Abstract
Entomopathogenic nematodes (EPN) applied inundatively to suppress insect pests are more likely to persist and establish in stable agroecosystems than in annual crops. We investigated a system of intermediate stability: three stumps harbouring the large pine weevil (Hylobius abietis L.; Coleoptera: Curculionidae), a major European forestry pest. We tested whether persistence of EPN Steinernema carpocapsae Weiser (Rhabditida: Steinernematidae) applied around stumps is maintained by recycling of EPN through pine weevils developing within stumps. Steinernema carpocapsae was detected in soil around and under the bark of treated tree stumps up to two years, but not 4–5 years after application. Differences in nematode presence between sites were better explained by tree species (pine or spruce) than soil type (mineral or peat). Presence of S. carpocapsae in soil was positively correlated with the number of H. abietis emerging from untreated stumps the previous year, which was greater for pine stumps than spruce stumps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Entomopathogenic nematodes (EPN) of the genera Steinernema (Rhabditida: Steinernematidae) and Heterorhabditis (Rhabditida: Heterorhabdidae) are lethal pathogens of insects with a wide potential host range (Bathon 1996; Smits 1996) that are used against pests in horticulture, agriculture and forestry (Grewal et al. 2005). The free living infective juvenile (IJ) invades the haemocoel of insects and releases symbiotic bacteria that cause toxaemia and/or septicaemia, killing the insect within days (Kaya and Gaugler 1993). EPN are mainly used as inundative biological control agents, with insect suppression being effected by the applied IJs. Following application to soil, numbers of IJs typically decrease rapidly and may reach <90 % of the original inoculum within days (Glazer 1992; Smits 1996; Griffin 2015). Applied nematodes may survive at low numbers for longer periods (Preisser et al. 2005), but longer term persistence of a population depends on recycling—reproduction in target and/or non-target hosts (Campbell et al. 1995; Griffin 2015; Koppenhöfer and Fuzy 2009; Peters 1996). Long-term persistence of EPN populations therefore crucially depends on the availability of host insects for reproduction, as well as suitable environmental conditions, and hence varies between agronomic systems. Stable ecosystems such as pasture and alfalfa favour long-term persistence and EPN populations can persist for years after application (Koppenhöfer and Fuzy 2009; Shields et al. 1999), while in annual crops, persistence beyond a year is less common (Susurluk and Ehlers 2008). Tree stumps as a breeding resource for certain forestry pests such as the large pine weevil, Hylobius abietis L. (Coleoptera: Curculionidae) represent a moderately stable environment, intermediate between annual and perennial crops.
The large pine weevil, H. abietis, is one of the most damaging forestry pests in Europe (Långström and Day 2004; Leather et al. 1999). Development takes place under the bark of recently dead conifers, including stumps of recently felled trees, while adults feed on the bark of saplings planted to restock clear-fell sites, often leading to extensive sapling damage and mortality (Leather et al. 1999; Månsson and Schlyter 2004). Clear-felled coniferous forest plantation sites can support large weevil populations (Leather et al. 1999; Örlander et al. 1997). Stumps can remain suitable for pine weevil oviposition for up to three years after felling (Nordenhem 1989) and emergence of adults occurs within 1–2 years of oviposition (Leather et al. 1999). Traditionally, seedlings are protected by chemical insecticides, but application of EPN to tree stumps, targeting immature weevils developing within, has shown promise for suppression of adult weevil populations (Brixey et al. 2006; Dillon et al. 2006, 2007, 2008a, b; Torr et al. 2007; Williams et al. 2013). Dillon et al. (2008a) investigated the fate over a five year period of four EPN species applied by hand to tree stumps harbouring pine weevil developmental stages. Incidence (percentage of soil cores positive) of all species remained high for the first two years (no difference between months 1, 12 and 24 post-application), but declined by year three post-application (Dillon et al. 2008a). Only Steinernema feltiae Filipjev (Rhabditida: Steinernematidae; native to clearfell sites) was recovered in years four and five. Pine weevil larvae can support EPN reproduction, yielding up to 98,000 IJs per insect (Dillon 2003). Dillon et al. (2008a) hypothesized that EPN populations initially remained high due to recycling in the target pest, and that the apparent disappearance after 4–5 years of Steinernenema carpocapsae Weiser and two Heterorhabditis species was due to a concomitant decrease in weevil numbers as stumps degraded. The Dillon et al. (2008a) study was conducted on a single site type: stumps of pine (Pinus sylvestris L. and Pinus contorta Douglas) on a deep peat soil.
