Abstract
Plant pollen is considered a food of high nutritional quality for several natural enemies, such as predatory insects and mites. In periods of prey absence or scarcity, omnivorous predators often exploit plant pollen as an alternative food. In the case of predators feeding on mixed diets, pollen may be consumed supplementary to the main prey. However, genetic variation may translate into quality differences in pollen derived from distinct plant species. We herein assessed the nutritional suitability of the pollen of four anemophilous plant species [cattail—Typha latifolia (L.), pine, corn, and olive] for the predatory mite Amblydromalus limonicus (Garman & McGregor) (Acari: Phytoseiidae), a phytoseiid mite with great potential for controlling thrips and whiteflies in greenhouse crops. Juvenile development and survival were not affected by the different pollens. Nevertheless, significant differences in adult performance (longevity and egg production) resulted in considerable effects of pollen species on the calculated intrinsic rates of increase (rm) for this predator. Cattail followed by olive pollen resulted in the highest rm values (0.2340 and 0.2001 day−1, respectively), while the lowest values were recorded for corn and pine pollen. Our results show that all pollens tested may be used as alternative food for sustaining the population of A. limonicus in the field. Recorded differences among pollens highlight the need for a careful consideration of the quality of pollen used in laboratory rearings and in field applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trophic interactions among plants and beneficial arthropods rely on the provision of plant resources to omnivorous predators which in return protect plants from herbivores (Sabelis et al. 2005). In this context, plant resources, such as pollen and nectar are food sources that ensure the maintenance of generalist predators at low prey densities (Coll and Guershon 2002; Wäckers 2005). In the presence of prey, the performance of omnivorous predators has been shown in many cases to be positively affected by plant pollen in mixed diets with animal prey (Eubanks and Styrsky 2005; Schmidt et al. 2013; van Rijn et al. 2002).
The reliance of generalist predators on more than one trophic level for the acquisition of essential nutrients is considered as one of their main advantages contrary to true carnivores that only need to feed on prey (Coll and Guershon 2002; Denno and Fagan 2003). Considering conservation biological control in agroecosystems, such predators should be easier to maintain in the crop by simply providing food of plant origin and/or artificial (other than target prey) food in periods of prey scarcity or decline (Messelink et al. 2014; Wäckers 2005), especially in crops lacking alternative food sources, such as pollen or extrafloral nectar. Pure pollen application on the crop (Nomikou et al. 2002; van Rijn et al. 2002) or pollen provision through the use of banker plants (Huang et al. 2011) are ideal methods facilitating the early establishment or maintenance of omnivorous predators within the crop during periods of low prey availability.
Type III and IV phytoseiid mites are among the best known examples of omnivorous predators feeding on both prey and plant food (McMurtry and Croft 1997; McMurtry et al. 2013). Pollen may be used by phytoseiids as a high-quality alternative food when prey is limited and/or to supplement their main diet. In addition, the provision of pollen in the field may be useful in the early establishment of predatory mite populations before the arrival of prey (Broufas and Koveos 2000; Hoy 2011; Nomikou et al. 2003; van Rijn and Tanigoshi 1999; van Rijn et al. 2002). Mass culturing certain phytoseiids in the lab may be mainly based on a pollen diet either in combination with prey or alone, depending on the species (Denno and Fagan 2003; Gerson et al. 2003; Hoy 2011).
Despite the well-documented high quality of several plant pollen species as alternative food for phytoseiids (McMurtry and Croft 1997; van Rijn and Tanigoshi 1999) to date we are aware of only one commercially available product, the narrow-leaved cattail (Typha angustifolia L.) based Nutrimite™ (Biobest N.V., Westerlo, Belgium), recommended for blowing applications to boost generalist phytoseiid populations in ornamentals, vegetables and plant nurseries. Cattail pollen (T. angustifolia and Typha latifolia L.) has been shown to favour the performance of phytoseiids in terms of intrinsic rates of population increase (e.g. Broufas and Koveos 2001; Goleva and Zebitz 2013; Lorenzon et al. 2012; Vangansbeke et al. 2014a). Similar to cattail, other anemophilous plants (e.g. corn, pine) produce high quantities of light pollen grains that could be easily collected for use as predator food. Contrary to entomophilous plant pollen species, some of which are marketed for plant pollination purposes (e.g. apple, almond, pear etc.), anemophilous pollen is cheaper and less labour-intensive to collect (Goleva and Zebitz 2013) and subsequently, to apply in the field. On the other hand, besides handling and storage conditions, genetic variation among plant species in pollen physical characteristics (e.g. pollen grain walls) and/or its chemical composition (e.g. amino acids, proteins, vitamins) may explain recorded differences in the quality of pollens for phytoseiid mites (Goleva and Zebitz 2013; Roulston and Cane 2000).
