Abstract
Scymnus subvillosus (Goeze) (Coleoptera: Coccinellidae) is an aphidophagous predator present in the Azores (Portugal), but occurring at low densities. Scymnus species belong to a poorly known Coccinellidae group of biological control agents. In this study we aimed to evaluate the suitability of Myzus persicae (Sulzer), Aphis fabae Scopoli and Melanaphis donacis (Passerini) (Homoptera: Aphididae) as prey for S. subvillosus. To achieve this, we determined (i) the temperature and prey-dependence for development and survival of the immature stages, (ii) the prey-dependence for reproductive performance at 25 °C and (iii) the voracity and nutritional physiology of the 4th larval instar fed on A. fabae. The development time from first instar larva to adult decreased with increasing temperature, ranging from 61.5 days at 15 °C to 10.4 days at 30 °C. To complete immature development on M. donacis, the lower development threshold (LDT) was estimated to be 11.7 °C and the sum of effective temperatures (SET) to be 196.3 degree-days (DD). At 15 °C, larvae failed to develop when fed on A. fabae or M. persicae but on M. donacis 22 % of the larvae survived. We also found that development time of immature stages was prey-dependent, with M. persicae being the least suitable prey. The reproductive parameters were prey-dependent, with A. fabae and M. donacis allowing better performance than M. persicae. Twelve-hour-old 4th instars of S. subvillosus ingested 3.23 mg of biomass per day corresponding to an average of 10.5 aphids of A. fabae, allowing for a daily mean weight gain of 0.71 mg. The conversion efficiency and relative growth rate obtained were approximately 21 and 48 %, respectively. The results obtained in the present study suggest that both A. fabae and M. donacis are more suitable prey for development and reproduction of S. subvillosus than M. persicae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ladybird predators (Coleoptera: Coccinellidae) are known as important biological control agents, especially against aphid populations (Michaud 2012). Several species are used in biological control under its various strategies (classical, augmentative, inundative), in different crops and cultivation systems, either outdoors or in greenhouses (Hodek and Michaud 2008; Cabral et al. 2009). These predators have been extensively studied due to their biological, ecological and behavioral characteristics such as polyphagy, high voracity and rapid numerical and aggregative response (Hodek and Honek 1996; Obrycki and Kring 1998; Dixon 2000).
Among Coccinellidae the small Scymnus species are poorly known. However, in recent years a substantial number of studies were performed under the perspective of their use as biological control agents (Uygun and Atlihan 2000; Wanntorp 2004; Pluke et al. 2005; Woin et al. 2006). Compared with larger species, the Scymnus spp., due to their small size and lower voracity, will be apparently less competitive and efficient as biological control agents. However, persisting at low densities of prey and having high longevities (Tawfik et al. 1973; Borges et al. 2011; 2013), these small coccinellids may be able to feed on aphid colonies at earlier and/or later stages (Agarwala and Yasuda 2001) and for a longer period of time, which would give them the possibility to exploit different spatial and temporal niches than the larger ladybirds.
Scymnus subvillosus (Goeze) is a widely distributed species, found in twenty seven European countries, twenty in Asia, eight in North Africa and also present in the Afrotropical Region (Gourreau 1974; Raimundo and Alves 1986; Kovář 2007). This aphidophagous predator is recorded for the Azores Archipelago (Portugal) (Soares et al. 2003a, 2006), but occurring at low densities. It has been found exploiting Melanaphis donacis (Passerini) (Hemiptera: Aphididae) on Arundo donax L. (Poales: Poaceae). On the Portuguese mainland, it is common in arboreal agro-ecosystems, including apple orchards, orange, peach, plum, walnut and oak, where it exploits Hyalopterus pruni (Geoffroy) (Homoptera: Aphididae) (Raimundo and Alves 1986). In Turkey, S. subvillosus is widespread (Atlihan and Güldal 2009), and it is an important natural enemy against 15 aphid species (Aslan and Uygun 2005). There it is common in agro-ecosystems and natural habitats as well, including stone fruits especially in Prunus spp. orchards, Malus spp., and Populus spp., on apple, plum, cherry, pine and alder (Aslan and Uygun 2005).