The present study complements the Dillon et al. (2008a) report of EPN persistence in a forest ecosystem, focussing on a single EPN species (S. carpocapsae) but extending the investigation to sites with diverse characteristics (soil type and tree species). For this study, nematodes were applied to stumps not by hand, but on a site-wide operational scale using spray nozzles connected to a tank of nematode suspension mounted on a forwarder. Specific objectives are: (1) confirm that the restricted spatial and temporal distribution of EPN reported by Dillon et al. (2008a) for Pinus spp. on peat in small scale trials is also applicable to commercial scale trials on sites with mineral soil and sites planted with Sitka spruce (Picea sitchensis Carr.), the species predominating in Irish and UK plantation forests (Anon. 2003, 2007). (2) Investigate the occurrence of EPN within the stump. Multiplication of EPN in pine weevils located in or under the bark is expected to release IJs into the space between the bark and the woody material of the tree-stump, but this has not been previously reported. (3) Investigate the relationship between EPN incidence in soil around stumps and the size of weevil populations within stumps. If EPN depend mainly on pine weevil as host, a positive correlation between weevil and EPN populations across sites is expected.
Materials and methods
Study sites
In 2007 and 2008, S. carpocapsae (All strain; Becker Underwood; Littlehampton, England) at a rate of 3.5 million IJs per stump applied in 500 ml water was applied to several clear-fell sites (Table 1) on an operational, site-wide scale (Williams et al. 2013). Steinernema carpocapsae was chosen for the study as it is the only species to date that has been applied operationally against pine weevil (Williams et al. 2013). On each site, a small number of stumps were marked and not treated, to serve as controls. These control stumps allowed an assessment of weevil populations within the stumps, based on the number of adult weevils caught in emergence traps erected over them (Williams et al. 2013). The soil type on each site (peat or mineral soil) was based on records of Coillte Teoranta (the site owner) and confirmed by visual evaluation on site. Conifer forests in northern Europe (including 44 % of Irish forests) are frequently planted on former peat bog, having high levels of organic matter. For our purposes, all other soils were classed as mineral soils, having lower organic content.
Soil sampling
Soil and bark samples were taken from stumps spaced at 5–15 m intervals along a diagonal transect. For the number of stumps sampled see Table 2. Soil cores were collected at four aspects (at right angles) around each sampled stump. One soil core each was taken at the bole (0 cm) and at 20, 40 and 60 cm along each aspect, resulting in 16 soil cores per sampled stump. Cores were taken to a depth of approximately 5 cm using a 50 ml plastic tube (2.9 cm inner diameter; Sarstaedt; Nürnbrecht, Germany) which was also used for transport and baiting. Soil cores were baited with final instar waxmoth larvae (Galleria mellonella L; Lepidoptera: Pyralidae) at room temperature (Dillon et al. 2006, 2007). Each core was baited twice for seven days with one bait insect each time. Live insects were incubated at 20 °C for a further seven days after removal from soil. Insects that showed signs of Steinernema infection (cadaver consistency and cream/tan colour) were incubated at 20 °C until IJs emerged. IJs were then measured for length (ten per cadaver) and scored as either S. carpocapsae (mean length: 558 μm, length range 438–650 μm) (Adams and Nguyen 2002) or a native Steinernema sp. (S. feltiae: mean 849, range 736–950; Steinernema kraussei Steiner: mean 951, range 797–1102) (Adams and Nguyen 2002). Steinernema feltiae and (rarely) S. kraussei are the only species so far detected in Irish conifer forests (Griffin et al. 1991; Gwynn and Richardson 1996; Dillon 2003; C. Harvey unpublished). For samples collected two years post-application (p.a.) or where no IJs emerged, cadavers were dissected, and spicules of male adult nematodes were used for identification (Adams and Nguyen 2002). Cadavers containing no adults or only females were scored as inconclusive and not included in analysis.
