Abstract
This work aimed to study the biology of Clitostethus arcuatus (Rossi) (Coleoptera: Coccinellidae) under different temperatures and evaluate the optimum temperature for its mass rearing. Studies were carried out in the laboratory at four constant temperatures (15°C, 20°C, 25°C and 30°C), 75 ± 5% relative humidity and a photoperiod of 16 h light:8 h dark, in which C. arcuatus was fed ad libitum with nymphs of all instar of Aleyrodes proletella L. (Homoptera: Aleyrodidae) on Brassicae oleracea L. (var. Costata). The following biological parameters were evaluated: development time and survival rates of pre-imaginal stages, adult longevity (female and male), length of the pre-oviposition and oviposition periods, fecundity, fertility and percentage of egg hatching. Population growth parameters, the lower development threshold and the sum of effective temperatures were estimated. Temperatures ranging from 20°C to 30°C were suitable for the development of C. arcuatus, suggesting that this species is well adapted to the temperatures usually found inside greenhouses or in open fields in temperate regions. Although the intrinsic rate of natural increase and doubling time were similar at 25°C and 30°C, the temperature of 25°C was shown to be the most suitable for mass rearing and development of populations under field conditions, since the percentage of egg hatching and the accumulated survival rates of the pre-imaginal stages were the highest. Considering the estimated lower threshold for pre-imaginal development (7.9°C) and the sum of effective temperatures [293.6 degree-days (°D)], it is predicted for Ponta Delgada (Azores, Portugal) that the first adults of C. arcuatus should emerge by the first fortnight of February and that up to 12 generations per year can occur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some whitefly species are considered to be major pests, by inflicting important crop losses worldwide, especially to horticultural plants in greenhouses (Mound and Halsey 1978; Evans and Castillo 1998; Katsoyannos et al. 1998; Martin 1999; Symondson et al. 1999; Liang and Liu 2002). The damages are caused by the insects’ direct feeding, which implies the extraction of large amounts of phloem sap. In consequence, there is a decrease of plant vigour and a great excretion of honeydew that serves as a medium for the development of black sooty mould fungi, which, in turn, interfere with photosynthesis. In addition, those whiteflies are involved in transmission of several plant viruses (Drost et al. 1998; Symondson et al. 1999; Viscarret et al. 2000; Liang and Liu 2002).
Among the Aleyrodidae found in the Azores, the citrus whitefly Aleurothrixus floccossus (Maskell), the greenhouse whitefly Trialeurodes vaporariorum (Westwood) and the cabbage whitefly Aleyrodes proletella L. (Homoptera: Aleyrodidae), are the most abundant species. In the Azores A. proletella is only referred for S. Miguel and Terceira islands (Borges et al. 2005). This species is also present in mainland Portugal (Guimarães 1996) and in several European countries (Mound and Halsey 1978).
The strategy of using chemical control has been proven to be ineffective, due to the whiteflies’ great reproductive potential, the difficulty of the growers to detect the small and translucent nymphs in the early stages of infestations and the negative impacts on biological control agents. Moreover, generally, the insecticides are not equally effective over all developmental stages of the insects (Wagner et al. 1991); therefore, it is very difficult to synchronize the spraying of insecticides with the specific developmental stage present on the crop. The increasing problems driven from chemical pesticides have led to a rising interest in classical biological control and integrated pest management (IPM) to control whitefly populations. The introduction of exotic natural enemies to control whitefly may be a possible strategy, but a number of studies have revealed that introduced species may affect the dynamics and the composition of native guilds, as the invaders may interact with them, directly or indirectly, including through intraguild predation (IGP) (Secord and Kareiva 1996; Evans 2004; Lucas 2005; Soares and Serpa 2007). Since biological invasions are considered to be the second greatest cause of global biodiversity loss after direct habitat destruction (Pimentel et al. 2000; Labrie et al. 2006), and, in small and isolated islands the lower species richness and abundance than in continental ecosystems increase the risk of biodiversity loss (Van Driesche and Hoddle 1997), the use of native natural enemies instead of the introduction of new species is strongly recommended.
Recent results on the suitability of the cabbage whitefly as prey for Coccinella undecimpunctata L. (Coleoptera: Coccinellidae), a predator present in the Azores, showed that this ladybird beetle did not thrive on A. proletella and, therefore, should not be used as a main biological control agent against this pest (Cabral et al. 2006).
