Abstract
Aging affects the energy metabolism differently in the cardiac and skeletal muscles. The study aim was to assess the effects of short-term calorie restriction (SCR) and refeeding on the expression of genes involved in the control of cardiac and skeletal muscle energy metabolism in old vs. young male rats. Young (4 mo) and old (24 mo) rats were subjected to 60% SCR for 30 days, and refed ad libitum for 2 or 4 days. In the cardiac (CM) and skeletal muscles (SM) we compared the gene expression (qPCR) of carnitine palmitoyltransferase-I (Cpt-I), peroxisome proliferator-activated receptor beta/delta (Ppar-β/δ), glucose transporter 4 (Glut4), peroxisome proliferator-activated receptor-γ coactivator-1α (Pgc-1α), and sirtuin 3 (Sirt3). In CM, aging increased Cpt-I expression but did not affect the other genes. In SM, Cpt-I, Glut4, Pgc-1α, and Sirt3 mRNA levels were lower in old than young rats. In CM of only young rats SCR increased Cpt-I expression which remained elevated after refeeding. Upon SCR, the expression of Ppar-β/δ, Glut4, Pgc-1α, and Sirt3 in CM increased in young but not old rats, and refeeding re-established control levels. In SM of young rats SCR increased Ppar-β/δ and Pgc-1α, and decreased Sirt3 expression, whereas refeeding generally decreased these mRNA levels. In SM of old rats SCR decreased only Pgc-1α expression. The adaptive response to SCR and subsequent refeeding is muscle tissue-specific and differs in young and old male rats. SCR appears to increase the efficiency of glucose and fatty acid utilization in the cardiac muscle of young, but not old male rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During aging regulatory mechanisms of carbohydrate and lipid homeostasis undergo distinct alterations that can be modulated by nutritional or pharmacological manipulations.
The mechanisms of body mass regulation have become an important object of animal and human studies in the context of both ‘epidemic of obesity’ and increase of the elderly population (Colleluori and Villareal 2021). In old people, there is an increasing prevalence of elevated body mass index (BMI), impaired glucose tolerance, insulin resistance leading to type 2 diabetes, other features of metabolic syndrome, and cardiovascular disease. In the treatment of obesity regular physical activity and reduction of the caloric intake are being advocated for the majority of patients (Zubrzycki et al. 2018). Nonetheless, weight loss in the elderly carries the risk of concomitant loss of lean body mass and bone mineral density (Colleluori and Villareal 2021). Long-term moderate calorie restriction (CR) in humans improves metabolic health and slows biological aging (Kebbe et al. 2021). However, even short-term CR may result in positive metabolic outcomes, including improved cardiovascular risk factors, insulin sensitivity, and antioxidant capacity (Kanikowska et al. 2021; Vion et al. 2021).
Sirtuins, which are NAD+-dependent protein deacetylases with critical roles in metabolism, stress responses, and aging processes, are also key players in CR-induced metabolic improvements (Hebert et al. 2013; Tauriainen et al. 2011). Among the seven mammalian sirtuins (SIRT1–SIRT7), the primarily mitochondrial SIRT3 deacetylates multiple mitochondrial enzymes in response to nutrient stresses such as fasting and CR (Hebert et al. 2013; Hirshey et al. 2010). In this way, SIRT3 regulates mitochondrial fatty acid β-oxidation (Hirschey et al. 2010), ketone body production (Shimazu et al. 2010), the urea cycle (Hallows et al. 2011), antioxidant capacity (Qiu et al. 2010), and multiple other processes (Hebert et al. 2013).
Importantly, SIRT3 activity may mitigate metabolic dysfunctions that are often associated with aging in the cardiac and skeletal muscles (Cao et al. 2022; Vargas-Ortiz et al. 2019). Aging of the cardiac muscle is associated with impairment of mitochondrial abundance and function, decreased fatty acid oxidation, cardiac lipotoxicity, and accumulation of reactive oxygen species (ROS), leading to increased risk of cardiovascular disease (Sithara and Drosatos 2021). Age-related changes in skeletal muscles include the loss of muscle mass and strength (sarcopenia), decreased protein synthesis and increased protein degradation, mitochondrial dysfunction, chronic low-grade inflammation, and insulin resistance (Bilski et al. 2022).
In the heart and skeletal muscles, energy metabolism is strictly regulated and depends on the amount and type of substrates. Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is involved in glucose and fatty acid metabolism, fibre type switching in skeletal muscle, cardiac diseases, and other processes (Cheng et al. 2018). In both types of muscles, PGC-1α co-activates peroxisome proliferator-activated receptor beta/delta (PPAR-β/δ) to modulate the transcription of genes involved in fatty acid oxidation and oxidative phosphorylation, as well as glucose uptake (Arany et al. 2005; Kleiner et al. 2009; Park et al. 2020; Wang et al. 2010). Studies in mice with inducible knockout of Ppar-β/δ revealed that in the adult heart PPAR-β/δ deficiency decreased mitochondrial biogenesis and reduced tissue levels of PGC-1α, resulting in attenuated fatty acid and glucose oxidation (Wang et al. 2010). Activation of PPAR-β/δ increases the transcription of genes involved in fatty acid catabolism in skeletal muscles of mouse (Dressel et al. 2003) and human (Krämer et al. 2007). PGC-1α and PPAR-β/δ co-activate the transcription of many genes, including the one encoding carnitine palmitoyltransferase- I (CPT-I), a crucial enzyme for oxidation of fatty acids as the primary energy source in muscles (Liu et al. 2007). CPT-I, located in the mitochondrial outer membrane, catalyzes the transfer of long chain acyl groups from coenzyme A to carnitine. In this form, the acyl groups pass into the mitochondrial intermembrane space, and further into matrix where they may be broken down via β-oxidation (Liu et al. 2007).
Aging is associated with increased glucose utilization as energy substrate for the heart (Sithara and Drosatos 2021). Glucose uptake in the cardiac and skeletal muscles takes place predominantly through the insulin-dependent glucose transporter type 4 (GLUT4) (Aerni-Flessner et al. 2012; Kern et al. 1990). Insulin stimulation translocates intracellular GLUT4 storage vesicles to the plasma membrane (Kern et al. 1990). Moreover, skeletal muscles play a critical role in glucose homeostasis. Since muscles comprise about 42% of body mass in normal-weight adults, they are responsible for most of the whole body insulin-induced glucose disposal (Bilski et al. 2022).