Baiting of bark samples from tree stumps treated with S. carpocapsae
At each sampled stump, approximately 100 cm2 of bark was stripped from the bole of the stump at the soil horizon at each aspect. Bark from each stump was pooled into a bulk sample, and placed in a 250 ml plastic cup for baiting. Ten wax moth larvae were added per cup and cups were covered with Parafilm (Bemis; Soignies, Belgium) and incubated at 20 °C. After three days, bait insect mortality was recorded. For sites A1-A4 in addition, four small pieces of bark (approx. 4 cm2 each), one from each of the four sampled aspects, were individually baited with a single wax moth larva. The insect was placed in a well (0.9 cm diam.) and covered by the piece of bark as described by Harvey and Griffin (2012), so that it was in contact with only the under surface of the bark. Since previous studies indicated that infection of insects under bark of tree stumps with native steinernematids is extremely rare (Dillon 2003; Dillon et al. 2008a, b; C. Harvey, personal observation), dead insects with cream colouration were scored as infected by S. carpocapsae.
Statistics
Statistical analysis was carried out using MiniTab Release 15 (MiniTab Solutions; Coventry, UK). To compare the proportion of samples scoring positive for the presence of S. carpocapsae over time (successive years after application) or between sites, these binary data (positive/negative) were compared using 2 × 2 contingency tables with Pearson’s χ2 test or, where the expected count of at least one cell in the table was <5, with Fisher’s exact test (α = 0.05). Yates’ correction was used for χ2-tests on 5 × 2 tables with expected counts <5. Significance levels of multiple pairwise comparisons of binary data between sampling time points and/or sites were adjusted for type-I family error rate using the Bonferroni procedure, with the significance level for n pairwise comparisons involving the same data set adjusted to 0.05/n (Rice 1989). The Mantel–Haenszel–Cochran (MHC) test was used to calculate odds ratios and detect effects of soil type on presence of EPN (in soil cores, at stumps and under bark) while controlling for the effect of tree stump species and vice versa. To investigate whether S. carpocapsae presence in soil cores and in bark samples was correlated with the size of previously recorded weevil populations in stumps, the percentage of soil cores and bark samples scoring positive for S. carpocapsae two years p.a. (2009) was regressed in binary logistic regression models for each response variable against the log10 (x + 1) of the mean number of adult weevils emerging from untreated control stumps on each of the corresponding sites in 2008 (predictive variable; see supplementary data, Dillon et al. 2012), which represented an indicator for the size of pine weevil populations within stumps on each site. The Wald test was used to test the model coefficient for significant difference from 0. Data for spruce and pine stumps at site A1 (Lackenrea) were used separately in the model. Pearson’s goodness-of-fit test (α = 0.05) was used to confirm validity of the logit link function used in the model and Pearson residuals were tested for normality (Anderson–Darling test, α = 0.05). To test for differences between the percentage of soil cores scoring positive for S. carpocapsae at four distances from the bole of stumps, a two-way χ2 test was used on data combined for each distance across sites A1–A4.
Results
Presence of entomopathogenic nematodes in soil samples
Steinernema carpocapsae was detected in soil samples from stumps at all four sites that were sampled up to two years p.a., but not on any of the six sites sampled 4–5 years p.a. (Table 2). Conversely, other Steinernema spp. were found at five out of six sites sampled 4–5 years p.a., but not earlier (Table 2). At the sites where soil was sampled in each of years one and two p.a. (A1 and A2), incidence of S. carpocapsae decreased significantly over time in the three samples taken five months, one year and two years after nematode application (A1: cores: χ 22 = 8.769, P = 0.012; stumps: χ 22 = 12.115, P = 0.002 [data combined for pine and spruce]; A2: cores χ 22 = 19.627, P < 0.001; stumps: χ 22 = 16.586, P < 0.001; Table 2).