In this study, we assessed the suitability of pollen as an alternative food source for a phytoseiid mite. We examined the effects of the pollen of four anemophilous plant species on the predator’s growth, survival and reproduction. Our experimental system consisted of the predatory mite Amblydromalus limonicus (Garman & McGregor) (Acari: Phytoseiidae), an efficient biological control agent for the control of thrips and whiteflies in greenhouse crops (e.g. Hoogerbrugge et al. 2011; Knapp et al. 2013; van Houten et al. 2008), and the pollen of corn, pine, olive and cattail plants, dusted on bean leaf discs under laboratory conditions. To our knowledge, data on the effects of pollen consumption on the life-history parameters of this predator is scarce and mainly focused on cattail (T. latifolia and T. angustifolia) pollen (e.g. Vangansbeke et al. 2014a, b). Using this system, we calculated the demographic parameters of the predator and classified the tested pollen species based on their suitability for A. limonicus.
Materials and methods
Predatory mites
The main rearing of A. limonicus was established with adults of the commercially available product Limonica® (Koppert BV, Berkel en Rodenrijs, The Netherlands) and had been maintained on detached French bean leaves (Phaseolus vulgaris L.) at 26 ± 1 °C and 16:8 (L:D) h. Cattail (T. latifolia) pollen was provided ad libitum on the leaf surface as a food source for the mites.
Pollen
Cattail (T. latifolia), olive (Olea europea L.), corn (Zea mays L., cv. Heleonora X1132R-I424) and Calabrian pine (Pinus brutia Ten.) pollens were collected from flowering plants in Northern Greece, as described in Broufas and Koveos (2000). Sampled plants had not been treated with pesticides. Pollen was air dried for 12 h, sieved (200 mm mesh), and subsequently stored at −20 °C.
Experiments
Cohorts of predatory mite eggs were obtained by allowing young (6–8 days old) females from the stock colony to lay eggs for 12 h on detached bean leaves placed upside down on wet cotton wool. Cattail pollen was provided as food during the egg-laying phase. Multiwell tissue culture plates (Corning®), each consisting of either six (3.51 cm in diameter) or 12 cells (2.21 cm in diameter) were used as experimental units for adults and juveniles, respectively. Within each cell, individual mites were transferred on floating bean leaf discs placed upside down on a cotton wool layer soaked with water. Twice daily each cell was refilled with water. A v-shaped plastic shelter (0.5 × 0.5 cm) was placed on each leaf disc. Pollen grains were offered ad libitum on each bean disc daily and old pollen grains were removed to avoid contamination by moulds. All experiments were conducted in a climate room at 26 ± 1 °C, 16:8 (L:D) h and 60–75 % RH.
Effect of pollen diet on juvenile development and survival
Eggs laid within 12 h by young females were individually placed on each leaf disc (1.4 cm in diameter) of the experimental arena. Upon larval hatching till adulthood, the developmental stage each predatory mite had reached, and survival was recorded at 12-h intervals. For each treatment (pollen species), 31 (for cattail and corn pollen) or 36 (for olive and pine pollen) replicates were used. Individuals lost or injured due to improper handling during inspections were excluded from data analysis.
Effect of pollen diet on adult survival and egg production
For each pollen treatment, a second group of mites reared under the same conditions as described above were used. For this group, the developmental stage of individuals was recorded every 24 h up to the deutonymphal stage. Subsequently, the newly molted adults were sexed and placed in pairs on the leaf discs (2.0 cm in diameter) of the experimental arenas. Daily, we recorded the number of eggs laid by each female throughout her life. After each counting, eggs were removed. There is a scarcity of published data on the life history traits of A. limonicus, specifically on the required number of successive matings for maximizing female reproductive success. Therefore, in order to ensure a continuous availability of sexually active, fertile males for female insemination, once a week males were replaced with young individuals (6–8 days old). Young males were collected from colonies reared on detached bean leaves, on the same plant pollen as the mites which they replaced in the experimental treatments.
