with adhering endospores (11.8 ± 13 %, 0–30). The mean number of spores per infected nematode was 0.8 (0–1.6, including nematodes with endospores inside). The densities of T. semipenetrans motile stages were below the Ht threshold value obtained from the previous sampling, with 128.9 ± 26.3 nematodes per 100 cc of soil (3.2–660). They were correlated to prevalence and to the numbers of nematodes with adhering endospores (r = 0.484 and 0.466, respectively; P < 0.001, n = 44), which were also correlated (r = 0.465; P < 0.001, n = 44). In July 2005 mean T. semipenetrans density in soil increased to 268.7 ± 48 nematodes per 100 cc of soil, with 72 ± 10 females per g of root (9.7–370). Prevalence levels were higher than in January, with 17.9 ± 2.5 % of parasitized nematodes (0–45 %), whereas the nematodes encumbered with endospores were 9.7 ± 2.1 % (0–40 %), with a mean of 0.9 ± 0.2 (0–4.8) adhering endospores per infected nematode.
Greenhouse assay
No effect of fertilization on Pasteuria sp. prevalence was observed among the treatments. The mean differences between final and initial prevalence levels ranged within ±2.2 % (range: −18.8 to 26.5) and were negatively correlated to the differences between final and initial nematode densities (r = −0.452, P < 0.05, n = 30, motile stages) showing opposite trends. The number of females per g of root and the densities of motile stages were significantly higher in the control pots, which also showed the highest fresh root weights (Fig. 5a–c), whereas prevalence and numbers of nematodes with endospores increased (Fig. 5d–f). When considering the pooled observations, a relationship was observed among the densities of females and those of motile stages at the beginning and at the end of the assay. The final percentages of parasitized nematodes were correlated to the initial female densities. A relationship was also observed between the initial densities of motile stages and final prevalence or nematodes with endospores, and between final prevalence and initial and final percent nematodes with adhering endospores (Table 2).
Effect of the number of sequential fertilizer applications on roots growth (a), densities of T. semipenetrans females (b) and motile stages (c), Pasteuria sp. prevalence (d), endospore infestations (e) and mean number of adhering propagules per nematode (f) (means of six replicates; vertical bars = SE)
Discussion
Population dynamics and spatial sampling data showed a cyclic, time-lagged density-dependent relationship between T. semipenetrans and the associated Pasteuria sp. The dramatic increase observed for T. semipenetrans in summer 1992 derived by an enhanced nematode reproduction, as shown by the females increase a month earlier, likely related to the increase of feeder roots in soil (Fig. 1 a, d). Prevalence showed similar trends following the nematode density increase with a 2–3 months time lag. In a classic density-dependent relationship any increase of parasite/predator is directly related to a higher host/prey abundance previously encountered (Hassell 1978). This implements a feedback which mutually links host and biological antagonist and that appeared at work in the populations sampled (Fig. 1a).
The first spatial sampling confirmed the Pasteuria regulating effect on T. semipenetrans, with host abundance and prevalence grouped by the calculated points of the AM-G model cycle applied. Due to the strict specificity and host dependence of Pasteuria endoparasites, this relationship suggests that a mechanism of endospore removal from soil is needed to avoid the build-up of durable propagules and host infestation by endospores of different ages, or even extinction. Factors affecting Pasteuria spp. endopores persistance in soil have been identified, including percolation (Dabiré et al. 2007; Mateille et al. 2009). It is likely that they interact with roots abundance, as suggested by the negative response of roots to increasing fertilizer applications observed in the greenhouse assay (Fig. 5a), and by the T. semipenetrans preference for young feeder roots (Duncan and Noling 1987). These factors affected the nematode multiplication because increasing the number of fertilizer applications the amounts of feeder roots needed to sustain plant nutrition were lower. This affected the number of females (Fig. 5b). The data also showed an increase in prevalence, likely related to an higher aggregation of nematodes in the reduced root systems in the pots.
As shown by plotting the time series data on their phase space, the nematode-parasite relationship was at equilibrium during the first period of study (Fig. 1b). First spatial sampling also showed that observations were distributed around the equilibrium point identified by the AM-G model and likely fluctuated around this area in time. Plotting the regular model fluctuations in time showed a three months out-of-phase time lag between the two organisms, indicating that the entire cycle of the Pasteuria and T. semipenetrans populations are completed within this period. The cycle amplitude, however, depends on the initial system conditions (nematode and propagule densities, data not shown). When transposed to field conditions, this property implies that endospore inoculations or, if available in mass, inundative treatments will require, at the densities observed, at least three months to affect T. semipenetrans densities (equivalent to five Pasteuria generations). This period, however, may likely increase, depending on temperatures, nematode numbers and prevalence levels eventually achieved in the soil microcosm after treatments.