Considering the above-mentioned reasons, we have selected another predator, also present in the Azores, that feeds primarily on whiteflies, the ladybird beetle Clitostethus arcuatus (Rossi) (Coleoptera: Coccinellidae) (Fürsch 1987; Soares et al. 2003; Borges et al. 2005). This species is widespread over central and meridional Europe, the Mediterranean region, Asia and North America (Raimundo and Alves 1986).
This study aimed to determine the effects of constant temperatures (15°C, 20°C, 25°C and 30°C) on biological and population growth parameters of the predator and to evaluate the optimum temperature for mass rearing of C. arcuatus. Thermal requirements for development were also determined so that we could estimate the annual number of generations that this species can achieve in its natural habitat.
Material and methods
Insects
Clitostethus arcuatus adults were collected on S. Miguel Island, Azores, Portugal, early in the summer before the experiments took place. Ladybird beetles were reared on a tri-trophic system (host plant Brassicae oleracea L. (var. Costata); prey A. proletella; predator C. arcuatus), inside a climate chamber at 25 ± 1°C, 75 ± 5% relative humidity and a photoperiod of 16 h light:8 h dark (16L:8D) using fluorescent lamps (Phillips TDL 23W/54 and TDL18W/54).
Biological parameters
The methodology used in this study was adapted from Soares et al. (2001). All experiments were done inside climate chambers at constant temperatures of 15°C, 20°C, 25°C and 30°C. Photoperiod was maintained at 16L:8D, while relative humidity was kept at 75 ± 5% inside each climate chamber.
For each temperature, at least 20 eggs of C. arcuatus (obtained from adults of the tri-trophic rearing system) were isolated inside Petri dishes (ø 5 cm, height 3 cm). Upon hatching, neonate larvae were fed ad libitum with mixed instar nymphs of A. proletella provided on cabbage leaves. Every 2 days, the food supply was replaced until adult emergence. To determine the pre-imaginal development times and accumulated survival rates, we observed individuals from egg to adult twice a day (09:00 h and 17:00 h).
To evaluate the longevity and reproductive parameters of the adults, we sexed and paired (N = 20) newly emerged individuals. Each pair was placed inside a 60 ml Petri dish (ø 5 cm, height 3 cm) and fed ad libitum with mixed instar nymphs of A. proletella provided on cabbage leaves. Every day, the food supply was replaced until the death of the adults. Each couple was observed daily so that we could record their longevity, the length of pre-oviposition and oviposition periods and the fecundity (number of laid eggs). The fertility (number of eggs with embryos) and percentage of egg hatching (percentage of eggs with embryos that hatched) were determined.
Population growth parameters
For each temperature, the following population growth parameters were estimated: the net reproductive rate (R0 = Σ l x m x ), the mean generation time (T), the intrinsic rate of natural increase (rm = lnR0(T)−1), the finite rate of increase (λ = er) and the doubling time (DT = ln2/rm). The l x corresponds to the age-specific survival of the females and the m x to the age-specific fertility (= born larvae/female). The expected number of larvae produced per female each day (l x m x ) was also estimated (Southwood and Henderson 2000; Vasicek et al. 2004; Kontodimas and Stathas 2005).
Thermal requirements
The lower development threshold (LDT) and the sum of effective temperatures (SET) were estimated using the development times of the pre-imaginal stages at each constant temperature (15°C, 20°C, 25°C and 30°C). It was assumed that, within the interval of temperatures favourable for the insects’ development, the relationship between the inverse of the development time (development rate) and the temperature is nearly linear (Honĕk and Kocourek 1990). According to this, the following linear relationship was considered:
where DT corresponds to the development time of the pre-imaginal, which is inversely proportional to the temperature (T), and a and b are the regression parameters. The LDT corresponds to T, when no development occurs, that is, when 1/DT = 0 (Honĕk and Kocourek 1988). The SET [degree-days (°D)] was calculated according to the following expression:
where DT x is the development time at temperature x and LDT X is the lower development threshold for the temperature x.
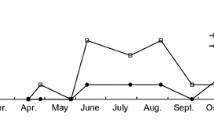
To estimate the number of generations of C. arcuatus that can occur in Ponta Delgada (Azores, Portugal), we used calendar monthly averages of temperature resulting from 12:00 GMT daily readings recorded during a 30-year period (1951–1980) provided by the Institute of Meteorology/Azores.