Effect of pollen diet on sex ratio
Progeny sex ratio during the first couple of days of phytoseiid oviposition period is male biased and becomes more or less stable and female biased later on (e.g. Broufas et al. 2007). Therefore, in order to estimate progeny sex ratio eggs laid by all females of each treatment (pollen species) were daily collected starting from the 4th day (after the onset of egg laying) for 15 consecutive days. These eggs were placed in groups (each group referring to a different day) on detached bean leaves as those used for the rearing. Depending on the treatment, pollen grains were offered ad libitum as food for the mites. Upon adult emergence, individuals were sexed. These data were used to calculate an overall sex ratio value for each pollen tested, which was subsequently used for the estimation of the demographic parameters.
Life table analysis
Demographic parameters were estimated by combining data from juvenile development, and adult survival and egg production. The intrinsic rates of increase (r m ) were estimated by iteratively solving the equation given by Birch (1948):
where x is the mean age class, m x the mean number of female progeny per female at age x, and l x the probability of survival to age x. Net reproductive rate (R0 = Σl x m x , number of female offspring produced per female), doubling time (DT = ln(2)/r m , number of days required for the population to double its numbers) and mean generation time (T = lnR0/r m ) were calculated as described by Southwood and Henderson (2000).
Statistical analysis
Two-way analysis of variance (ANOVA) was used to evaluate the effect of sex (S) and pollen (P) species on total developmental time. Since sex (S) significantly affected developmental time, one-way ANOVA was subsequently used for each sex and developmental stage to evaluate the effect of pollen species on developmental time (SPSS 2011). Student–Newman–Keuls test was further used to compare means within each sex and developmental stage as well as total juvenile development. Before analyses, all data sets were graphically (normal Q–Q plot) tested for normality, and for homogeneity of variances by Levene’s test. Subsequently, the non-parametric Kruskal–Wallis test was used to analyze data not fulfilling the criteria of parametric analysis and means were separated with Mann–Whitney-U tests. For each pollen species, the duration of each developmental stage was compared between sexes with Student t-tests. Significance levels were α = 0.05 for all tests. Similar analyses were performed to evaluate the effect of pollen species on female longevity, duration of pre- and post-oviposition periods (Kruskal–Wallis test, followed by Mann–Whitney-U test), total egg production and duration of oviposition period (one-way ANOVA, followed by Student–Newman–Keuls test). Survival percentages and sex ratios were compared among pollen species with χ 2 test. All possible pairwise comparisons were performed and type I error was corrected using the Bonferroni method (Sokal and Rohlf 1995). The Jackknife procedure was used to estimate a standard error for the r m , R0, T and DT values for the different pollens tested and comparisons were performed by Student–Newman–Keuls test (Meyer et al. 1986; Sokal and Rohlf 1995).
Results
Effects of pollen diet on juvenile development and survival
Two-way analysis of variance revealed that there was a significant effect of sex (S: F = 25.55; df = 1, 108; P < 0.001) and pollen (P: F = 4.61, df = 3, 108, P = 0.011) but not of their interaction (S × P: F = 1.99, df = 3, 108, P = 0.119) on juvenile developmental time of A. limonicus. Total juvenile development of males ranged between 6.4 to 6.6 days depending on pollen treatment, whereas development of females took longer to complete (6.8–7.9 days, Table 1). Total developmental time of males and females was not different when juveniles were fed with cattail or olive pollen (cattail: t = −1.188, df = 25, P = 0.246; olive: t = −1.612, df = 29, P = 0.118), while males emerged faster than females when juveniles were fed with corn (t = −3.669, df = 24, P < 0.05) or pine (t = −3.788, df = 30, P < 0.05) pollen (Table 1). Survival of A. limonicus was relative high (93.9–100 %) for all pollen diets (Table 2).