The Pasteuria prevalence and host-parasite interactions appeared controlled by a few basic biological characters of the organisms involved. The parasite persisted for a long time and after 12 years it was associated to declining densities of T. semipenetrans. Sampling data, however, suggest the interference of additional factors. In 2005 both the nematode and Pasteuria sp. populations were below their equilibrium values and all densities of T. semipenetrans were under their Ht value (Fig. 4c). The latter is of major significance for the persistence of infection, which was in fact reduced. It was not possible to determine, however, if the nematode density reduction was affected by tree decline (less abundant feeder roots were observed in 2005 due to poor tree maintenance), indirectly lowering prevalence, or if the nematode decline results by parasitism only.
The constants considered in the AM-G model represent mechanisms at work in the real environment. Caution, however, is suggested in the use of non-linear, complex models that are highly dependent on small changes that affect cycles amplitudes and final results (Gressel 2005). In nature, nematode growth rates are not constant but are a function of many variables including the population density, food abundance and temperatures. The parasite transmission and mortality rates are also affected by physical variables like soil texture or water content (Mateille et al. 2009). Introducing temperature functions in the model may increase its adherence to real data, but it must be checked whether either T. semipenetrans and Pasteuria spp. coevolved towards the same optimal development temperatures, or if different optima are needed for parasitism and/or nematode lifestages.
The counter-clockwise cycle of the AM-G model plotted points (Fig. 5a, b) suggests that stochastic fluctuations of host densities may affect the levels of parasitism achieved after a given time lapse. At a critical distance from the equilibrium, a temporary host local extinction may occur if the satellite approaches the prevalence axis. In these situations, densities far below a single nematode per 100 cc of soil are achieved, a level at which: (i) the host population can be considered locally extinct and (ii) Pasteuria remains in the microcosm as a durable endospore, a trait that probably evolved for this purpose.
The Pasteuria lifespan (1/µ, ca. 46 days) corresponds to its longevity in the host rather than as endospore in soil (which is a suspended life state). Other parameter applied from data by Kaplan (1994) was the hourly rate of propagule production (1/λ), equal to 0.5 days required for production of one endospore. It yields 282–423 endospores per nematode, produced in a 24–36 h sporulation time, a value fitting the number of propagules reported (Fattah et al. 1989; Kaplan 1994) and confirmed during this study (data not shown).
In conclusion, the density-dependent relationship and the long term persistence of the Pasteuria sp. studied confirm its potential as an effective biocontrol agent of the citrus nematode. Modelled data (Fig. 4b) offer a quantitative basis for the development of a T. semipenetrans management strategy through inundative endospore treatments at rates around 2–4 × 105 endospores per liter of soil, a perspective made feasible today by the cultivation of these bacteria.
References
Anderson RM, May RM (1981) The population dynamics of microparasites and their invertebrate hosts. Phil Trans R Soc London 291:451–524
Atibalentja N, Noel GR, Liao TF, Gertner GZ (1998) Population changes in Heterodera glycines and its bacterial parasite Pasteuria sp. in naturally infested soil. J Nematol 30:81–92
Ciancio A (1995) Density-dependent parasitism of Xiphinema diversicaudatum by Pasteuria penetrans in a naturally infested field. Phytopathology 85:144–149
Ciancio A, Quénéhervé P (2000) Population dynamics of Meloidogyne incognita and infestion levels by Pasteuria penetrans in a naturally infested field in Martinique. Nematropica 30:77–86
Ciancio A, Roccuzzo G (1992) Observations on a Pasteuria sp. parasitic in Tylenchulus semipenetrans. Nematologica 38:403–404
Ciancio A, Mankau R, Mundo-Ocampo M (1992) Parasitism of Helicotylenchus lobus by Pasteuria penetrans in naturally infested soil. J Nematol 24:29–35