Statistical analysis
To correct heteroscedasticity, data regarding pre-imaginal development time, adult longevity, and pre-oviposition and oviposition periods were transformed by log (x), and fecundity and fertility data by log (x + 1) prior to analysis of variance (ANOVA) (Hill and Hill 2002). Where statistical differences existed between data sets (P < 0.05), Fisher’s least significant difference (LSD) tests were used to separate the differing means. Accumulated survival rates (from egg to adulthood) and percentage of egg hatching were analysed by the multiple comparison test for proportions, where significant results are represented by giving a q 0.05,∞,4 value > 3.633 (Zar 1996). All analyses were performed with SPSS, 12.0.1 for Windows (SPSS 2004). The values displayed are regular averages and standard errors.
Results
Biological parameters
Within the range of temperatures tested, and with the exception of the third and fourth larval stages reared at 25°C and 30°C, the pre-imaginal development times decreased significantly with temperature increase, the total time of development being longer at 15°C (44.3 ± 2.25 days) and shorter at 30°C (13.2 ± 0.58 days) (Table 1).
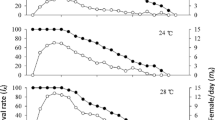
Accumulated survival rate was significantly higher at the temperature of 25°C (56%) than at the other temperatures (q = 7.32, q = 6.15 and q = 9.15, P < 0.05, respectively for 15/25°C, 20/25°C and 25/30°C comparisons). No significant differences (all P > 0.05) were observed between accumulated survival rates at 15°C (21%), 20°C (26%) and 30°C (14%) (Fig. 1).
Survival rates of C. arcuatus pre-imaginal stages under different constant temperatures (15°C, 20°C, 25°C and 30°C). L1, L2, L3 and L4 first, second, third and fourth larval stages, respectively, PP + P pre-pupa and pupa. *Values with different letters are significantly different at P < 0.05 (multiple comparison tests for proportions)
Adult male mean longevity was significantly higher at 15°C, but it did not differ significantly between the other temperatures tested (Table 2). Adult female mean longevity also decreased with temperature increase, being significantly higher at 15°C than was the longevity observed at 25°C and 30°C (Fig. 2, Table 2). Independently of temperature and age, the age-specific survival curve of females (l x ) showed a constant rate of mortality corresponding to a typical type II hypothetical survivorship curve (Fig. 2).
The pre-oviposition period decreased significantly with temperature increase, while oviposition period was significantly higher at 20°C than at the other temperatures. Fecundity did not differ significantly between the temperatures of 20°C, 25°C and 30°C, but it was significantly lower at 15°C (Table 2). However, the expected number of larvae produced per female each day (l x m x ) differed between all temperatures: at 15°C the females maintained a constant and low rate of larvae production, while at 25°C and 30°C the number of expected larvae peaked during the first 15 days of their lives; at 20°C two peaks of larvae production were identified (Fig. 2).
Percentage of egg hatching was significantly higher at 25°C (97.06%) than at the other temperatures (q = 10.46, q = 6.28 and q = 7.43, P < 0.05, respectively for 15/25°C, 20/25°C and 25/30°C comparisons).
Population growth parameters
The net reproductive rate (R0) was higher when C. arcuatus was maintained under 20°C; However, the intrinsic rate of natural increase (rm) and the finite rate of increase (λ) were higher and the doubling time (DT) was lower at the rearing temperatures of 25°C and 30°C (Table 3).
Thermal requirements
Within the range of temperatures tested, the LDT (°C) and the SET (°D) for C. arcuatus were effectively determined by the linear model, as shown by the high coefficients of determination obtained for all the developmental stages (all R2 > 0.94) (Table 4). The lower development thresholds of C. arcuatus pre-imaginal stages ranged from 1.7°C (third larval stage) to 9.1°C (first and second larval stages); for the total pre-imaginal development it was 7.9°C (Table 4).
The number of degree-days required for the development of each pre-imaginal stage ranged from 20.9°D (second larval stage) to 75.5°D (pre-pupa and pupa), and to complete the development from egg to adult emergence it was 293.6°D (Table 4).