The temperature and the nutritional quality of prey are decisive factors in the biological performance of insect predators, altering for instance the development time of pre-imaginal stages and reproductive performances of adults (e.g., fecundity, fertility), and in this way contributing to differential population growth (McCaffrey and Horsburgh 1986; Niijima et al. 1986; Gibson et al. 1992; Hodek 1993; Mohaghegh et al. 2001; Skirvin and Fenlon 2003; Kontodimas and Stathas 2005; Michaud 2005; Cabral et al. 2006; Jalali et al. 2010). The estimation of the biological characteristics of natural enemies, such as time of development and the thermal constant may contribute to select the most appropriate biocontrol agent to be used under certain environmental conditions (Perdikis and Lykouressis 2002) as well as the best conditions, either thermal or nutritional, for mass production.
In this study we aimed to access the ecophysiological suitability of M. persicae, A. fabae and M. donacis as prey for S. subvillosus. To achieve this, we determined (i) the temperature and prey-dependence for development and survival of the immature stages, (ii) the prey-dependence for reproductive performance at 25 °C and (iii) the voracity and nutritional physiology of the 4th instar fed on A. fabae.
Materials and methods
Insects
Scymnus subvillosus adults were collected in the field, on A. donax, in S. Miguel Island, Azores (Portugal). The predator was found foraging and reproducing on M. donacis. We further tested the suitability of two other important crop pests found in the Azores, Myzus persicae (Sulzer) and Aphis fabae Scopoli (Homoptera: Aphididae). Ladybird adults were reared in the laboratory at 25 ± 1 °C, 75 ± 5 % RH and a light regime of 16L:8D under fluorescent lamps. The predator was fed on a mixed diet of M. persicae and A. fabae, provided ad libitum, and complemented with pollen and honey. This diet was maintained only a few days before starting the experiments. The mixed diet was provided to avoid food adaptation (Rana et al. 2002) and also to supply a wider group of nutrients. The prey species were reared on Vicia faba L. (Fabales: Fabaceae) at 15 ± 1 °C, 75 ± 5 % RH and a light regime of 16L:8D under fluorescent lamps.
Temperature and prey-dependence for development of the immature stages of S. subvillosus
To determine the effect of temperature and prey on the development of the immature stages of S. subvillosus, the development time from egg to adult emergence was followed by means of 12 experimental treatments, corresponding to one of the following temperatures, 15, 20, 25 and 30 °C, and prey regimes of A. fabae, M. donacis or M. persicae (N = 30). Each larva was kept individually in a plastic box (diameter: 5 cm, height: 3 cm) and was fed once a day ad libitum, with a mixture of several stages of A. fabae, M. donacis or M. persicae. In the treatment of 15 °C and due to a high level of infertility, the eggs were collected from couples reared at 25 °C. All assays were performed at 75 ± 5 % RH, with a photoperiod of 16L:8D. Immature stages were observed twice a day (10:00 am and 17:00 pm) to record moulting and adult emergence.
The larval development time and survival were determined for each diet at each tested temperature. The lower developmental threshold (LDT) was determined. The relationship between the inverse of the development time and the temperature is nearly linear (Honek and Kocourek 1990). According to this, the following linear relationship was considered (Mota et al. 2008):
where DT corresponds to the development time of a pre-imaginal stage, which is inversely proportional to the temperature (T), and a and b are the regression parameters. The LDT corresponds to the temperature where no development occurs, that is -(b/a) from the above function (Honek and Kocourek 1988).
The sum of effective temperatures (SET) represents the lower developmental threshold for completion of a developmental stage (Nedvĕd and Honĕk 2012) and was calculated according to the model (Mota et al. 2008):
DT x development time at temperature x
LDT lower developmental threshold
The suitability of A. fabae, M. donacis and M. persicae as prey for S. subvillosus was evaluated by determining development data such as development time of immature stages (N = 30), age-specific survival rate, weight of females upon emergence and reproductive parameters, e.g., pre-oviposition time, fecundity and fertility. To evaluate reproductive performance of adults, eight couples were formed and reared for 20 days on each prey diet. Each couple was kept inside a plastic box (diameter: 5 cm, height: 3 cm) and fed ad libitum with A. fabae, M. donacis or M. persicae. Couples were observed once a day to obtain data on pre-oviposition time, fecundity and fertility. All assays were performed in a climate chamber at 25 ± 1 °C, 75 ± 5 % RH and a light regime of 16L:8D under fluorescent lamps.