Although short-term calorie restriction (SCR) has been advocated as a weight-loss regimen in overweight or obesity (Kanikowska et al. 2021; Vion et al. 2021; Zubrzycki et al. 2018), its molecular effects may also be affected by aging. The metabolism of cardiac and skeletal muscles strongly affects health and is subjected to age-related changes. Therefore, we decided to assess the effects of 60% CR lasting 30 days in these muscle types in young and old male rats. The duration of CR was chosen to approximately reflect 4–6 month-long CR regiments in humans (Zubrzycki et al. 2018). We measured the blood serum concentrations of key metabolites and hormones reflecting whole-body metabolic outcomes. Next, we focused on SCR’s effects on cardiac and skeletal muscle expression levels of key genes involved in mitochondrial fatty acid β-oxidation (the rate-limiting enzyme Cpt-I and its transcriptional regulator Ppar-β/δ) and glucose uptake (Glut4 and its transcriptional regulator Pgc-1α). In addition, we analysed the gene and protein expression of SIRT3 as a crucial regulator of mitochondrial metabolism and effector of CR. We also measured the effects of refeeding SCR-subjected rats with full-diet for two or four days. Our results provide new data on aging-related differences in the response to SCR and refeeding in skeletal and cardiac muscles of male rats.
Materials and methods
Animals and tissue collection
The experiment was performed on young (4-month-old at the onset of the experiment) and old (24-month-old) male Wistar-Han rats maintained at Specific Pathogen Free conditions in the Academic Laboratory Animal Centre in Gdansk, Poland, at 22 °C under a 12 h/12 h light/dark cycle. Littermates were housed in cages individually or in pairs of similar weight. All animals were fed a standard, sterilized chow, which provided 52% of metabolic energy from carbohydrates, 11% from fat, and 37% from proteins (Labofeed B; Wytwornia Pasz Morawski, Kcynia, Poland). Rats had free access to water throughout the experiment. Food intake was measured every second day during 3-week-long pre-trial period to determine the baseline food intake, which was approximately 21 g/day and 25 g/day for the young and old rats, respectively. Rats from control groups (C; n = 16 and n = 12, young and old, respectively) were fed ad libitum during the whole experiment. Calorie-restricted animals received pre-weighted portions of chow equivalent to 60% of the baseline food intake, for 30 days on a daily basis, 2 h after lights on, and it was found that they consumed entire daily portions. At the end of the SCR period some animals were euthanized (SCR group; n = 20/14, young/old) while others were refed ad libitum for 2 (SCR + 2; n = 14/14) or 4 days (SCR + 4; n = 14/14). Under full anaesthesia (90 mg/kg ketamine and 10 mg/kg xylazine, i.p.), blood was collected from the heart, followed by decapitation. Tissue samples of cardiac muscle and vastus lateralis muscle (mixed fast-twitch oxidative-glycolytic fibres) were collected, immediately frozen in liquid nitrogen, and stored at − 80 °C until analyses. All animal experimental procedures had been authorized by the Local Ethical Committee in Gdansk (protocol no 11/2011) and were conducted in agreement with the institutional and European Community guidelines.
Gene expression
Frozen tissues samples of the cardiac and skeletal muscles were homogenised (MagNALyser homogenizer, Roche Diagnostics, Indianapolis, IN, USA) and total RNA was extracted from homogenates using the Total RNA kit (A&A Biotechnology, Gdynia, Poland). 2 µg of RNA were reverse transcribed to cDNA with the RevertAid kit (Thermo Fischer Scientific, Fitchburg, WI, USA) and oligo(dT)18 primers (Sigma-Aldrich, Munich, Germany). Relative quantification of Cpt-I, Glut4, Pgc-1α, Ppar-β/δ, and Sirt3 genes’ expression was performed by real-time PCR (StepOne Plus thermal cycler, Applied Biosystems-Life Technologies, Grand Island, NY, USA), using SybrGreen Master Mix (A&A Biotechnology). The mRNA levels were assessed relative to the mean expression of housekeeping genes: acidic ribosomal phosphoprotein P0 (36B4) and cyclophilin A (CycloA), according to the 2−ΔΔCT method (Livak and Schmittgen 2001). The primers were designed using Primer3Plus software based on BLAST, ENSMBL, and AceView databases. The primer pairs were (5′→3′): Cpt-I ATGTTTGACCCAAAGCAGTACCCC and TCGCCTGCGATCATGTAGGAAAC; Glut4 AGGCCGGGACACTATACCCTATTC and AAACTGAAGGGAGCCAAGCACAG; Pgc-1α CACGTTCAAGGTCACCCTACAGC and TAAATCACACGGCGCTCTTCAAT; Ppar-β/δ ACAAGGCCTCAGGCTTCCACTAC and TCCGATCGCACTTCTCGTACTTG; Sirt3 AAGCTGGTTGAAGCTCATGGGTC and TCCAGGGAGGTCCCAAGAATGAG; 36B4 CTCAGTGCCTCACTCCATCA and GGGGCTTAGTCGAAGAGACC; CycloA TGTCTCTTTTCGCCGCTTGCTG and CACCACCCTGGCACATGAATCC.
SIRT3 protein measurement by western blotting
Semi-quantification of SIRT3 protein levels was performed in duplicate for every young and old rats’ group. Protein samples were obtained through tissue lysis with Mammalian Cell Extraction Kit (BioVision, Milpitas, CA, USA), then separated by 10% SDS-PAGE, transferred to PVDF membranes (Invitrogen-Life Technologies, Carlsbad, CA, USA), and blocked with 5% non-fat milk in TBS. The membranes were incubated with anti-SIRT3 antibodies (SAB5700222, Merck Millipore, Saint Louis, MI, USA) at 4 °C overnight. Following washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (A9169, Merck Millipore, Burlington, MA, USA) for 2 h at room temperature. GAPDH protein levels were measured as loading control (G9295, Merck Millipore). Bands were visualized using Chemiluminescent Peroxidase Substrate (Merck Millipore) and developed against light-sensitive X-ray films. QuantityOne software (Bio-Rad, Hercules, CA, USA) was used for densitometric analysis.
Determination of carnitine palmitoyltransferase I activity
CPT-I activity was measured in cardiac and skeletal muscles of control and SCR rats (5 animals per group). 100 mg tissue samples were minced and homogenised in a Dounce homogenizer in 1 ml of cold buffer (pH 7.4), containing 250 mM sucrose, 10 mM TRIS, and 0.5 mM EDTA. Homogenates were centrifuged at 300 g for 3 min at 4 °C. CPT-I activity and total protein content were measured in the supernatant at 412 nm at 37 °C using a spectrophotometer with a thermostatic holder (Beckman Coulter Inc., USA). The reaction mixture was composed of 1 M Tris–HCl (pH 8.0), 1 mM DTNB, 100 µM palmitoyl-CoA, and 50–100 µl of supernatant. The enzyme activity was calculated as the difference in the absorbance of two measurements: with or without 1.67 mM l-carnitine, in relation to the total amount of protein in the tissue homogenates (nmol/ min/ mg protein).