Effects of pollen diet on adult longevity and egg production
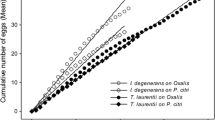
Pollen diet during juvenile and adult life significantly affected the longevity of A. limonicus females which ranged from 13.9 days on olive pollen to 23.7 days on cattail pollen (Table 3; Fig. 1). Upon adulthood, it took the females a short period of time (1.1–2.3 days) to start laying eggs which, however, was dependent on pollen diet (Table 3). Subsequently, oviposition period lasted for 8.7–19.5 days, and females died 3.0–6.7 days after the final egg was laid. Female longevity averaged 22–24 days on pollen of cattail, corn and pine, but was substantially shorter on olive pollen (13.9 days). On the other hand, females produced a low number of eggs (13.4–16.8 eggs/per female) on all pollen diets except on cattail pollen (26.4 eggs/per female) (Table 3). The sex ratio [females/(females + males)] of A. limonicus offspring was not affected by pollen diet of juveniles and adults (χ2 = 2.46, df = 3, P > 0.05; 0.64 on cattail pollen, 0.70 on olive pollen, 0.66 on corn pollen and 0.61 on pine pollen).
Effects of pollen diet on life-history parameters
Recorded differences in adult longevity and egg production for the different pollen diets resulted in considerable variation in calculated values for life-history parameters of A. limonicus. The intrinsic rate for increase (r m ) ranged between 0.1415 day−1 for pine pollen to 0.2340 day−1 for cattail pollen (Table 4). Values of net reproductive rate (R0), mean generation time (T), and doubling time (DT) ranged in a similar manner to r m among treatments (Table 4). Based on r m values, pollens tested may be classified from the best quality to the worst as follows: cattail > olive > corn > pine.
Discussion
In this study, we assessed the suitability of pollen of four anemophilous plant species as food for the predatory mite A. limonicus. We showed that all tested pollens could be used to efficiently rear this predator under laboratory conditions. Nevertheless, we recorded an inter-specific variation in the way pollen affected the performance of this predator, mainly with regard to female egg production. This variation is reflected in the calculated intrinsic rates of population increase which allowed us to classify the pollen species tested from the best to the worst in quality for A. limonicus.
To our knowledge, cattail pollen is the only pollen species already having been assessed for A. limonicus with regard to intrinsic rates of population increase. In our study, T. latifolia pollen was shown to be the superior pollen among those tested resulting in the highest r m value (0.234 day−1). Vangansbeke et al. (2014a) reported a slightly higher r m value (0.262 day−1) when A. limonicus was fed with T. angustifolia pollen (Nutrimite™) on bean leaf arenas at 25 ± 1 °C, while T. latifolia pollen results were variable in terms of r m values ranging from 0.157 and 0.166 day−1 at 23 ± 1 °C on modified Munger cells and bean leaf discs, respectively (Vangansbeke et al. 2014b) to 0.258 day−1 at 25 ± 1 °C on modified Munger cells (Nguyen et al. 2015). Such variation may be related to different experimental conditions and/or genetic variation among predator (A. limonicus) populations.
Different pollen species have resulted in variable oviposition rates of A. limonicus females. Feeding A. limonicus for three days on sweet pepper pollen on cucumber leaf discs at 25 °C resulted in 1.5 eggs per female per day (van Houten et al. 1995), while on sweet pepper leaf discs oviposition rates ranged from 0.9–1.1 eggs per female per day on sweet corn pollen to 1.7 eggs per female per day on cattail (T. latifolia) pollen during a three-day experimental period (Leman and Messelink 2015). Similar values between 0.21 eggs per female per day on lemon pollen and 2.27 eggs per female per day on almond pollen have been reported for A. limonicus by Swirski and Dorzia (1968) in a ten-day oviposition trial using different pollen species (castor bean, avocado and Carpobrotus edulis (L.) L. Bolus pollen were included in the study) on plastic substrate. In our study, corn pollen on bean leaf discs promoted high survival and rapid juvenile growth of A. limonicus, while peak oviposition rate reached 1.7 eggs per female per day resulting in an intrinsic rate of increase of 0.170 day−1. On the other hand, peak oviposition rates for A. limonicus reared on the other pollen species ranged between 2.6 eggs per female per day on cattail pollen to 1.5 eggs per female per day on pine pollen. Besides effects of different pollen species used and experimental conditions, plant sap feeding by A. limonicus suggested in a previous study (Messelink et al. 2006), may have additionally affected recorded oviposition rates on different substrates. On the other hand, egg cannibalism, which has been shown to be diet-dependent and to result in reduced population growth rates of A. limonicus (Vangansbeke et al. 2014a), may have shaped to a certain extent the reported results on oviposition rates.