Voracity and nutritional physiology of the 4th instar of S. subvillosus fed on A. fabae
The larvae used in this experiment were reared ad libitum with A. fabae under the abiotic conditions previously described. To determine the daily survival rate (Sr) of the aphids and weight loss due to dehydration (PWd), a control test was performed in which ten aphid females were placed inside a plastic box (N = 15) for a period of 12 h. The weight of the aphids was recorded at the beginning and at the end of the experiment and the number of aphids surviving was recorded. The test of consumption started with 12 h old 4th instar larvae. Then larvae were subjected to starvation for a period of 12 h, followed by a 24 h test in which they were allowed to consume prey. Because the aphid prey dehydrated considerably over a 24 h period, the predator was provided with ten aphids at each of two feeding times (8:00 am and 8:00 pm). To determine the biomass consumption, the aphids were weighed before and after the 24 h feeding period. The number of prey consumed was recorded. At the beginning and the end of the test the prey biomass (PW) and predator larval weight (LW) were also recorded.
Voracity was estimated as the number of prey entirely or partially eaten (P). The voracity (V), biomass ingested (BI), relative growth rate (RGR %) and conversion efficiency (CE %) were also calculated (Borges 2008) :
where PW i and PW f are prey initial and final weights, respectively, and LW i and LW f are the predator larval initial and final weights, respectively.
Statistical analyses
Data normality and variance homogeneity were evaluated by the Kolmogorov–Smirnov and Levene’s tests, respectively. When normality and homogeneity of variance were confirmed, ANOVA was used and for multiple comparisons the Tukey test was performed. When data was not normal, the Kruskal–Wallis test was used, with multiple comparisons made by the Mann–Whitney test with the correction of Bonferroni (Zar 1996). In the present study, three diets were tested, therefore the critical P value for the Bonferroni correction was 0.017 (0.05/3). To compare the effect of prey and temperature on the developmental time of the predator, a non-parametric two-way ANOVA, a special extension of a Kruskal–Wallis was performed (Zar 1996). In this case it is not possible to perform multiple comparison tests. SPSS v. 15.0 was used to perform statistical analyses.
Results
Temperature and prey-dependence for development of the immature stages of S. subvillosus
The larvae failed to develop into adults when fed on A. fabae and M. persicae at 15 °C, whereas when fed on M. donacis, developmental time was 61.5 ± 1.96 days. For this reason the temperature and prey-dependence for development of the immature stages of S. subvillosus was statistically compared only at 20, 25 and 30 °C. No significant interaction was detected between temperature and prey (extension of the Kruskal–Wallis temperature × prey interaction: \(\chi_{4}^{2}\) = 8.76, p = 0.067). Our results show that independently of the prey provided, development time of S. subvillosus immatures decreased with increasing temperature, as expected within the range of temperatures tested (extension of the Kruskal–Wallis: \(\chi_{2}^{2}\) = 89.86, p < 0.0001). For a given temperature, the development time of immature stages was independent of prey (extension of the Kruskal–Wallis: \(\chi_{2}^{2}\) = 1.19, p = 0.55) (Table 1).
The LDT and sum of effective temperatures (SET) for immature development of S. subvillosus fed on M. donacis ranged from 9.4 °C for L1 to 12 °C for L4, and from 16.7 degree-days (DD) for L2 to 70.6 DD for pupa, respectively (Table 2). To complete immature development, the LDT was estimated to be 11.7 °C and SET to be 196.3 DD (Table 2). The thermal parameters of larvae fed on A. fabae and M. persicae were not calculated because data from three temperatures only (20, 25 and 30 °C) may lead to a less accurate estimate of the LDT and SET values.
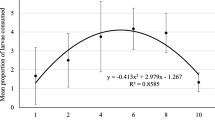
We found that at 15 °C larvae failed to develop into adults when fed on A. fabae and M. persicae but on M. donacis 22 % of larvae survived. At 20 and 25 °C larval survival was high, ranging from 74 to 85 % (Fig. 1).