Dabiré RK, Ndiaye S, Mounport D, Mateille T (2007) Relationships between abiotic soil factors and epidemiology of the biocontrol bacterium Pasteuria penetrans in a root-knot nematode Meloidogyne javanica-infested field. Biol Control 40:22–29
Davies KG, Redden M (1997) Diversity and partial characterization of putative virulence determinants in Pasteuria penetrans, the hyperparasite of root-knot nematodes. J Appl Microbiol 83:227–235
Davies KG, Flynn CA, Laird V, Kerry BR (1990) The lifecycle, population dynamics and host specificity of a parasite of Heterodera avenae, similar to Pasteuria penetrans. Rev Nématol 13:303–309
Duncan LW, Noling JW (1987) The relationship between development of the citrus root system and infestation by Tylenchulus semipenetrans. Rev Nématol 10:61–66
Fattah FA, Saleh HM, Aboud HM (1989) Parasitism of the citrus nematode, Tylenchulus semipenetrans, by Pasteuria penetrans in Iraq. J Nematol 21:431–433
Gaspard JT, Mankau R (1986) Nematophagous fungi associated with Tylenchulus semipenetrans and the citrus rhizosphere. Nematologica 32:359–363
Gressel J (2005) Problems in qualifying and quantifying assumptions in plant protection models: resultant simulations can be mistaken by a factor of million. Crop Prot 24:1007–1015
Hassell MP (1978) The dynamics of arthropod predator-prey systems. Princeton University Press, Princeton
Jaffee BA, McInnis TM (1991) Sampling strategies for detection of density-dependent parasitism of soil-borne nematodes by nematophagous fungi. Rev Nematol 14:147–150
Jaffee B, Phillips R, Muldoon A, Mangel M (1992) Density-dependence host–pathogen dynamics in soil microcosms. Ecology 73:495–506
Kaplan DT (1994) Partial characterization of a Pasteuria sp. attacking the citrus nematode, Tylenchulus semipenetrans, in Florida. Fundam Appl Nematol 17:509–512
Luc JE, Pang W, Crow WT, Giblin-Davis RM (2010) Effects of formulation and host nematode density on the ability of in in vitro-produced Pasteuria endospores to control its host Belonolaimus longicaudatus. J Nematol 42:87–90
Mateille T, Fould S, Dabiré KR, Diop MT, Ndiaye S (2009) Spatial distribution of the nematode biocontrol agent Pasteuria penetrans as influenced by its soil habitat. Soil Biol Biochem 41:303–308
McSorley R, Dankers WH, Parrado JL, Reynolds JS (1985) Spatial distribution of the nematode community on perrine marl soil. Nematropica 15:77–92
Noel GR, Atibalentja N, Domier LL (2005) Emended description of Pasteuria nishizawae. Int J Syst Evol Microbiol 55:1681–1685
Oostendorp M, Dickson DW, Mitchell DJ (1991) Population development of Pasteuria penetrans on Meloidogyne arenaria. J Nematol 23:58–64
Sorribas FJ, Verdejo-Lucas S, Forner JB, Alcaidel A, Pons J, Ornat C (2000) Seasonality of Tylenchulus semipenetrans Cobb and Pasteuria sp. in citrus orchards in Spain. J. Nematol 32:622–632
Sorribas FJ, Verdejo-Lucas S, Pastor J, Ornat C, Pons J, Valero J (2008) Population densities of Tylenchulus semipenetrans related to physicochemical properties of soil and yield of clementine mandarin in Spain. Plant Dis 92:445–450
Stirling G (2014) Biological control of plant-parasitic nematodes, 2nd edition. Soil ecosystem management in sustainable agriculture. CABI, Oxfordshire
Verdejo Lucas S (1992) Seasonal population fluctuations of Meloidogyne spp. and the Pasteuria penetrans group in kiwi orchards. Plant Dis 76:1275–1279
Acknowledgments
Research partially funded by MiPAF, PF Lotta biologica, PF Orticoltura and Project BIOMED. The authors thank R. Bonsignore, A. Loffredo and L. C. Rosso for the assistance provided during this study. The Universitat Politecnica de Catalunya and CNR are gratefully acknowledged for short-term mobility grants (CO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Francisco Cazorla.
An erratum to this article is available at http://dx.doi.org/10.1007/s10526-017-9786-z.
Rights and permissions
About this article
Cite this article
Ciancio, A., Roccuzzo, G. & Longaron, C.O. Regulation of the citrus nematode Tylenchulus semipenetrans by a Pasteuria sp. endoparasite in a naturally infested soil. BioControl 61, 337–347 (2016). https://doi.org/10.1007/s10526-015-9704-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-015-9704-1