Measurements of serum concentrations of metabolites
Blood samples were allowed to clot and were subsequently centrifuged (2000 g, 15 min, 4 °C). Sera were frozen at − 80 °C until analyses. Measurements of the concentrations of glucose, free fatty acids (FFA), triglycerides (TG), total cholesterol (Ch), HDL-cholesterol (HDL), urea, and albumin in rat blood sera were performed by commercially available standard diagnostic tests at the Clinical Laboratory of the Medical University of Gdansk, Gdansk, Poland.
Serum leptin and insulin concentrations
The concentrations of leptin and insulin in blood sera were measured by rat-specific ELISA tests (Merck Millipore, St Charles, MO, USA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism v.6 (GraphPad Software, San Diego, CA, USA). Because not all sets of data followed normal distribution (Shapiro-Wilk test), analyses of group pairs were performed using the Mann–Whitney test. Correlation coefficients between two groups were calculated using Spearman rank correlation test. Data are presented as mean ± SEM. Statistical significance was considered for p < 0.05.
Results
Body mass and concentrations of metabolites, leptin and insulin in blood serum
Mean body mass was substantially higher in old vs. young animals (526 ± 11.2 g and 375 ± 6.1 g, respectively, p < 0.001). The body mass decreased after SCR by 6.1% and 10.9% in young and old rats, respectively (p < 0.001 for both). Young rats restored the pre-SCR body mass after 2 days of ad libitum feeding, whereas the body mass of old rats was lower than the pre-SCR by 7.4% (p < 0.01) after 4 days of refeeding.
Table 1 presents the blood serum levels of major biochemical factors and hormones in control and treated rats. In old as compared to young control rats, glucose and insulin concentrations were comparable, TG, FFA, Ch, HDL, total protein, and leptin concentrations were higher, whereas urea and albumin concentrations were lower in old rats (Table 1).
SCR decreased serum glucose concentration by 42% and 32% in young and old rats, respectively (Table 1). Subsequent ad libitum feeding increased the concentration in young rats; however, in old rats glucose remained below control level throughout 4 days of refeeding. Insulin concentration in young rats decreased after SCR and reverted to control level after 2 days of refeeding. In old rats, insulin concentration decreased after SCR, returned to control values after 2 days of refeeding, but decreased again after 4 days.
SCR decreased TG concentration by 48% and 63% in young and old rats, respectively (Table 1). Two days of ad libitum feeding increased the concentration by 22% and 53%, but did not restore the control levels throughout 4 days of refeeding. SCR and refeeding had no effect on serum FFA concentration in young rats (Table 1). In old rats the FFA concentration increased by 45% after SCR, and reverted to control level upon refeeding.
HDL-cholesterol decreased after SCR by 27% in young rats and by 31% in the old ones (p < 0.01 for both; Table 1). Refeeding of young rats increased the HDL-cholesterol to control levels, however, in old rats the concentration remained below control throughout 4 days of refeeding (p < 0.01). The TG/HDL ratio decreased after SCR by 27% (p < 0.05) and 38% (p < 0.001) in young and old rats, respectively, and increased upon refeeding up to control levels.
Cholesterol, urea, and albumin concentrations in blood serum were unaffected by SCR and refeeding in either age group (Table 1). SCR caused small but significant decreases in total protein concentrations in blood serum: by 4.3% (p < 0.001) in young rats and by 3.1% (p < 0.05) in old animals. Control protein level was restored by refeeding in old rats, but not in the young ones.
SCR reduced serum leptin concentrations by 73% and 42% in young and old rats, respectively (Table 1). Refeeding elevated leptin levels, however, did not restore the pre-SCR values throughout 4 days of refeeding.
Age-related differences in gene expression between cardiac and skeletal muscles
In cardiac muscle, Cpt-I gene expression was higher by 75% in control old than young rats (Fig. 1a), and CPT-I activity tended to increase in old rats (p = 0.06) (Fig. 1e). There were no differences in the mRNA levels of Ppar-β/δ (Fig. 1c), Glut4, Pgc-1α (Fig. 2a, c), and Sirt3 (Fig. 3a). In skeletal muscle, Cpt-I gene expression was lower in control old rats (Fig. 1b), even though CPT-I enzymatic activity was unaffected by aging (Fig. 1e). Ppar-β/δ expression did not differ between young and old animals (Fig. 1d), whereas Glut4 and Pgc-1α (Fig. 2b, d) mRNA levels were 2-fold lower in control old rats. Sirt3 expression was lower by 44% in skeletal muscle of old as compared to young rats (Fig. 3b).
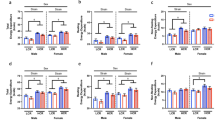
Effects of short-term calorie restriction and refeeding on gene expression of Cpt-I and Ppar-β/δ (a–d) and CPT-I activity (e) in the cardiac and skeletal muscles. The mRNA expression levels were normalised to young control animals (expression level = 1); data are means ± SEM of 6–20 rats per group. C control rats, SCR short-term calorie restricted animals, SCR + 2 calorie-restricted and refed for 2 days, SCR + 4 calorie-restricted and refed for 4 days. Statistical significance: a vs. control rats (of the same age), b vs. calorie restricted animals, c vs. animals subjected to calorie restriction and refed for 2 days, *p < 0.05, **p < 0.01, ***p < 0.001
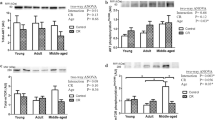
Effects of short-term calorie restriction and refeeding on gene expression of Glut4 (a, b) and Pgc-1α (c, d) in the cardiac and skeletal muscles. The mRNA expression levels were normalised to young control animals (expression level = 1); data are means ± SEM of 6–20 animals per group. C control rats, SCR short-term calorie restricted animals, SCR + 2 calorie-restricted and refed for 2 days, SCR + 4 calorie-restricted and refed for 4 days. Statistical significance: a vs. control rats (of the same age), b vs. calorie restricted animals, c vs. animals subjected to calorie restriction and refed for 2 days, *p < 0.05, **p < 0.01, ***p < 0.001
Effects of short-term calorie restriction and refeeding on sirtuin 3 gene (a, b) and protein (c, d) expression in the cardiac and skeletal muscles. a, b Sirt3 mRNA expression levels were normalised to young control animals (expression level = 1); data are means ± SEM of 6–20 animals per group. c, d SIRT3 protein levels were measured in duplicate relative to GAPDH and analysed semi-quantitatively through densitometry; representative blots are shown. C control rats, SCR short-term calorie restricted animals, SCR + 2 calorie-restricted and refed for 2 days, SCR + 4 calorie-restricted and refed for 4 days. Statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001
Effects of SCR and refeeding on gene expression in cardiac and skeletal muscles of young and old rats
In the cardiac muscle of young rats, SCR increased Cpt-I expression by 90% (Fig. 1a) and CPT-I activity by 100% (Fig. 1e). In old SCR animals, Cpt-I expression (Fig. 1a) and the enzyme activity (Fig. 1e) in the cardiac muscle were unaffected by the diet, while 4-day refeeding decreased Cpt-I expression by 30%. In skeletal muscle of young rats, Cpt-I mRNA level and CPT-I activity were unaffected by SCR, while refeeding for 2 days decreased Cpt-I expression by 46% (Fig. 1b, e). In the old rats’ skeletal muscle SCR and refeeding did not change Cpt-I expression (Fig. 1b) or enzymatic activity (Fig. 1e).