Female body weight did not significantly differ with prey species (Table 3). Pre-oviposition period was significantly longer when females were fed on M. persicae and no significant differences were found for A. fabae and M. donacis. Fecundity did not significantly differ with prey species. Females of S. subvillosus laid an average of 80 eggs and 159 eggs when fed on M. persicae and A. fabae, respectively. Despite the absence of significant differences in the mean number of eggs laid, we found that the proportion of ovipositing females differed: 90, 70 and 50 % for M. donacis, A. fabae and M. persicae, respectively. The fertility of S. subvillosus eggs was significantly higher on A. fabae and M. donacis (Table 3).
Voracity and nutritional physiology of 4th instars of S. subvillosus fed on A. fabae
The mean weight gain by a 12-hour old 4th larval instar was 0.71 mg, corresponding roughly to 50 % of its initial body weight (Table 4). The 3.23 mg of aphid biomass ingested, corresponding to ca. 10.5 prey items (females), is converted to larval biomass at a rate of 21 % (Table 4).
Discussion
Environmental temperature and food quality are important factors determining physiological processes in coccinellid predators (Hodek and Evans 2012; Nedved and Honek 2012), with direct implications on developmental rate and survival (Blackman 1967; Honek and Kocourek 1990; Obrycki and Orr 1990; Chapman 1998; Kalushkov 1998; Kalushkov and Hodek 2001; Soares et al. 2001; Isikber and Copland 2002; Evans 2003; Soares et al. 2003b; Soares et al. 2004; Ungerová et al. 2010) and reproductive performance, such as fecundity and fertility (Blackman 1967; Niijima et al. 1986; Hodek 1993; Michaud 2005). As we expected, developmental time of the immature stages of S. subvillosus decreased with increasing temperature. Our results also indicated that developmental rate and survival were concomitantly temperature and prey-dependent, which is consistent with previous studies (Atlihan et al. 1999; Satar and Uygun 2012). For instance, survival rate was higher when larvae were fed on M. donacis. The most remarkable example was the inability of S. subvillosus to complete its development at 15 °C when fed with M. persicae and A. fabae. The importance of prey quality for developmental rate could be revealed by differences in the sum of effective temperatures (SET). In our experiment, S. subvillosus required 196.3 DD to develop from 1st instar to adult when fed M. donacis, but on Aphis gossypii Glover it required 230 DD (Satar and Uygun 2012).
Growth and development of an insect species occur only across a specific range of temperatures (Nedvĕd and Honĕk 2012) and thus thermal parameters such as the lower developmental threshold and thermal constant are useful indicators to predict its potential distribution and abundance (Messenger 1970). The LDT of S. subvillosus was estimated to be 11.7 °C for total immature development when fed on M. donacis. Satar and Uygun (2012) reported a value of 10.26 °C from egg to adult of S. subvillosus fed on A. gossypii. In the present study, and contrarily to these authors, the highest LDT was found for the 4th instar. These values are quite similar to other Scymnus species: Scymnus hoffmanni Weise: 10.1 °C; Scymnus frontalis (Fabricius): 11.7 °C fed on Diuraphis noxia (Mordvilko); Scymnus syriacus Marseul: 11.3 °C; Scymnus levaillanti Mulsant: 11.7 °C fed on A. gossypii (Kawauchi 1985; Naranjo et al. 1990; Emami et al. 1998; Uygun and Atlihan 2000). Atlihan and Chi (2008) estimated a lower LDT of 7.1 °C for S. subvillosus when fed on H. pruni. This discrepancy can be partially explained by regional acclimatization. Satar and Uygun (2012) collected their S. subvillosus in the Mediterranean region where mild winters occur whereas Atlihan and Chi (2008) collected individuals in Turkey (Van region) where there is a harsh and long winter. The combination of a high LDT and a low SET guarantees a fast development at high temperatures, in contrast to cold adapted species whose LDT is low and SET high (Turgill 1995).