Discussion
Environmental temperature is one of the most important factors determining physiological process on poikilothermic species (Honĕk and Kocourek 1990; Chapman 1998). Temperature determines the biological performances of insects, such as development time of pre-imaginal stages, life expectation and reproductive parameters of the adults (e.g. fecundity, fertility and percentage of egg hatching), having by this way a differential contribution of each single individual for the population growth (Perdikis and Lykouressis 2002; Kontodimas and Stathas 2005; Cabral et al. 2006). The estimation of the performances of natural enemies, including development time, temperature thresholds, thermal constant and population growth parameters, can contribute to the selection of the most suitable biological control agent to be used under different environmental conditions (Perdikis and Lykouressis 2002), as well to set the best thermal condition for insect mass rearing.
Our results showed that total development time of C. arcuatus pre-imaginal stages significantly decreased with increasing temperature, as expected within the range of favourable temperatures. Furthermore, regardless of temperature, the proportion of the development time of eggs ranged between 22% and 25%, of L1–L4 it ranged between 50% and 56% and of pre-pupa and pupa it ranged between 25% and 29%. These results are consistent with the suggestion that the proportion of the development time of pre-imaginal stages for ladybird beetles is typical of each stage and independent of temperature (Hodek and Honĕk 1996; Dixon 2000).
The development times of pre-pupa and pupa and of the fourth larval stage were higher than those of the other pre-imaginal stages. Liotta (1981) obtained similar results when studying this species under field conditions in Sicily. Bellows et al. (1992), using a population from Israel fed with Siphoninus phillyreae (Haliday) (Homoptera: Aleyrodidae) nymphs and the experimental temperatures of 21.1°C, 28.2°C and 32.2°C, also achieved similar results. The same trend of pre-imaginal development time was observed for other Coccinellidae (Coleoptera), such as Harmonia axyridis Pallas (Schanderl et al. 1985), Coccinella septempunctata L. and Hippodamia convergens Guerin-Meneville (Michels and Behle 1991) and Coccinella trifasciata LeConte (Harris) (Miller and LaMana 1995).
Accumulated survival rates of C. arcuatus pre-imaginal stages were higher at 25°C and lower at 15°C and 30°C. Bellows et al. (1992) reported for C. arcuatus higher rates of survival at 28.2°C (∼80%) and 32.2°C (∼40%) in contrast to our results at 30°C (14%). Although the diets provided in both studies are distinct, the results obtained suggest a possible acclimation of the Azorean populations to the mild environmental temperatures of this archipelago, since the highest survival rate was observed at 25°C.
The fecundity of C. arcuatus was higher at 20°C, while Bellows et al. (1992) observed that the fecundity of this species peaked at 28.2°C. Furthermore, although the percentage of egg hatching varied among the temperature tested, no trend in the variation was observed in the current study, while Bellows et al. (1992) reported a decrease in egg hatching rates with increasing temperatures. Again, these discrepancies could be explained by the possible acclimation of the Azorean populations to the temperatures usually found in their natural habitats that fall into the range of temperatures tested in this study.
According to Hodek and Honĕk (1996), the decrease of the pre-oviposition period with temperature increase is a common trend among coccinellid females that do not undergo diapause. The pre-oviposition period of C. arcuatus at 15°C was much longer than at the other temperatures. One possible explanation for this fact may be related to the thermal requirements for egg maturation in C. arcuatus, suggesting that oviposition and reproduction may be negatively affected at temperatures beneath 15°C.
Considering the estimated lower threshold for pre-imaginal development (7.9°C) and the sum of effective temperatures (293.6°D), we predict for Ponta Delgada (Azores, Portugal) that the first adults of C. arcuatus should emerge during the first fortnight of February and that up to 12 generations per year can occur.
The intrinsic rate of natural increase (rm) and the finite rate of increase (λ) were higher at 30°C and lower at 15°C; nevertheless, the values of these rates were quite similar at 25°C and 30°C. The net reproductive rate (R0) was higher at 20°C and much lower at 15°C. Bellows et al. (1992) estimated for C. arcuatus a maximum net reproductive rate (R0) at 28.2°C (79.18 descendants per female in each generation), which is higher than the obtained in the current study. This discrepancy can be explained by the different nutritional quality of diets provided in both studies. According to several studies, essential foods show varying degrees of favourability to coccinellids, enabling different development rates, survival and fecundity, factors that limit the capacity of a population to grow (Dixon 2000; Soares et al. 2004; Cabral et al. 2006).