Two years p.a., S. carpocapsae was recovered from 0.9 to 5.2 % of soil cores, representing 11–43 % of sampled stumps (Table 2). Differences between sites (A1–A4, treating spruce and pine on A1 separately) were significant based on both cores (χ 24 = 27.311, P < 0.001) and stumps (Yates’ χ2 = 12.522, df = 4; P = 0.014). Two years p.a., soil type had a significant effect on the proportion of soil cores scoring positive for EPN when controlling for the effect of stump species (MHC = 7.825, odds ratio [peat:mineral] = 2.494, P = 0.005), but the odds ratio for the effect of stump species when controlling for soil type was more than three times as great, with soil cores from pine stumps eight times as likely to score positive for S. carpocapsae as cores from spruce stumps (MHC = 17.935, odds ratio [pine:spruce] = 8.009, P < 0.001) (Table 3). When data for stumps were used instead, soil type had no significant effect, though the effect of stump species remained highly significant (soil: MHC = 1.067, odds ratio peat:mineral] = 1.887, P = 0.302; stump species: MHC = 17.680, odds ratio [pine:spruce] = ∞, P < 0.001) (Table 3). There was a significant positive relationship between the incidence of S. carpocapsae in soil cores and the number of adult weevils emerging the previous year (Wald test; Coef. = 1.66, Z = 5.62, P < 0.001; Fig. 1a).
Scatterplots for the percentage of soil cores scoring positive for S. carpocapsae (a) or the percentage of stumps with bark samples scoring positive for S. carpocapsae (b) plotted against the log10 (x + 1) of the mean number of pine weevils emerging from untreated control stumps on sampled sites A1–A4 (spruce and pine from site A1 used separately). Black points show spruce sites, grey points show pine sites. Horizontal error bars give SE of mean weevil emergence
Presence of entomopathogenic nematodes in bark samples
Steinernema carpocapsae was recovered from bark one and two years p.a., but no EPN were found there after four or five years (Table 2). For most of the stumps on sites A2 and A3 where S. carpocapsae was recovered from bulk bark samples it was also detected when only the inside of the bark was baited (site A2, one year p.a.: 15/20 stumps; site A2, two years p.a.: 6/11; site A3, two years p.a.: 9/12). The proportion of stumps where bark samples were positive for S. carpocapsae at sites A1 and A2 decreased from one to two years of application (Table 2), significantly so for site A2 (χ 21 = 6.944, P = 0.008; Table 2). Two years p.a., the proportion of stumps with S. carpocapsae in bulk bark samples ranged from 0 to 67 %, a highly significant difference between sites (A1–A4, treating spruce and pine on A1 separately) (Yates’ χ2 = 19.203, df = 4, P < 0.001). Two years p.a., stump species had a significant effect on the proportion of stumps with EPN detected under their bark (MHC = 15.108, odds ratio [pine:spruce] = ∞, P < 0.001), but soil type did not (MHC = 0.204, odds ratio [peat:mineral] = 1.424, P = 0.651) (Table 3). The percentage of stumps with positive bark samples tended to be higher on sites with high weevil emergence compared with sites with low emergence (Fig. 1b). However, link functions of binary logistic models regressing the proportion of tree stumps with bark positive for S. carpocapsae against mean number of weevils emerging from untreated stumps in 2008 did not provide an adequate fit for the data (Pearson’s goodness-of-fit test, P < 0.05).
Dispersal of EPN from treated stumps
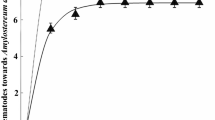
At all sites where S. carpocapsae was recovered, and at all sampling times, the percentage of cores positive for S. carpocapsae tended to be highest directly at the bole of the stump (0 cm distance) and lowest at 60 cm distance from the bole of a stump. Nematodes were detected 60 cm from the stump bole within five months p.a. (data not shown). The percentage of soil cores positive for S. carpocapsae (sites A1–A4) decreased significantly with increasing distance from the bole of the stump two years p.a. (χ 23 = 68.57, P < 0.001; Fig. 2).