Egg production requires nutritional intake beyond a maintenance level, and thus high quality food is important for supporting reproductive capacity (Seagraves 2009). Our results showed that M. donacis and A. fabae are equally suitable prey whereas M. persicae leads to a lower biological performance. Despite the absence of significant differences in the mean number of eggs laid, the proportion of ovipositing females differed: 90, 70 and 50 % for M. donacis, A. fabae and M. persicae, respectively. This finding may have implications for biocontrol. Indeed females presenting a high rate of oviposition may lead to potentially higher biocontrol capacity of the resulting hatched larvae. In the Scymninae the pre-oviposition period varies with temperature and prey suitability even among closely related species (Naranjo et al. 1990; Uygun and Atlihan 2000; Nedvĕd and Honĕk 2012). This is consistent with our results in which pre-oviposition period was significantly longer on M. persicae than on A. fabae and M. donacis.
From an ecophysiological point of view, the three prey species tested in our study can be considered as essential foods. Indeed, in light of the criteria described by Hodek and Evans (2012), the consumption of those prey species supported growth and development of larvae and reproduction by adults. Other essential prey reported for S. subvillosus includes Aphis sambuci L., H. pruni, and A. gossypii (Atlihan and Chi 2008; Atlihan and Güldal 2009; Klausnitzer 1992; Satar and Uygun 2012). Essential foods, however, show varying degrees of favorability, enabling different developmental rates, body mass, fecundity, and survival (Hodek 1993; Hodek and Honěk 1996; Kalushkov 1998; Kalushkov and Hodek 2004; Soares et al. 2005; Cabral et al. 2006; Ungerová et al. 2010). Overall A. fabae and M. donacis appear to be more suitable food resources than M. persicae. This result could reflect a possible case of prey specialization due to the co-occurrence of S. subvillosus and A. fabae and M. donacis (contrarily to M. persicae) in the Azorean coastal habitats where larvae of the predator have been found feeding on aphid colonies (I. Borges, pers. obs.). Indeed, according to Rana et al. (2002), prey specialization due to selection through consecutive generations increases fitness in ladybirds.
From a practical point of view, the estimation of voracity, expressed as the number of aphids killed, is an important predictor of a predator’s potential as a biological control agent. However, expressing voracity as the amount of biomass intake, allows i) to correct voracity given that aphids do not have the same body size and ii) to estimate some physiological parameters (Frazer 1988). Despite the small size of the 4th instar of S. subvillosus, an average of 10.5 aphids was killed during 24 h. Considering an average aphid weight of 0.6 mg, this would correspond to 6.3 mg of prey biomass. However, only a small amount of prey biomass was effectively taken up: 3.23 mg. Considering the general model of biomass/energy flow, we estimated that 21 % of prey biomass ingested is converted to predator biomass, allowing a relative growth rate of approximately 48 %. Borges (2008) obtained a similar value for the conversion efficiency for Scymnus nubilus Mulsant (26 %). The low proportion of prey biomass ingested increases the potential of the predator to kill more aphids. If all prey items were fully consumed, as with large ladybirds, only six aphids would be required for the daily needs instead of the average of 10.5 obtained in this study. From a practical point of view this fact can be advantageous when considering the use of the smaller ladybird beetles in biological control.
In conclusion, our results show a concomitant influence of temperature and prey on developmental time and survival rate of immature stage of S. subvillosus. Myzus persicae, A. fabae and M. donacis are essential prey species for S. subvillosus and thus the predator can be a suitable biological control agent against these aphids. However, diets differ in suitability, with M. donacis and A. fabae being equally suitable and better than M. persicae. Considering our results and previously published data, the optimal temperature for population growth of S. subvillosus and mass production, could be somewhere between 20 to 25 °C.