Rearing A. limonicus on natural prey, factitious food or artificial diet has been shown to result in comparable intrinsic rates of increase to that recorded in our study for cattail pollen. The highest r m value (0.256 day−1) ever reported for A. limonicus has been reached on two factitious foods, i.e. Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs or Carpoglyphus lactis (L.) (Acari: Astigmata) (Vangansbeke et al. 2014a, b), similarly to feeding on natural prey (Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) larvae) (0.248 day−1) (Vangansbeke et al. 2014b). The r m value for cattail pollen reported here (0.234 day−1) is lower than the values above, and higher than the values reported for A. limonicus fed on Artemia franciscana Kellogg cysts (0.215 day−1) or on a liquid artificial diet enriched with dry decapsulated A. franciscana cysts (0.212 day−1) (Nguyen et al. 2015; Vangansbeke et al. 2014b). Based on the above, we conclude that cattail and olive pollen should be considered as alternative foods for A. limonicus of high to moderately high quality, respectively, followed by corn and pine pollen (r m values: 0.170 and 0.141 day−1, respectively).
Variation in pollen suitability as an alternative food source has also been shown for several other phytoseiid species (e.g. Broufas and Koveos 2000; Goleva and Zebitz 2013; Kolokytha et al. 2011). Inter-specific variation in pollen innate characteristics (nutrient content and/or specific morphological/chemical traits) as well as species-specific physiological (e.g. digestion mechanisms) or anatomical (e.g. mouthpart morphology) characteristics of phytoseiid predators may explain the reported variation in the effects of different pollen species on life-history traits of different phytoseiids (Goleva and Zebitz 2013; Roulston and Cane 2000; Roulston et al. 2000). With regard to pollination type, while pollen of anemophilous compared to entomophilous plant pollen is considered to be of lower nutritional quality (Roulston et al. 2000), this quality difference was not reflected in the calculated intrinsic rates of increase for Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) when fed with several anemophilous and entomophilous plant pollen species (Goleva and Zebitz 2013). On the contrary, the considerable intra-pollination type variation was attributed to other pollen-related traits (i.e. morphological and/or chemical). Similarly, we assume that the recorded variation in our study was affected by the nutrient content and/or the morphology of the grains of the pollen species tested. Interestingly, Goleva and Zebitz (2013) characterised a high number of pollen species as suitable as, or more suitable for A. swirskii than cattail pollen. In accordance, we highlight the need for further investigation of additional pollen species of even higher quality than cattail pollen for A. limonicus.
Biological pest control with the use of A. limonicus requires a series of predator releases in the absence of alternative and/or supplementary food. Application of pollen as a supplementary food in the crop may help sustaining populations of the predatory mite and thus contribute to the cost effectiveness of biological control. Pollen application on the terminal leaf of cucumber plants has been shown by van Rijn et al. (1999) to improve thrips control. In addition to cattail pollen, we herein identified three anemophilous pollen species with variable suitability for A. limonicus. High quality pollen may be used to minimize A. limonicus cannibalism in periods of prey decline, but also to boost predator populations in the presence of prey. Low quality pollen on the other hand, may sustain A. limonicus in periods of prey scarcity or when prey is present, but predator satiation levels are expected to be lower on this food. Since pollen may also serve as alternative food for prey (e.g. thrips) in certain cases the appropriate pollen species for a given predator should ideally be a food of intermediate to low quality for the prey, as was recently shown for A. franciscana cysts evaluated as supplemental food for A. swirskii against T. angustifolia (Vangansbeke et al. 2015). In laboratory trials, pine pollen was shown to be the most suitable pollen species tested for the population growth of F. occidentalis (Hulshof et al. 2003). Sweet corn pollen on chrysanthemum leaf discs on the other hand resulted in a lower oviposition rate of F. occidentalis compared with cattail pollen, while providing similar thrips control at low predator densities when offered as food for A. swirskii in chrysanthemum greenhouse plants (Leman and Messelink 2015). Therefore, the effects of different pollen species on both the predator (A. limonicus) and its main prey (F. occidentalis) should be tested under realistic greenhouse conditions in order to conclude on the best combination for efficient pest control. Besides the pollen species tested in this study, other species or a combination of pollen species or pollen with artificial diets may be worthwhile for testing in the greenhouse to improve biological pest control with A. limonicus. Furthermore, long-term experiments extending to several successive generations of the predator reared on each of the different pollens tested are required to verify these results before they can be used in a mass-rearing system.