Discussion
Our results confirm the finding by Dillon et al. (2008a) that S. carpocapsae declines to undetectable levels 4–5 years after application to coniferous tree stumps for pine weevil control. They also support Dillon et al. (2008a) suggestion that this is due to a concomitant decrease in the availability of weevils for reproducing as stumps degraded. We did find other steinernematid nematodes 4–5 years after application, probably S. feltiae or S. kraussei, the only other Steinernema spp. so far detected in Irish conifer forests or clearfells (Dillon 2003; Griffin et al. 1991; C. Harvey unpublished data). Similarly, Dillon et al. (2008a) found that the only EPN recovered 4–5 years after application was S. feltiae, either an indigenous applied strain or a native strain that naturally colonised the site. It is possible that the abundance of these native EPN species is linked to the availability of soil-associated insect hosts, which may increase in diversity and number as clearfell sites proceed through stages of succession (Butterfield 1997; Irwin et al. 2014; Niemelä et al. 1993; Pawson et al. 2006).
Though inundatively applied IJs can survive in soil for months (Dillon et al. 2008a; Kung et al. 1990; Poinar and Hom 1986), up to 90 % of them are expected to die within hours of application (Smits 1996). Therefore, most (if not all) of the S. carpocapsae found at our first sampling time five months p.a. and beyond had likely originated from reproducing through insect hosts in the field (Gaugler 1988; Smits 1996; Susurluk and Ehlers 2008). Nematodes were applied in July 2007 to coincide with the occurrence of late instar larvae and pupae of pine weevil in the stumps. Steinernema carpocapsae can reproduce in immature pine weevil in the field and a single infected weevil larva can yield more than 85,000 IJs (Dillon 2003; Pye and Burman 1978). Our data support the hypothesis that S. carpocapsae was reproducing in immature pine weevils: incidence of IJs in soil and bark was positively associated with the size of weevil populations in stumps (as indicated by adult weevil emergence from stumps the year previous). Infection rates of pine weevil by S. carpocapsae in the weeks after application are proportionately similar for pine and spruce stumps (Brixey et al. 2006; Dillon et al. 2006, 2008b) and appear not to be affected by weevil population size per stump (Williams et al. 2013). Thus, differences in S. carpocapsae reproduction between spruce and pine stumps are likely driven by long-term differences in overall weevil population size between the two stump species, with higher weevil populations in pine (Dillon 2003; Leather et al. 1999; Williams et al. 2013). While soil type is more important than tree species in determining effectiveness of S. carpocapsae against the pine weevil (Williams et al. 2013), tree species (as a predictor of weevil populations in stumps) has a greater effect on continued presence of the nematodes in this ecosystem.
Since weevil populations in stumps may remain high for 2–3 years after nematodes are applied (Leather et al. 1999) they may facilitate repeated cycles of reproduction of applied S. carpocapsae under the bark. Longer term presence of S. carpocapsae in the soil (1–2 years p.a.) may thus be explained by IJs migrating into the soil following reproduction under stump bark. Nematode populations fluctuate with the target pest population (Campbell et al. 1995), but reproduction in non-target insects may also be important. For example, Hodson et al. (2012) found a positive correlation between persistence of S. carpocapsae in pistachio orchards and pitfall catches of non-target tenebrionid beetles. Plantation forests and clearfell sites can harbour a considerable diversity and number of potential non-target insects (Dillon et al. 2012; Fahy and Gormally 1998; Niemelä and Koivula 2007; Sippola et al. 2002). However, Dillon et al. (2012) found no evidence of an impact of EPN on non-target beetle populations in a study that included some of the sites sampled for this study (sites A1–A4). Consequently, reproduction of S. carpocapsae through such non-target hosts is unlikely to contribute significantly to the EPN persistence we observed.