References
Agarwala BK, Yasuda H (2001) Larval interactions in aphidophagous predators: effectiveness of wax cover as defence shield of Scymnus larvae against predation from syrphids. Entomol Exp Appl 100:101–107
Aslan M, Uygun N (2005) The aphidophagous coccinellid (Coleoptera: Coccinellidae) species in Kahramanmaraş, Turkey. Turk J Zool 29:1–8
Atlihan R, Chi H (2008) Temperature-dependent development and demography of Scymnus subvillosus (Coleoptera: Coccinellidae) reared on Hyalopterus pruni (Homoptera: Aphididae). J Econ Entomol 101:325–333
Atlihan R, Güldal H (2009) Prey density-dependent feeding activity and life history of Scymnus subvillosus. Phytoparasitica 37:35–41
Atlihan R, Denizhan E, Yaşar B (1999) Effects of different preys on development and fecundity of Scymnus subvillosus Goeze (Coleoptera: Coccinellidae). In: Proceedings of the IV Turk Congress of Biol Control (Adana, Turkey), pp 397–406 (Turkish, with English summary)
Blackman RL (1967) The effects of different prey on Adalia bipunctata L. and Coccinella 7-punctata L. Ann Appl Biol 59:207–219
Borges IMM (2008) Life history evolution in aphidophagous and coccidophagous Coccinellidae (Coleoptera). PhD Thesis. University of the Azores, Portugal
Borges I, Soares AO, Magro A, Hemptinne JL (2011) Prey availability in time and space is a driving force in life history evolution of predatory insects. Evol Ecol 25:1307–1319
Borges I, Hemptinne JL, Soares AO (2013) Contrasting population growth parameters of the aphidophagous Scymnus nubilus and the coccidophagous Nephus reunioni. BioControl 58:351–357
Cabral S, Soares AO, Moura R, Garcia P (2006) Suitability of Aphis fabae, Myzus persicae (Homoptera: Aphididae) and Aleyrodes proletella (Homoptera: Aleyrodidae) as prey for Coccinella undecimpunctata (Coleoptera: Coccinellidae). Biol Control 39:434–440
Cabral S, Soares AO, Garcia P (2009) Predation by Coccinella undecimpunctata L. (Coleoptera: Coccinellidae) on Myzus persicae Sulzer (Homoptera: Aphididae): effect of prey density. Biol Control 50:25–29
Chapman RF (1998) The insects: structure and function, 4th edn. Cambridge University Press, UK
Dixon AFG (2000) Insect predator–prey dynamics: ladybirds and biological control. Cambridge University Press, Cambridge, UK
Emami MS, Sahragard A, Haji-Zadeh J (1998) Effect of different temperatures on the development of Scymnus syriacus (Coleoptera: Coccinellidae). Appl Entomol Phytopathol 66:21–22
Evans EW (2003) Searching and reproductive behaviour of female aphidophagous ladybirds (Coleoptera: Coccinellidae): a review. Eur J Entomol 100:1–10
Frazer BD (1988) Predators. In: Minks AK, Harrewijn P (eds) Aphids: their biology, natural enemies and control, vol B. Elsevier Science, Amsterdam, The Netherlands, pp 217–230
Gibson R, Elliot N, Schaefer P (1992) Life history and development of Scymnus frontalis (Fabricius) (Coleoptera: Coccinellidae) on four species of aphid. J Kansas Entomol Soc 65:410–415
Gourreau JM (1974) Systématique de la tribu des Scymnini (Coccinellidae). Institut National de la Recherche Agronomique, Paris, France
Hodek I (1993) Habitat and food specificity in aphidophagous predators. Biocontrol Sci Technol 3:91–100
Hodek I, Evans EW (2012) Food relationship. In: Hodek I, van Emden HF, Honěk A (eds) Ecology and behavior of the ladybird beetles (Coccinellidae). Blackwell Publishing Ltd., UK, pp 141–274
Hodek I, Honěk A (1996) Ecology of Coccinellidae. Kluwer Academic, Dordrecht, The Netherlands
Hodek I, Michaud JP (2008) Why is Coccinella septempunctata so successful? (a point-of-view). Eur J Entomol 105:1–12
Honěk A, Kocourek F (1988) Thermal requirements for development of aphidophagous Coccinellidae (Coleoptera), Chrysopidae, Hemerobiidae (Neuroptera), and Syrphidae (Diptera): some general trends. Oecologia 76:455–460