References
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26
Broufas GD, Koveos DS (2000) Effect of different pollens on development, survivorship and reproduction of Euseius finlandicus (Acari: Phytoseiidae). Environ Entomol 29:743–749
Broufas GD, Koveos DS (2001) Cold hardiness characteristics in a strain of the predatory mite Euseius (Amblyseius) finlandicus (Acari: Phytoseiidae) from Northern Greece. Ann Entomol Soc Am 94:82–90
Broufas GD, Pappas ML, Koveos DS (2007) Development, survival, and reproduction of the predatory mite Kampimodromus aberrans (Acari: Phytoseiidae) at different constant temperatures. Environ Entomol 36:657–665
Coll M, Guershon M (2002) Omnivory in terrestrial arthropods: mixing plant and prey diets. Annu Rev Entomol 47:267–297
Denno RF, Fagan WF (2003) Might nitrogen limitation promote omnivory among carnivorous arthropods? Ecology 84:2522–2531
Eubanks MD, Styrsky JD (2005) Effects of plant feeding on the performance of omnivorous predators. In: Wäckers FL, van Rijn PCJ, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, UK, pp 148–177
Gerson U, Smiley RL, Ochoa R (2003) Mites (Acari) for pest control. Oxford Blackwell Science, Oxford, UK
Goleva I, Zebitz CPW (2013) Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp Appl Acarol 61:259–283
Hoogerbrugge H, van Houten Y, Knapp M, Bolckmans K (2011) Biological control of thrips and whitefly on strawberries with Amblydromalus limonicus and Amblyseius swirskii. IOBC/WPRS Bull 68:65–69
Hoy MA (2011) Agricultural acarology: introduction to integrated mite management. CRC Press, Boca Raton, USA
Huang N, Enkegaard A, Osborne LS, Ramakers PMJ, Messelink GJ, Pijnakker J, Murphy G (2011) The banker plant method in biological control. Crit Rev Plant Sci 30:259–278
Hulshof J, Ketoja E, Vänninen I (2003) Life history characteristics of Frankliniella occidentalis on cucumber leaves with and without supplemental food. Entomol Exp Appl 108:19–32
Knapp M, van Houten Y, Hoogerbrugge H, Bolckmans K (2013) Amblydromalus limonicus (Acari: Phytoseiidae) as a biocontrol agent: literature review and new findings. Acarologia 53:191–202
Kolokytha PD, Fantinou AA, Papadoulis GT (2011) Effect of several different pollens on the bio-ecological parameters of the predatory mite Typhlodromus athenas Swirski and Ragusa (Acari: Phytoseiidae). Environ Entomol 40:597–604
Leman A, Messelink G (2015) Supplemental food that supports both predator and pest: a risk for biological control? Exp Appl Acarol 65:511–524
Lorenzon M, Pozzebon A, Duso C (2012) Effects of potential food sources on biological and demographic parameters of the predatory mites Kampimodromus aberrans, Typhlodromus pyri and Amblyseius andersoni. Exp Appl Acarol 58:259–278
McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their roles in biological control. Annu Rev Entomol 42:291–321
McMurtry JA, de Moraes GJ, Sourassou NF (2013) Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst Appl Acarol 18:297–320
Messelink GJ, van Steenpaal SEF, Ramakers PMJ (2006) Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. BioControl 51:753–768
Messelink GJ, Bennison J, Alomar O, Ingegno BL, Tavella L, Shipp L, Palevsky E, Wäckers FL (2014) Approaches to conserving natural enemy populations in greenhouse crops: current methods and future prospects. BioControl 59:377–393
Meyer JS, Ingersoll CG, McDonald LL, Boyce MS (1986) Estimating uncertainty in population growth rates: jackknife vs. bootstrap techniques. Ecology 67:1156–1166
Nguyen DT, Vangansbeke D, De Clercq P (2015) Performance of four species of phytoseiid mites on artificial and natural diets. Biol Control 80:56–62
Nomikou M, Janssen A, Schraag R, Sabelis MW (2002) Phytoseiid predators suppress populations of Bemisia tabaci on cucumber plants with alternative food. Exp Appl Acarol 27:57–68