On all of the sites in our study, S. carpocapsae was applied within two years of felling, when the number of weevils per tree stump tends to peak (Dillon et al. 2007; Leather et al. 1999; Williams et al. 2013). As weevils emerge and stumps degrade, the pest population inevitably drops to a level that no longer supports large nematode populations (Leather et al. 1999). Thus, the ephemeral and contained nature of weevil populations in tree stumps should provide a natural limit to EPN presence on clearfell sites, especially if reproduction in non-target insects is infrequent. We detected S. carpocapsae up to 60 cm from the bole of treated tree stumps, but incidence dropped steeply with increasing distance, a trend similar to previous studies (Torr et al. 2007; Dillon et al. 2008a). On the clearfell sites we sampled, the absence of a stable pool of suitable hosts for reproduction outside of tree stumps may have prevented S. carpocapsae from establishing. However, there is potential for local pockets of nematode recycling where dead-wood with susceptible longhorn beetles occurs (Harvey et al. 2012).
Dispersal and prolonged persistence of control agents is usually not a desired outcome of inundative biological control, mainly because of the increased risk of non-target impacts (Bathon 1996; Smits 1996; van Lenteren et al. 2003). Where EPN reproduction is primarily restricted to the target pest habitat, however, as appears to be the case in our studied system, recycling in the pest may enhance and extend the controlling effect, thereby reducing the need for repeated application while minimising damage to non-target hosts outside of this habitat (Klein and Georgis 1992; Smits 1996). We conclude that, for the large pine weevil, the persistence of inundatively applied EPN is dependent on the target pest population, resulting in limited risk of dispersal and longer term establishment while at the same time potentially enhancing control efficacy. The same may be true of other pests with transient populations occurring in cryptic habitats.
References
Adams BJ, Nguyen KB (2002) Taxonomy and systematics. In: Gaugler R (ed) Entomopathogenic nematology, 1st edn. CAB publishing, Wallingford, pp 1–35
Anon. (2003) National inventory of woodland and trees. Forestry Commission, Wexford
Anon. (2007) National forest inventory Republic of Ireland. Forest Service, Midlothian
Bathon H (1996) Impact of entomopathogenic nematodes on non-target Hosts. Biocontrol Sci Technol 6:421–434
Brixey JM, Moore R, Milner AD (2006) Effect of entomopathogenic nematode (Steinernema carpocapsae Weiser) application technique on the efficacy and distribution of infection of the large pine weevil (Hylobius abietis L.) in stumps of Sitka spruce (Picea sitchensis Carr.) created at different times. For Ecol Manage 226:161–172
Butterfield J (1997) Carabid community succession during the forestry cycle in conifer plantations. Ecography (Cop.) 20:614–625
Campbell JF, Lewis E, Yoder F, Gaugler RJ (1995) Entomopathogenic nematode (Heterorhabditidae and Steinernematidae) seasonal population dynamics and impact on insect populations in turfgrass. Biol Control 5:598–606
Dillon AB (2003) Biological control of the large pine weevil, Hylobius abietis L., (Coleoptera: Curculionidae) using entomopathogenic nematodes. In: Ph.D. thesis submitted at National University of Ireland Maynooth, Ireland
Dillon AB, Ward D, Downes MJ, Griffin CT (2006) Suppression of the large pine weevil Hylobius abietis (L.) (Coleoptera: Curculionidae) in pine stumps by entomopathogenic nematodes with different foraging strategies. Biol Control 38:217–226
Dillon AB, Downes MJ, Ward D, Griffin CTJ (2007) Optimizing application of entomopathogenic nematodes to manage large pine weevil, Hylobius abietis L. (Coleoptera: Curculionidae) populations developing in pine stumps, Pinus sylvestris. Biol Control 40:253–263