Honěk A, Kocourek F (1990) Temperature and development time in insects: a general relationship between thermal constants. Zoologische Jahrbücher, Abteilung für Systematik, Ökologie und Geographie der Tiere 117:401–439
Işıkber AA, Copland MJW (2002) Effects of various aphid foods on Cycloneda sanguinea. Entomol Exp Appl 102:93–97
Jalali MA, Tirry L, De Clercq P (2010) Effect of temperature on the functional response of Adalia bipunctata to Myzus persicae. BioControl 55:261–269
Kalushkov P (1998) Ten aphid species (Sternorrhyncha: Aphidae) as prey for Adalia bipunctata (Coleoptera: Coccinellidae). Eur J Entomol 95:343–349
Kalushkov P, Hodek I (2001) New essential aphid prey for Anatisocellata and Calvia quatuordecimguttata (Coleoptera: Coccinellidae). Biocontrol Sci Technol 11:35–39
Kalushkov P, Hodek I (2004) The effects of thirteen species of aphids on some life history parameters of the ladybird Coccinella septempunctata. BioControl 49:21–32
Kawauchi SE (1985) The threshold temperature and thermal constant for development from the egg to the adult form of Coccinella septempunctata brucki, Propylea japonica and Scymnus (Pullus) hoffmanni (Coleoptera, Coccinellidae). Kurume Univ J 32:45–51
Klausnitzer B (1992) Coccinellidenals Prädatoren der Holunderblattlaus (Aphis sambuci L.) im Wärmefrühjahr. Entomol Nachr Ber 36:185–190
Kontodimas DC, Stathas GJ (2005) Phenology, fecundity and life table parameters of the predator Hippodamia variegata reared on Dysaphis crataegi. BioControl 50:223–233
Kovář I (2007) Coccinellidae. In: Löbl I, Smetana A (eds) Catalogue of Palaearctic Coleoptera, vol 4. Apollo Books, Denmark, pp 568–630
McCaffrey JP, Horsburgh RL (1986) Functional response of Orius insidiosus (Hemiptera: Anthocoridae) to the European red mite, Panonychus ulmi (Acari: Tetranychidae), at different constant temperatures. Environ Entomol 15:532–535
Messenger PS (1970) Bioclimatic inputs to biological control and pest management programs. In: Guthrie FE (ed) Rabb RL. Concepts of Pest Management North Carolina State University Press, Raleigh, USA, pp 84–102
Michaud JP (2005) On the assessment of prey suitability in aphidophagous Coccinelidae. Eur J Entomol 102:385–390
Michaud JP (2012) Coccinellids in biological control. In: Hodek I, van Emden HF, Honěk A (eds) Ecology and behavior of the ladybird beetles (Coccinellidae). Blackwell Publishing Ltd., UK, pp 488–519
Mohaghegh J, De Clercq P, Tirry L (2001) Functional response of the predator Podisus maculiventris (Say) and Podisus nigrispinus (Dallas) (Het.:Pentatomidae) to the beet armyworm, Spodoptera exigua (Hübner) (Lep.: Noctuidae): effect of temperature. J Appl Entomol 125:131–134
Mota A, Soares AO, Garcia P (2008) Temperature dependence for development of the whitefly predator Clitostethus arcuatus (Rossi). BioControl 53:603–613
Naranjo SE, Gibson RL, Walgenbach DD (1990) Development, survival and reproduction of Scymnus frontalis (Coleoptera: Coccinellidae), an imported predator of Russian wheat aphid, at four fluctuating temperatures. Ann Entomol Soc Am 83:527–531
Nedvĕd O, Honĕk A (2012) Life history and development. In: Hodek I, van Emden HF, Honěk A (eds) Ecology and behavior of the ladybird beetles (Coccinellidae). Blackwell Publishing Ltd., UK, pp 54–109
Niijima K, Matsuka M, Okada I (1986) Artificial diets for an aphidophagous coccinellid, Harmonia axyridis, and its nutrition (Minireview). In: Hodek I (ed) Ecology of Aphidophaga. Academia, Prague, Czech Republic and W. Junk, Dordrecht, The Netherlands, pp 37–50
Obrycki JJ, Kring TT (1998) Predaceous coccinellidae in biological control. Annu Rev Entomol 43:295–321
Obrycki JJ, Orr CJ (1990) Suitability of three prey species for Nearctic populations of Coccinella septempunctata, Hippodamia variegata, and Propylea quatuordecimpunctata (Coleoptera: Coccinellidae). J Econ Entomol 4:1292–1297
Perdikis DC, Lykouressis DP (2002) Thermal requirements of the polyphagous predator Macrolophuspygmaeus (Hemiptera: Miridae). Environ Entomol 31:661–667