In cardiac muscle of young rats, Ppar-β/δ expression increased by 70% after SCR, and decreased to control level upon refeeding, whereas in old rats there were no changes (Fig. 1c). In skeletal muscle of young rats SCR caused a 70% increase in Ppar-β/δ expression, which decreased upon refeeding to a level 79% lower than control after 4 days, while in old rats there were no changes (Fig. 1d).
In cardiac muscle of young rats, SCR increased Glut4 expression by 97%, and refeeding decreased the expression to control level (Fig. 2a). In contrast, the treatments did not affect the cardiac expression of Glut4 in old rats. In skeletal muscle of young rats SCR did not affect, while refeeding decreased Glut4 expression (Fig. 2b). In skeletal muscle of old rats there were no changes in Glut4 mRNA levels upon treatment (Fig. 2b).
In cardiac muscle of young rats, SCR increased Pgc-1α expression by 230%, and refeeding decreased the expression to control level (Fig. 2c). In old rats, Pgc-1α expression did not change upon SCR, but increased by 70% after refeeding for 2 days, and decreased to control level after 4 days (Fig. 2c). In skeletal muscle of young rats, SCR increased Pgc-1α expression by 75%, and refeeding decreased the expression below control level (Fig. 2d). In old rats, SCR decreased Pgc-1α expression 2.5-fold, and refeeding for 4 days restored the control level.
In cardiac muscle of young rats on SCR, the mRNA level of Pgc-1α strongly correlated with Glut4 expression (r = 0.82, p = 0.00002), however, not in the old SCR animals. In skeletal muscle of old SCR rats the expression of Pgc-1α positively correlated with the expression of Glut4 (r = 0.79, p = 0.02), however, no significant correlation was found for the young SCR animals.
In cardiac muscle, SCR increased Sirt3 mRNA expression by 288% and 45% in young and old rats, respectively (p < 0.05 and p = 0.18, respectively; Fig. 3a). Refeeding for 2 and 4 days restored control levels of Sirt3 expression in both age groups. Similarly, SIRT3 protein levels were upregulated by about 50% after SCR and reverted to control levels after refeeding of both young and old rats (Fig. 3c). In skeletal muscle of young animals, SCR decreased by 43% the Sirt3 mRNA, which remained below control level after 4 days of refeeding (Fig. 3b). In the old rats’ skeletal muscle, neither SCR nor refeeding affected Sirt3 gene expression. SIRT3 protein expression in skeletal muscle of young rats was elevated after SCR and refeeding for 2 and 4 days by 45%, 75%, and 50%, respectively, as compared to control. However, in old rats SCR and 2-day refeeding were associated with 50% lower SIRT3 protein expression in skeletal muscle (Fig. 3d).
In cardiac muscle of young SCR rats, Sirt3 expression correlated with those of Pgc-1α (r = 0.73, p = 0.0006) and Glut4 (r = 0.94, p = 10− 6). In the cardiac muscle of old rats Sirt3 expression correlated with those of Ppar-β/δ (r = 0.65, p = 0.029) and Glut4 (r = 0.64, p = 0.048). In skeletal muscle of old SCR rats, Sirt3 expression correlated with those of Ppar-β/δ (r = 0.86, p = 0.006) and Glut4 (r = 0.88, p = 0.004).
Discussion
Our study compared transcriptional-level changes in response to SCR followed by ad libitum feeding for up to 4 days in cardiac and skeletal muscles of young and old male rats. Additionally, we assessed gene expression and protein levels of sirtuin 3 that was shown to play an important role in the control of mitochondrial lipid and carbohydrate metabolism. The results point to age-related differences in the metabolic adaptations to SCR in these two types of striated muscle tissue (Fig. 4).
Firstly, we compared young and old rats to identify the transcriptional changes evoked by aging. We found that aging of the cardiac muscle was associated with increases in Cpt-I mRNA expression and matching enzymatic activity, similarly to the results reported in old Sprague-Dawley rats (Zhao et al. 2014). On the contrary, in vastus lateralis skeletal muscle of old rats we observed decreased Cpt-I expression, though without corresponding decline in enzymatic activity. Other researchers have reported either decreased CPT-I activity in skeletal muscle of old Fischer 344 rats (Kim et al. 2009) and old men (Kaczor et al. 2006) or increased CPT-I mRNA and protein levels in the skeletal muscle of old Sprague-Dawley rats (Zhao et al. 2014). It should be noted that control of the muscle-type CPT-Ib (analysed in our study) is largely performed at transcriptional level, through fatty acid-induced activation of PPAR-β/δ in a PGC-1-dependent manner, as demonstrated in C2C12 skeletal muscle cell cultures (Dressel et al. 2003), and preferentially through PPARα in primary cultures of cardiomyocytes (Brandt et al. 1998). In contrast, the liver-type CPT-Ia has a much higher affinity to malonyl-coenzyme A, which strongly inhibits its activity (Brandt et al. 1998). Since we observed no age-related change in Ppar-β/δ expression, it is likely that the age-related changes in Cpt-I gene expression could be PPAR-β/δ-independent in both the cardiac and skeletal muscles. Because aging of skeletal muscle was associated with parallel decreases of Cpt-I and Pgc-1α mRNA levels, a lower PGC-1α availability could have contributed to the age-related reduction of Cpt-I expression.
We observed an age-related decrease of Glut4 expression in skeletal muscle, in line with other studies in rat (Larkin et al. 2001; Qiang et al. 2007) and mice (Sczelecki et al. 2014). Our finding of the age-related reduction of Pgc-1α expression, one of the transcriptional regulators of Glut4 expression (Leick et al. 2010), as well as the reduction of Sirt3, could both contribute to this result. Interestingly, in our SPF rat colony we found no difference in the blood glucose levels between young and old rats despite the fact that skeletal muscles are the major site of glucose disposal. In pulmonary hypertension rat model signalling through SIRT3 and AMPK in skeletal muscle was shown to be indispensable for the blood glucose-lowering effect of nitrite (Lai et al. 2016).