Despite rm, T, λ and DT values being quite similar at 25°C and 30°C, the fact is that C. arcuatus at 25°C displays higher larval survival rates, male and female longevities, percentage of egg hatching and number of expected larvae during the first 15 days of its life. For these reasons the optimum temperature for mass rearing of C. arcuatus, using A. proletella as prey and B. oleracea as host plant, is considered to be 25°C. In general the results obtained reveal that C. arcuatus is well adapted to temperatures between 20°C and 30°C, which are quite frequent inside greenhouses and open horticultural crops in Azores.
References
Bellows Jr TS, Paine TD, Gerling D (1992) Development, survival, longevity and fecundity of Clitostethus arcuatus (Coleoptera: Coccinellidae) on Siphoninus phillyreae (Homoptera: Aleyrodidae) in laboratory. Environ Entomol 21:659–663
Borges PAV, Cunha R, Gabriel R, Martins AF, Silva L and Vieira V (eds) (2005) A list of the terrestrial fauna (Mollusca and Arthropoda) and flora (Bryophyta, Pteridophyta and Spermatophyta) from the Azores. Direcção Regional do Ambiente and Universidade dos Açores, Horta, Angra do Heroísmo and Ponta Delgada, p 317
Cabral S, Soares AO, Moura R., Garcia P (2006) Suitability of Aphis fabae, Myzus persicae (Homoptera: Aphididae) and Aleyrodes proletella (Homoptera: Aleyrodidae) as prey for Coccinella undecimpunctata (Coleoptera: Coccinellidae). Biol Control 39:434–440
Chapman RF (1998). The insects: structure and function, 4th edn. Cambridge University Press, UK, p 770
Dixon AFG (2000) Insect predator-prey dynamics. Ladybird beetles and biological control. Cambridge University Press, Cambridge, MA, p 257
Drost YC, van Lenteren JC, van Roermund HJW (1998) Life-history parameters of different biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae) in relation to temperature and host plant: a selective review. Bull Entomol Res 88:219–229
Evans EW (2004) Habitat displacement of North American ladybirds by an introduced species. Ecology 85:637–647
Evans GA, Castillo JA (1998) Parasites of Aleurotrachelus socialis (Homoptera: Aleyrodidae) from Colombia including descriptions of two new species (Hymenoptera: Aphelinidae: Platygasteridae). Fla Entomol 81:171–178
Fürsch H (1987) Die Scymninae der Kanaren, Azoren und Madeira. Acta Coleopterol 3:1–14
Guimarães JM (1996) The diagnostic value of the cement gland and other abdominal structures in aleyrodid taxonomy. Bull OEPP/EPPO Bull 26:413–419
Hill MM, Hill A (2002) Investigação por questionário. Edições Sílabo, Lisboa, p 377
Hodek I, Honĕk A (1996) Ecology of Coccinellidae. Kluwer Academic Publishers, Dordrecht, Netherlands, p 464
Honĕk A, Kocourek F (1988) Thermal requirements for development of aphidophagous Coccinellidae (Coleoptera), Chrysopidae, Hemerobiidae (Neuroptera), and Syrphidae (Diptera): some general trends. Oecologia 76:455–460
Honĕk A, Kocourek F (1990) Temperature and development time in insects: a general relationship between thermal constants. Zool Jb Syst 117:401–439
Katsoyannos P, Kontodimas DC, Stathas GJ (1998) The inundative release of Cales noacki Howard (Hymenoptera: Aphelinidae), for curative treatment of Aleurothrixus floccosus (Maskell) (Homoptera: Aleyrodidae) on heavily infested citrus in Greece. Ann Benaki Phytopathol Inst (N.S.) 18:111–122
Kontodimas DC, Stathas GJ (2005) Phenology, fecundity and life table parameters of the predator Hippodamia variegata reared on Dysaphis crataegi. BioControl 50:223–233
Labrie G, Lucas E, Coderre D (2006) Can development and behavioral characteristics of the multicolored Asian lady beetle Harmonia axyridis explain its invasive success? Biol Inv 8:743–754
Liang G, Liu T (2002) Repellency of a kaolin particle film, surround, and a mineral oil, sunspray oil, to silverleaf whitefly (Homoptera: Aleyrodidae) on melon in the laboratory. J Econ Entomol 95:317–324