Pluke RWH, Escribano A, Michaud JP, Stansly PA (2005) Potential impact of lady beetles on Diaphorina citri (Homoptera: Psyllidae) in Puerto Rico. Fla Entomol 88:123–128
Raimundo AAC, Alves MLG (1986) Revisão dos coccinelídeos de Portugal. Universidade de Évora, Évora, Portugal
Rana JS, Dixon AFG, Jarosik V (2002) Costs and benefits of prey specialization in a generalis insect predator. J Anim Ecol 71:15–22
Satar G, Uygun N (2012) The effects of various temperatures on development and fecundity of Scymnus subvillosus (Goeze) (Coleoptera: Coccinellidae) feeding on Aphis gossypii Glover (Hemiptera: Aphididae). Türk biyo müc derg 3:169–182
Seagraves MP (2009) Lady beetle ovipositon behaviour in response to the tropic environment. Biol Control 51:313–322
Skirvin DJ, Fenlon JS (2003) The effect of temperature on the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae). Exp Appl Acarol 31:37–49
Soares AO, Coderre D, Schanderl H (2001) Fitness of two phenotypes of Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 98:287–293
Soares AO, Elias RB, Resendes R, Figueiredo H (2003a) Contribution to the knowledge of the Coccinellidae (Coleoptera) fauna from the Azores islands. Arquipélago 20:47–53
Soares AO, Coderre D, Schanderl H (2003b) Effect of temperature and intraspecific allometry on predation by two phenotypes of Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Environ Entomol 32:939–944
Soares AO, Coderre D, Schanderl H (2004) Dietary self-selection behaviour by the adults of the aphidophagous ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae). J Anim Ecol 73:478–486
Soares AO, Coderre D, Schanderl H (2005) Influence of prey quality on the fitness of two phenotypes of Harmonia axyridis adults. Entomol Exp Appl 114:227–232
Soares AO, Borges I, Cabral S, Figueiredo H, Resendes R (2006) New Records of Coccinellidae (Coleoptera) to the Azores islands. Rel Com Depart Biol 20:87–91
Tawfik MFS, Abdul-Nasr S, Saad BM (1973) The biology of Scymnus interruptus Goeze (Coleoptera: Coccinellidae). Bull Entomol Soc Egypt 57:9–26
Turgill DL (1995) Why do tropical poikilothermic organisms tend to have a higher threshold temperature for development than temperate ones? Funct Ecol 9:136–137
Ungerová D, Kalushkov P, Nedvěd O (2010) Suitability of diverse prey species for development of Harmonia axyridis and the effect of container size. IOBC/WPRS Bull 58:165–174
Uygun N, Atlıhan R (2000) The effect of temperature on development and fecundity of Scymnus levaillanti. BioControl 45:453–462
Wanntorp HE (2004) “Musical chairs”: the Swedish species of Scymnus subg. Neopullus (Coleoptera, Coccinellidae) change places. Entomol Tidskrift 125:103–109
Woin N, Volkmar C, Ghogomu T (2006) Numerical response of predatory ladybirds (Coccinellidae) to aphid outbreaks and their diversity in major rice ecosystems of Cameroon. Arch Phytopathol Plant Protect 39:189–196
Zar JH (1996) Biostatistical analysis. Prentice-Hall, London, UK
Acknowledgments
This study was partially funded by a grant from the Direção Regional dos Recursos Florestais of Secretaria Regional dos Recursos Naturais, Governo Regional dos Açores, Portugal to the research projects: PICA: i) Projeto Investigação para Combate a Afídeos em Viveiros Florestais (Direção Regional dos Recursos Florestais, Secretaria Regional dos Recursos Naturais) and ii) “PRO-BIO: Profiling Reliable Organisms as Bioindicators: an integrated approach for island systems” financed by the FLAD (Fundação Luso-Americana para o Desenvolvimento).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq.
Rights and permissions
About this article
Cite this article
Sebastião, D., Borges, I. & Soares, A.O. Effect of temperature and prey in the biology of Scymnus subvillosus . BioControl 60, 241–249 (2015). https://doi.org/10.1007/s10526-014-9640-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-014-9640-5