Nomikou M, Janssen A, Sabelis MW (2003) Phytoseiid predators of whiteflies feed and reproduce on non-prey food sources. Exp Appl Acarol 31:15–26
Roulston TH, Cane JH (2000) Pollen nutritional content and digestibility for animals. Plant Syst Evol 222:187–209
Roulston TH, Cane JH, Buchmann SL (2000) What governs protein content of pollen: pollinator preferences, pollen–pistil interactions, or phylogeny? Ecol Monogr 70:617–643
Sabelis MW, van Rijn PCJ, Janssen A (2005) Fitness consequences of food-for-protection strategies in plants. In: Wäckers FL, van Rijn PCJ, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, UK, pp 109–134
Schmidt JM, Peterson JA, Lundgren JG, Harwood JD (2013) Dietary supplementation with pollen enhances survival and Collembola boosts fitness of a web-building spider. Entomol Exp Appl 149:282–291
Sokal RR, Rohlf FJ (1995) The principles and practice of statistics in biological research, 3rd edn. Freeman, New York, USA
Southwood TRE, Henderson PA (2000) Ecological methods, 3rd edn. Blackwell, Oxford, UK
SPSS (2011) SPSS. IBM SPSS statistics base 20, ©copyright IBM Corporation
Swirski E, Dorzia N (1968) Studies on the feeding, development and oviposition of the predaceous mite Amblyseius limonicus Garman and McGregor (Acarina: Phytoseiidae) on various kinds of food substances. Isr J Agric Res 18:71–75
van Houten YM, van Rijn PCJ, Tanigoshi LK, van Stratum P, Bruin J (1995) Preselection of predatory mites to improve year-round biological control of western flower thrips in greenhouse crops. Entomol Exp Appl 74:225–234
van Houten YM, Rothe J, Bolckmans KJF (2008) The generalist predator Typhlodromalus limonicus (Acari: Phytoseiidae): a potential biological control agent of thrips and whiteflies. IOBC/WPRS Bull 32:237–240
van Rijn PCJ, Tanigoshi LK (1999) Pollen as food for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): dietary range and life history. Exp Appl Acarol 23:785–802
van Rijn PCJ, van Houten YM, Sabelis MW (1999) Pollen improves thrips control with predatory mites. IOBC/WPRS Bull 22:209–212
van Rijn PCJ, van Houten YM, Sabelis MW (2002) How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 83:2664–2679
Vangansbeke D, Nguyen DT, Audenaert J, Verhoeven R, Deforce K, Gobin B, Tirry L, De Clercq P (2014a) Diet-dependent cannibalism in the omnivorous phytoseiid mite Amblydromalus limonicus. Biol Control 74:30–35
Vangansbeke D, Nguyen DT, Audenaert J, Verhoeven R, Gobin B, Tirry L, De Clercq P (2014b) Performance of the predatory mite Amblydromalus limonicus on factitious foods. BioControl 59:67–77
Vangansbeke D, Nguyen DT, Audenaert J, Verhoeven R, Gobin B, Tirry L, De Clercq P (2015) Supplemental food for Amblyseius swirskii in the control of thrips: feeding friend or foe? Pest Manage Sci. doi:10.1002/ps.4000
Wäckers FL (2005) Suitability of (extra-) floral nectar, pollen, and honeydew as insect food sources. In: Wäckers FL, van Rijn PCJ, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, UK, pp 17–74
Acknowledgments
The editor and two anonymous reviewers are acknowledged for their constructive comments on an earlier version of our manuscript. Vassiliki Mantali, Anneta Triantafyllou and Georgia Tavlaki are thanked for technical assistance during the course of the experiments. K. Samaras and M.L. Pappas were supported by the Onassis Public Benefit Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq.
Rights and permissions
About this article
Cite this article
Samaras, K., Pappas, M.L., Fytas, E. et al. Pollen suitability for the development and reproduction of Amblydromalus limonicus (Acari: Phytoseiidae). BioControl 60, 773–782 (2015). https://doi.org/10.1007/s10526-015-9680-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-015-9680-5