Dillon AB, Rolston AN, Meade CV, Downes MJ, Griffin CT (2008a) Establishment, persistence, and introgression of entomopathogenic nematodes in a forest ecosystem. Ecol Appl 18:735–747
Dillon AB, Moore CP, Downes MJ, Griffin CT (2008b) Evict or infect? Managing populations of the large pine weevil, Hylobius abietis, using a bottom-up and top-down approach. For Ecol Manage 255:2634–2642
Dillon AB, Foster A, Williams CD, Griffin CT (2012) Environmental safety of entomopathogenic nematodes—effects on abundance, diversity and community structure of non-target beetles in a forest ecosystem. Biol Control 63:107–114
Fahy O, Gormally M (1998) A comparison of plant and carabid beetle communities in an Irish oak woodland with a nearby conifer plantation and clearfelled site. For Ecol Manage 110:263–273
Gaugler R (1988) Ecological considerations in the biological control of soil-inhabiting insects with entomopathogenic nematodes. Agric Ecosyst Environ 24:351–360
Glazer I (1992) Survival and efficacy of Steinernema carpocapsae in an exposed environment. Biocontrol Sci Technol 2:101–107
Grewal PS, Ehlers RU, Shapiro-Ilan DI (eds) (2005) Nematodes as biocontrol agents, 1st edn. CAB Publishing, Wallingford
Griffin CT (2015) Behaviour and population dynamics of entomopathogenic nematodes following application. In: Campos Herrera R (ed) Nematode pathogenesis of insects and other pests—ecology and applied technologies for sustainable plant and crop protection, 1st edn. Springer, Berlin, pp 57–95
Griffin CT, Moore JF, Downes MJ (1991) Occurrence of insect-parasitic nematodes (Steinernematidae, Heterorhabditidae) in the Republic of Ireland. Nematologica 37:92–100
Gwynn RL, Richardson PN (1996) Incidence of entomopathogenic nematodes in soil samples collected from Scotland, England and Wales. Fundam Appl Nematol 19:427–431
Harvey CD, Griffin CT (2012) Host activity and wasp experience affect parasitoid wasp foraging behaviour and oviposition on nematode-infected larvae of the forestry pest Hylobius abietis. Ecol Entomol 37:269–282
Harvey CD, Alameen KM, Griffin CT (2012) The impact of entomopathogenic nematodes on a non-target, service-providing longhorn beetle is limited by targeted application when controlling forestry pest Hylobius abietis. Biol Control 62:173–182
Hodson AK, Siegel JP, Lewis EE (2012) Ecological influence of the entomopathogenic nematode, Steinernema carpocapsae, on pistachio orchard soil arthropods. Pedobiologia 55:51–58
Irwin S, Pedley S, Coote L, Dietzsch A, Wilson M, Oxbrough A, Sweeney O, Moore K, Martin R, Kelly D, Mitchell FG, Kelly T, O’Halloran J (2014) The value of plantation forests for plant, invertebrate and bird diversity and the potential for cross-taxon surrogacy. Biodivers Conserv 23:697–714
Kaya HK, Gaugler R (1993) Entomopathogenic nematodes. Annu Rev Entomol 38:181–206
Klein MG, Georgis R (1992) Persistence of control of Japanese Beetle (Coleoptera: Scarabaeidae) larvae with steinernematid and heterorhabditid nematodes. J Econ Entomol 85:727–730
Koppenhöfer AM, Fuzy EM (2009) Long-term effects and persistence of Steinernema scarabaei applied for suppression of Anomala orientalis (Coleoptera: Scarabaeidae). Biol Control 48:63–72
Kung SP, Gaugler R, Kaya HK (1990) Soil type and entomopathogenic nematode persistence. J Invertebr Pathol 55:401–406
Långström B, Day KR (2004) Damage, control and management of weevil pests, especially Hylobius abietis. In: Leutier F (ed) Bark and wood boring insects in living trees in Europe, a synthesis, 1st edn. Springer, Berlin, pp 415–444
Leather SR, Day KR, Salisbury AN (1999) The biology and ecology of the large pine weevil, Hylobius abietis (Coleoptera: Curculionidae): a problem of dispersal? Bull Entomol Res 89:3–16
Månsson PE, Schlyter F (2004) Hylobius pine weevils adult host selection and antifeedants: feeding behaviour on host and non-host woody scandinavian plants. Agr For Entomol 6:165–171