It has been shown that prolonged moderate CR without malnutrition (decrease of daily calorie intake by 25%) positively impacts human health and lifespan (Kebbe et al. 2021). However, long-term adherence to this form of therapy may be a major obstacle for the majority of human patients. A more feasible option is short-term CR (up to 6 months), which exerts positive effects beyond reduction of body weight in rats (Pires et al. 2014; Wang et al. 2016) and humans (Vion et al. 2021; Zubrzycki et al. 2018). The duration of SCR regimen in most animal studies varies from 21 days (e.g. Pires et al. 2014) to 2 months (e.g. Wang et al. 2016). With the aim of comparing sexually-mature young and old male rats, we used a 30-day SCR protocol, with additional 2 or 4 days of refeeding to mimic the effects of returning to full diet.
The SCR-associated lowering of glucose, insulin, and TG blood serum levels, as well as TG/HDL ratio in both age groups indicate that even this short-term diet may improve cardio-metabolic risk factors. SCR caused only mild increases in FFA levels, reflecting lipid mobilization from adipose tissue, which suggests that the SCR was a mild intervention. At the same time SCR decreased the serum concentrations of HDL-cholesterol, a potent anti-atherogenic, anti-oxidant and anti-inflammatory factor (Yang et al. 2019). Other authors reported no change in HDL-cholesterol level in obese Wistar rats after 28-day CR (de Castro et al. 2020) or increases after 12-week CR regimens in Wistar rats (Chen et al. 2015). The authors ascribed the HDL elevation to increased lipoprotein lipase-mediated catabolism of TG-rich lipoproteins and transfer of the resultant lipid moieties to HDL particles (Chen et al. 2015). However, HDL proteomic analyses in metabolic syndrome patients who underwent a 12-week lifestyle therapy indicated that there could be improvements in HDL function preceding any significant changes in plasma HDL levels (Mathew et al. 2018). Of note, a recent study showed that the metabolic outcomes of dietary interventions depend on the timing of food intake, since rats who were for 10 weeks intermittently fasted during the night (i.e. active phase) had reduced HDL-cholesterol levels, in contrast to those fasted during the day (Hanjani et al. 2021). Nevertheless, because the TG/HDL ratios were reduced after our SCR regimen in both studied age groups, it can be concluded that the diet resulted in overall improvement of cardio-metabolic risk.
Interestingly, we noted that SCR caused greater relative reduction of body weight in old than in young rats. Regrettably, we found no other published data directly comparing SCR in young and old animals. We hypothesise that the smaller reduction in body weight of young animals could reflect more efficient energy preservation. Alternatively, the result may be related to some degree of dehydration in old animals, despite water accessibility.
Our study proved that the aging process differently shapes the effects of SCR on the expression and enzymatic activity of CPT-I in rat cardiac and skeletal muscles. In line with the elevated TG levels, we found that SCR increased gene expression and activity of CPT-I in the cardiac muscle of young but not old rats. Moreover, we noted that SCR increased the expression of Ppar-β/δ (which activates the transcription of Cpt-I in the muscle (Dressel et al. 2003)) in cardiac and skeletal muscles of young but not old rats. However, in the skeletal muscle of both studied age groups Cpt-I expression and the enzyme activity did not change after the SCR diet. Similar results were observed in young male KKAy obese/diabetic mice after 8 weeks of 70% CR (Huang et al. 2006). On the contrary, increased abundance and activity of CPT-I in the skeletal muscle was reported in 17- and 24-month-old male Fisher 344 rats subjected to 3-week-long 60% CR (Kim et al. 2009), and in female rats fed for 27 weeks with high-fat diet and subjected for the last 7 weeks to 60% CR (Pattanakuhar et al. 2019). Thus, aging inhibits the induction of Ppar-β/δ and Cpt-I expression in response to SCR in rat cardiac muscle.
The SCR-associated decrease of blood glucose can be explained by greater glucose uptake by skeletal muscles of calorie-restricted animals (Wang et al. 2016). However, in our study in the skeletal muscle of young rats Glut4 expression was unaffected by SCR. This result falls in line with other reports showing that CR in rodents did not increase the muscle abundance of the GLUT4 glucose transporter (Argentino et al. 2005; Sequea et al. 2012; Wang et al. 1997). 20-day-long CR was shown to cause greater insulin-stimulated translocation of GLUT4 to cell surface membrane in rat epitrochlearis muscle, indicating increased insulin signalling (Dean et al. 1998). PGC-1α was reported to stimulate Glut4 expression in murine and rat cultured muscle cells and skeletal muscle (Michael et al. 2001). Our finding of increased expression of Pgc-1α upon SCR of young rats is similar to the up-regulation of Pgc-1α mRNA levels reported by Song et al. (2014) in the gastrocnemius muscle of young male Sprague-Dawley rats subjected for 8 weeks to 60% CR. In the skeletal muscle of old rats in our study, SCR exacerbated the age-related decline in Pgc-1α expression, with parallel (though insignificant) reduction of Glut4 mRNA. Interestingly, lifelong CR has been reported to increase Pgc-1α mRNA level in the skeletal muscle of old male B6D2F1 mice but not in the young and middle-aged animals, whereas PGC-1α protein expression was not affected by CR in any of the age groups (Miller et al. 2012). It should be noted that GLUT4 and PGC-1α are regulated mostly post-transcriptionally, e.g. through phosphorylation, acetylation, or interactions with other proteins (Bogan and Kandror 2010; Krämer and Handschin 2019; Rodgers et al. 2005).
In the cardiac muscle of young rats, SCR stimulated Glut4 expression, suggesting that SCR may potentiate the use of glucose in the heart of young, but not old rats. This could be achieved through the upregulation of SIRT3, whose endothelial expression was shown to regulate cardiomyocyte glucose availability in the mouse heart (Zeng et al. 2020). In support of this hypothesis, in our study the expression of Sirt3 in the heart of young SCR rats correlated with Glut4 expression. Moreover, Glut4 gene expression was induced by PGC-1α in murine C2C12 myoblasts, potentiating glucose transport into these cells (Michael et al. 2001). Both Sirt3 and Glut4 correlated with Pgc-1α mRNA levels, similarly to what was shown in mice (Wende et al. 2017). This may indicate that SCR may co-ordinately regulate these three factors affecting cardiac glucose metabolism. PGC-1α may also stimulate fatty acid oxidation in the heart (Arany et al. 2005), thus the observed Pgc-1α increase may contribute to the greater utilization of lipids, which was reported in the cardiac muscle of CR mice (Lee et al. 2002). Ageing blunted the cardiac response to SCR, as old rats showed little change in Glut4, Sirt3 or Pgc-1α expression.