Liotta G (1981) Osservazioni bio-etologiche su Clitostethus arcuatus (Rossi) (Col. Coccinellidae) in Sicilia. Redia 64:171–185
Lucas E (2005) Intraguild predation among aphidophagous predators. Eur J Entomol 102:351–364
Martin NA (1999) Whitefly, Biology, identification and life cycle. Crop Food Res Broadsheet No. 91:8
Michels Jr GJ, Behle RW (1991) A comparison of Coccinella septempunctata and Hippodamia convergens larval development on greenbugs at constant temperatures. Southwest Entomol 16:73–80
Miller JC, LaMana ML (1995) Assessment of temperature-dependent development in the general population and among isofemale lines of Coccinella trifasciata (Col.: Coccinellidae). Entomophaga 40:183–192
Mound LA, Halsey SH (1978) Whitefly of the world. A systematic catalogue of the Aleyrodidae (Homoptera) with host plant and natural enemy data. British Museum (Natural History) and Wiley, Chichester, UK, p 340
Perdikis DC, Lykouressis DP (2002) Thermal requirements of the polyphagous predator Macrolophus pygmaeus (Hemiptera: Miridae). Environ Entomol 31:661–667
Pimentel D, Lach L, Zuniga R, Morrisson D (2000) Environmental and economics costs of nonindigenous species in the United States. Bioscience 50:53–65
Raimundo AAC, Alves MLLG (1986) Revisão dos Coccinelídeos de Portugal. Universidade de Évora, Évora, p 103
Schanderl H, Ferran A, Larroque M-M (1985) Les besoins trophiques et thermiques des larves de la coccinelle Harmonia axyridis Pallas. Agronomie 5:417–421
Secord D, Kareiva P (1996) Perils and pitfalls in the host specificity paradigm. Bioscience 46:448–453
Soares A, Serpa A (2007) Interference competition between ladybird beetle adults (Coleoptera: Coccinellidae): effects on the growth and reproductive capacity. Popul Ecol 49:37–43
Soares AO, Coderre D, Schanderl H (2001) Fitness of two phenotypes of Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Eur J Entomol 98:287–293
Soares AO, Elias RB, Resendes R, Figueiredo H (2003) Contribution to the knowledge of the Coccinellidae (Coleoptera) fauna from the Azores islands. Arquipélago-Life Mar Sci 20A:47–53
Soares AO, Coderre D, Schanderl H (2004) Dietary self-selection behaviour by the adults of the aphidophagous ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae). J Anim Ecol 73:478–486
Southwood TRE, Henderson PA (2000) Ecological methods. Blackwell Science, Oxford, UK, p 575
SPSS (2004) SPSS Base 12.0.1 for Windows: user’s guide. Chicago, Illinois, USA
Symondson WOC, Gasull T, Liddell JE (1999) Rapid identification of adult whiteflies in plant consignments using monoclonal antibodies. Ann Appl Biol 134:271–276
Van Driesche RG, Huddle M (1997) Should arthropod parasitoids and predators be subject to host range testing when used as biological control agents? Agric Hum Val 14:211–226
Vasicek A, La Rossa F, Paglioni A, Mason SC (2004) Comparación de los parámetros biológicos y demográficos de Nasonovia ribisnigri (Mosley) y Aulacorthum solani (Kaltenbach) (Homoptera: Aphididae) en tres Compositae hortícolas. Bol San Veg Plagas 30:155–161
Viscarret MM, Botto EN, Polaszek A (2000) Whiteflies (Hemiptera: Aleyrodidae) of economic importance and their natural enemies (Hymenoptera: Aphelinidae, Signiphoridae) in Argentina. Rev Chil Entomol 26:5–11
Wagner TL, Olson RL, Willers JL (1991). Modelling arthropod development time. J Agric Entomol 8:251–270
Zar JH (1996) Biostatistical analysis. Prentice-Hall, London, UK, p 662
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mota, J.A., Soares, A.O. & Garcia, P.V. Temperature dependence for development of the whitefly predator Clitostethus arcuatus (Rossi). BioControl 53, 603–613 (2008). https://doi.org/10.1007/s10526-007-9101-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9101-5