Niemelä J, Koivula MKDJ (2007) The effects of forestry on carabid beetles (Coleoptera: Carabidae) in boreal forests. J Insect Conserv 11:5–18
Niemelä J, Langor D, Spence JR (1993) Effects of clear-cut harvesting on boreal ground-beetle assemblages (Coleoptera: Carabidae) in Western Canada. Conserv Biol 7:551–561
Nordenhem H (1989) Age, sexual development, and seasonal occurrence of the pine weevil Hylobius abietis (L.). J Appl Entomol 108:260–270
Örlander G, Nilsson U, Nordlander G (1997) Pine weevil abundance on clear-cuttings of different ages: a 6-year study using pitfall traps. Scand J For Res 12:225–240
Pawson SM, Brockerhoff EG, Norton DA, Didham RK (2006) Clear-fell harvest impacts on biodiversity: past research and the search for harvest size thresholds. Can J For Res 36:1035–1046
Peters A (1996) The natural host range of Steinernema and Heterorhabditis spp. and their impact on insect populations. Biocontrol Sci Technol 6:389–402
Poinar GO, Hom A (1986) Survival and horizontal movement of infective stage Neoaplectana carpocapsae in the field. J Nematol 18:34–36
Preisser EL, Dugaw CJ, Dennis B, Strong DR (2005) Long-term survival of the entomopathogenic nematode Heterorhabditis marelatus. Env Entomol 34:1501–1506
Pye AE, Burman M (1978) Neoaplectana carpocapsae: Infection and reproduction in large pine weevil larvae, Hylobius abietis. Exp Parasitol 46:1–11
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Shields EJ, Testa A, Miller JM, Flanders KL (1999) Field efficacy and persistence of the entomopathogenic nematodes Heterorhabditis bacteriophora Oswego and H. bacteriophora NC on Alfalfa Snout Beetle larvae (Coleoptera: Curculionidae). Environ Entomol 28:128–136
Sippola AL, Siitonen J, Punttila P (2002) Beetle diversity in timberline forests: a comparison between old-growth and regeneration areas in Finnish Lapland. Ann Zool Fennici 39:69–86
Smits PH (1996) Post-application persistence of entomopathogenic nematodes. Biocontrol Sci Technol 6:379–388
Susurluk A, Ehlers R-U (2008) Field persistence of the entomopathogenic nematode Heterorhabditis bacteriophora in different crops. BioControl 53:627–641
Torr P, Heritage S, Wilson MJ (2007) Steinernema kraussei, an indigenous nematode found in coniferous forests: efficacy and field persistence against Hylobius abietis. Agric For Entomol 9:181–188
van Lenteren JC, Babendreier D, Bigler F, Burgio G, Hokkanen HMT, Kuske S, Loomans AJM, Menzler-Hokkanen I, van Rijn PCJ, Thomas MB, Tommasini MG, Zeng QQ (2003) Environmental risk assessment of exotic natural enemies used in inundative biological control. BioControl 48:3–38
Williams CD, Dillon AB, Girling RD, Griffin CT (2013) Organic soils promote the efficacy of entomopathogenic nematodes, with different foraging strategies, in the control of a major forest pest: A meta-analysis of field trial data. Biol Control 65:357–364
Acknowledgments
We thank the Irish Environmental Protection Agency (EPA) and the Department of Environment, Heritage and Local Government (DoEHLG) for support. This study was funded by the EPA STRIVE Programme (Project Code 2007-PhD-B-6) under the National Development Plan 2007–2013. Nematodes were applied under license (TA003) from the Pesticide Control Services of the Irish Department of Agriculture and Food. Coillte Teoranta provided clearfell sites and Dr Aoife Dillon at the Coillte Forest Protection Section provided information and technical advice on the sites.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Ralf Ehlers.
Rights and permissions
About this article
Cite this article
Harvey, C.D., Griffin, C.T. Local host-dependent persistence of the entomopathogenic nematode Steinernema carpocapsae used to control the large pine weevil Hylobius abietis . BioControl 61, 185–193 (2016). https://doi.org/10.1007/s10526-015-9709-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-015-9709-9