SIRT3 is a key factor involved in the adaptation to nutrient stresses such as CR (van de Ven et al. 2017). We found that SCR stimulated SIRT3 gene and protein expression in the cardiac muscle of young rats. Other researchers demonstrated that induction of SIRT3 may be central to the beneficial outcome of CR, such as metabolic adaptations and antioxidant defence (Qiu et al. 2010; Tauriainen et al. 2011). Moreover, upregulation of SIRT3 by CR may potentiate the use of glucose in the cardiac muscle, as the endothelial expression of SIRT3 was shown to regulate cardiomyocyte glucose availability in the mouse heart (Zeng et al. 2020). In support of this hypothesis, in our cohort of young SCR rats Sirt3 correlated with Glut4 expression. Importantly, the intensity of CR may differently affect the cardiac SIRT3 expression, since SIRT3 protein levels in myocardium of female SpragueDawley rats increased after two months of 25% CR, but decreased after 45% CR (Yu et al. 2018). However, in our cohort of old Wistar-Han rats, SCR diminished SIRT3 induction in the cardiac muscle. Even though we did not measure SIRT3 activity, it should be noted that SIRT3 is activated through its deacetylation catalysed by SIRT1, whose levels drop upon ageing in many tissues (Kwon et al. 2017).
Differently than in the cardiac muscle, the skeletal muscle expression of Sirt3 decreased after SCR of young rats, even though SIRT3 protein levels were elevated. The results of our 4-week long CR model differ from that of 12-week-long CR, which stimulated Sirt3 gene expression in mixed-type skeletal muscle of young C57BL/6 male mice (Jing et al. 2011). As noted above, CR effect on SIRT3 levels depends on the diet intensity, and possibly genetic background of the subjects. In contrast to the young ones, in old rats SIRT3 protein was lower after SCR, though without corresponding mRNA changes. This discrepancy may be explained by the aging-related decline in the efficiency of protein synthesis or increased protein degradation (Bilski et al. 2022).
We investigated the studied regulatory factors after 2 or 4 days of refeeding to obtain more information about the changes in their expression upon transition from calorie-restricted to normal diet. Refeeding of young rats quickly reversed the SCR-induced transcriptional changes in the cardiac muscle. Only Cpt-I expression remained elevated above control level after 4 days of refeeding. Notably, in the cardiac muscle of old rats neither CR nor refeeding caused any significant transcriptional changes, with the exception of Pgc-1α expression transiently elevated upon 2-day-long refeeding. There is a scarcity of literature data on the transcriptional effects of refeeding in the cardiac muscle. Our data suggest that in the cardiac muscle of young rats, the transcriptional changes in genes regulating energy substrate utilization are not preserved upon return to normal feeding. In the cardiac muscle of old rats, the response to dietary manipulations appears to be blunted. However, we found no other reports that compared the refeeding response in various age groups of rodents.
In the skeletal muscle of young rats, refeeding reduced the studied genes’ expression below control level, corresponding to reported Pgc-1α mRNA reduction in tibialis anterior muscle of young male Sprague-Dawley rats (Zheng et al. 2012). The transcriptional changes observed in our study could contribute to the phenomenon of catch-up fat after calorie restriction, which favours disproportionate recovering of fat mass rather than lean mass in model animals and humans. It was reported that young male Sprague-Dawley rats, subjected to refeeding after 2 weeks of 50% CR, developed insulin resistance in skeletal muscles and insulin hyperresponsiveness in adipose tissue, leading to glucose redistribution from skeletal muscle to white adipose tissue (Cettour-Rose et al. 2005). With regard to old rats’ skeletal muscle, like in the cardiac muscle, the response to dietary manipulations was blunted.
We conducted our study only in male rats, since inclusion of females would require the assessment of the estrous cycle, supported by measurements of sex hormones. The results of this research cannot be extended to the female sex. Rat skeletal and cardiac muscles exhibit sexual dimorphism, which may manifest at different ages. For example, Vijay et al. (2015) reported that the cardiac expression of genes associated with oxidative phosphorylation was lower in male than in female Fisher 344 rats at 78 weeks of age, though not at 8 or 21 weeks. Moreover, analyses in isolated cardiac mitochondria showed higher mitochondrial efficiency of energy production in young female as compared to male Wistar rats (Colom et al. 2015). Another study, using DNA microarray analysis, revealed that expression of genes for myofibrillar and glycolytic proteins in skeletal muscle was higher in young male Sprague-Dawley rats compared to females, whereas females exposed to high fat diet showed greater induction of genes involved in oxidative metabolism and cellular defences (Oh and Yun 2012). The sexual dimorphism in skeletal and cardiac muscles contributes to different sex- and age-dependent outcomes of calorie restriction regimens (Bartke et al. 2019; Boldrin et al. 2017). It is therefore likely that the SCR effects revealed in our study in male rats would differently affect the skeletal and cardiac muscles in females.
In conclusion, changes in the expression of key genes involved in energy substrate utilization induced by SCR and refeeding suggest the presence of important age-related differences in the adaptive responses to nutritional challenge both in cardiac and skeletal muscles. SCR appears to increase the efficiency of glucose and fatty acid utilization in the cardiac muscle, however, this response is reduced in old rats. This may be a consequence of the aging process per se, or some other factors, such as increased adiposity of the old rats (Wronska and Kmiec 2012). The results of our study on the expression of factors controlling glucose and lipid metabolism suggest that SCR regimens might be much more effective in the young and middle-aged humans than in the elderly.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Aerni-Flessner L, Abi-Jaoude M, Koenig A, Payne M, Hruz PW (2012) GLUT4, GLUT1, and GLUT8 are the dominant GLUT transcripts expressed in the murine left ventricle. Cardiovasc Diabetol 11:63. https://doi.org/10.1186/1475-2840-11-63
Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM (2005) Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 1:259–271. https://doi.org/10.1016/j.cmet.2005.03.002
Argentino DP, Muñoz MC, Rocha JS, Bartke A, Turyn D, Dominici FP (2005) Short-term caloric restriction does not modify the in vivo insulin signaling pathway leading to Akt activation in skeletal muscle of Ames dwarf (Prop1(df)/Prop1(df)) mice. Horm Metab Res 37:672–679. https://doi.org/10.1055/s-2005-870577
Bartke A, Evans TR, Musters CJM (2019) Anti-aging interventions affect lifespan variability in sex, strain, diet and drug dependent fashion. Aging 11:4066–4074. https://doi.org/10.18632/aging.102037
Bilski J, Pierzchalski P, Szczepanik M, Bonior J, Zoladz JA (2022) Multifactorial mechanism of sarcopenia and sarcopenic obesity. Role of physical exercise, microbiota and myokines. Cells 11:160. https://doi.org/10.3390/cells11010160
Bogan JS, Kandror KV (2010) Biogenesis and regulation of insulin-responsive vesicles containing GLUT4. Curr Opin Cell Biol 22:506–512. https://doi.org/10.1016/j.ceb.2010.03.012
Boldrin L, Ross JA, Whitmore C, Doreste B, Beaver C, Eddaoudi A, Pearce DJ, Morgan JE (2017) The effect of calorie restriction on mouse skeletal muscle is sex, strain and time-dependent. Sci Rep 7:5160. https://doi.org/10.1038/s41598-017-04896-y
Brandt JM, Djouadi F, Kelly DP (1998) Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. J Biol Chem 273:23786–23792. https://doi.org/10.1074/jbc.273.37.23786
Cao M, Zhao Q, Sun X, Qian H, Lyu S, Chen R, Xia H, Yuan W (2022) Sirtuin 3: Emerging therapeutic target for cardiovascular diseases. Free Radic Biol Med 180:63–74. https://doi.org/10.1016/j.freeradbiomed.2022.01.005
Cettour-Rose P, Samec S, Russell AP, Summermatter S, Mainieri D, Carrillo-Theander C, Montani JP, Seydoux J, Rohner-Jeanrenaud F, Dulloo AG (2005) Redistribution of glucose from skeletal muscle to adipose tissue during catch-up fat: a link between catch-up growth and later metabolic syndrome. Diabetes 54:751–756. https://doi.org/10.2337/diabetes.54.3.751
Chen JH, Ouyang C, Ding Q, Song J, Cao W, Mao L (2015) A moderate low-carbohydrate low-calorie diet improves lipid profile, insulin sensitivity and adiponectin expression in rats. Nutrients 7:4724–4738. https://doi.org/10.3390/nu7064724
Cheng CF, Ku HC, Lin H (2018) PGC-1alpha as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci 19:3447. https://doi.org/10.3390/ijms19113447
Colleluori G, Villareal DT (2021) Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp Gerontol 155:111561. https://doi.org/10.1016/j.exger.2021.111561
Colom B, Oliver J, Garcia-Palmer FJ (2015) Sexual dimorphism in the alterations of cardiac muscle mitochondrial bioenergetics associated to the ageing process. J Gerontol A Biol Sci Med Sci 70:1360–1369. https://doi.org/10.1093/gerona/glu014
de Castro JM, Assumpção JAF, Stein DJ, Toledo RS, da Silva LS, Caumo W, Carraro CC, da Rosa Araujo AS, Torres ILS (2020) Nicotinamide riboside reduces cardiometabolic risk factors and modulates cardiac oxidative stress in obese Wistar rats under caloric restriction. Life Sci 263:118596. https://doi.org/10.1016/j.lfs.2020.118596
Dean DJ, Brozinick JT Jr, Cushman SW, Cartee GD (1998) Calorie restriction increases cell surface GLUT-4 in insulin-stimulated skeletal muscle. Am J Physiol 275:E957–964. https://doi.org/10.1152/ajpendo.1998.275.6.E957
Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE (2003) The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol 17:2477–2493. https://doi.org/10.1210/me.2003-0151
Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM, Zhao S, Guan KL, Denu J (2011) Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell 41:139–149. https://doi.org/10.1016/j.molcel.2011.01.002
Hanjani NA, Zamaninour N, Najibi N, Hosseini AF, Nasirinezhad F, Vafa MR (2021) The effects of calorie restriction and time-restricted feeding on IGF1 serum level and lipid profile in male Wister rats with previous obesity. Int J Prev Med 12:157. https://doi.org/10.4103/ijpvm.IJPVM_38_20
Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49:186–199. https://doi.org/10.1016/j.molcel.2012.10.024
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464(7285):121–125. https://doi.org/10.1038/nature08778
Huang H, Iida KT, Sone H, Yokoo T, Yamada N, Ajisaka R (2006) The effect of exercise training on adiponectin receptor expression in KKAy obese/diabetic mice. J Endocrinol 189:643–653. https://doi.org/10.1677/joe.1.06630
Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR (2011) Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A 108:14608–14613. https://doi.org/10.1073/pnas.1111308108
Kaczor JJ, Ziółkowski W, Antosiewicz J, Hac S, Tarnopolsky MA, Popinigis J (2006) The effect of aging on anaerobic and aerobic enzyme activities in human skeletal muscle. J Gerontol A Biol Sci Med Sci 61:339–344. https://doi.org/10.1093/gerona/61.4.339
Kanikowska D, Kanikowska A, Swora-Cwynar E, Grzymisławski M, Sato M, Bręborowicz A, Witowski J, Korybalska K (2021) Moderate caloric restriction partially improved oxidative stress markers in obese humans. Antioxid (Basel) 10:1018. https://doi.org/10.3390/antiox10071018
Kebbe M, Sparks JR, Flanagan EW, Redman LM (2021) Beyond weight loss: current perspectives on the impact of calorie restriction on healthspan and lifespan. Expert Rev Endocrinol Metab 16:95–108. https://doi.org/10.1080/17446651.2021.1922077
Kern M, Wells JA, Stephens JM, Elton CW, Friedman JE, Tapscott EB, Pekala PH, Dohm GL (1990) Insulin responsiveness in skeletal muscle is determined by glucose transporter (Glut4) protein level. Biochem J 270:397–400. https://doi.org/10.1042/bj2700397
Kim JY, Kim DH, Choi J, Park JK, Jeong KS, Leeuwenburgh C, Yu BP, Chung HY (2009) Changes in lipid distribution during aging and its modulation by calorie restriction. Age (Dordr) 31:127–142. https://doi.org/10.1007/s11357-009-9089-0
Kleiner S, Nguyen-Tran V, Baré O, Huang X, Spiegelman B, Wu Z (2009) PPAR{delta} agonism activates fatty acid oxidation via PGC-1{alpha} but does not increase mitochondrial gene expression and function. J Biol Chem 284:18624–18633. https://doi.org/10.1074/jbc.M109.008797
Krämer AI, Handschin C (2019) How epigenetic modifications drive the expression and mediate the action of PGC-1alpha in the regulation of metabolism. Int J Mol Sci 20:5449. https://doi.org/10.3390/ijms20215449
Krämer DK, Al-Khalili L, Guigas B, Leng Y, Garcia-Roves PM, Krook A (2007) Role of AMP kinase and PPARdelta in the regulation of lipid and glucose metabolism in human skeletal muscle. J Biol Chem 282:19313–19320. https://doi.org/10.1074/jbc.M702329200
Kwon S, Seok S, Yau P, Li X, Kemper B, Kemper JK (2017) Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J Biol Chem 292:17312–17323. https://doi.org/10.1074/jbc.M117.778720
Lai YC, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, St Croix CM, Garcia-Ocaña A, Goncharova EA, Tofovic SP, Mora AL, Gladwin MT (2016) SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation 133:717–731. https://doi.org/10.1161/CIRCULATIONAHA.115.018935
Larkin LM, Reynolds TH, Supiano MA, Kahn BB, Halter JB (2001) Effect of aging and obesity on insulin responsiveness and GLUT-4 glucose transporter content in skeletal muscle of Fischer 344 x Brown Norway rats. J Gerontol A Biol Sci Med Sci 56:B486–492. https://doi.org/10.1093/gerona/56.11.b486
Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA (2002) Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci U S A 99:14988–14993. https://doi.org/10.1073/pnas.232308999
Leick L, Fentz J, Biensø RS, Knudsen JG, Jeppesen J, Kiens B, Wojtaszewski JF, Pilegaard H (2010) PGC-1{alpha} is required for AICAR-induced expression of GLUT4 and mitochondrial proteins in mouse skeletal muscle. Am J Physiol Endocrinol Metab 299:E456–465. https://doi.org/10.1152/ajpendo.00648.2009
Liu HY, Zheng G, Zhu H, Woldegiorgis G (2007) Hormonal and nutritional regulation of muscle carnitine palmitoyltransferase I gene expression in vivo. Arch Biochem Biophys 465:437–442. https://doi.org/10.1016/j.abb.2007.06.026
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Mathew AV, Li L, Byun J, Guo Y, Michailidis G, Jaiswal M, Chen YE, Pop-Busui R, Pennathur S (2018) Therapeutic lifestyle changes improve HDL function by inhibiting myeloperoxidase-mediated oxidation in patients with metabolic syndrome. Diabetes Care 41:2431–2437. https://doi.org/10.2337/dc18-0049
Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM (2001) Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A 98:3820–3825. https://doi.org/10.1073/pnas.061035098
Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL (2012) A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell 11:150–161. https://doi.org/10.1111/j.1474-9726.2011.00769.x
Oh TS, Yun JW (2012) DNA microarray analysis reveals differential gene expression in the soleus muscle between male and female rats exposed to a high fat diet. Mol Biol Rep 396:6569–6580. https://doi.org/10.1007/s11033-012-1486-2
Park JS, Holloszy JO, Kim K, Koh JH (2020) Exercise training-induced PPARbeta increases PGC-1alpha protein stability and improves insulin-induced glucose uptake in rodent muscles. Nutrients 12:652. https://doi.org/10.3390/nu12030652
Pattanakuhar S, Sutham W, Sripetchwandee J, Minta W, Mantor D, Palee S, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2019) Combined exercise and calorie restriction therapies restore contractile and mitochondrial functions in skeletal muscle of obese-insulin resistant rats. Nutrition 62:74–84. https://doi.org/10.1016/j.nut.2018.11.031
Pires RC, Souza EE, Vanzela EC, Ribeiro RA, Silva-Santos JC, Carneiro EM, Boschero AC, Amaral ME (2014) Short-term calorie restriction improves glucose homeostasis in old rats: involvement of AMPK. Appl Physiol Nutr Metab 39:895–901. https://doi.org/10.1139/apnm-2013-0520
Qiang W, Weiqiang K, Qing Z, Pengju Z, Yi L (2007) Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPK alpha. Exp Mol Med 39:535–543. https://doi.org/10.1038/emm.2007.59
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. https://doi.org/10.1016/j.cmet.2010.11.015. 12:662-7
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434:113–118. https://doi.org/10.1038/nature03354
Sczelecki S, Besse-Patin A, Abboud A, Kleiner S, Laznik-Bogoslavski D, Wrann CD, Ruas JL, Haibe-Kains B, Estall JL (2014) Loss of Pgc-1α expression in aging mouse muscle potentiates glucose intolerance and systemic inflammation. Am J Physiol Endocrinol Metab 306:E157–167. https://doi.org/10.1152/ajpendo.00578.2013
Sequea DA, Sharma N, Arias EB, Cartee GD (2012) Calorie restriction enhances insulin-stimulated glucose uptake and Akt phosphorylation in both fast-twitch and slow-twitch skeletal muscle of 24-month-old rats. J Gerontol A Biol Sci Med Sci 67:1279–1285. https://doi.org/10.1093/gerona/gls085
Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM, Jacobson MP, Verdin E (2010) SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab 12:654–661. https://doi.org/10.1016/j.cmet.2010.11.003
Sithara T, Drosatos K (2021) Metabolic complications in cardiac aging. Front Physiol 12:669497. https://doi.org/10.3389/fphys.2021.669497
Song J, Ke SF, Zhou CC, Zhang SL, Guan YF, Xu TY, Sheng CQ, Wang P, Miao CY (2014) Nicotinamide phosphoribosyltransferase is required for the calorie restriction-mediated improvements in oxidative stress, mitochondrial biogenesis, and metabolic adaptation. J Gerontol A Biol Sci Med Sci 69:44–57. https://doi.org/10.1093/gerona/glt122
Tauriainen E, Luostarinen M, Martonen E, Finckenberg P, Kovalainen M, Huotari A, Herzig KH, Lecklin A, Mervaala E (2011) Distinct effects of calorie restriction and resveratrol on diet-induced obesity and Fatty liver formation. J Nutr Metab 2011:525094. https://doi.org/10.1155/2011/525094
van de Ven RAH, Santos D, Haigis MC (2017) Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol Med 23:320–331. https://doi.org/10.1016/j.molmed.2017.02.005
Vargas-Ortiz K, Pérez-Vázquez V, Macías-Cervantes MH (2019) Exercise and Sirtuins: A way to mitochondrial health in skeletal muscle. Int J Mol Sci 20:2717. https://doi.org/10.3390/ijms20112717
Vijay V, Han T, Moland CL, Kwekel JC, Fuscoe JC, Desai VG (2015) Sexual dimorphism in the expression of mitochondria-related genes in rat heart at different ages. PLoS ONE 10:e0117047. https://doi.org/10.1371/journal.pone.0117047
Vion J, Sramkova V, Montastier E, Marquès MA, Caspar-Bauguil S, Duparc T, Martinez LO, Bourlier V, Harant I, Larrouy D, Moussaoui N, Bonnel S, Vindis C, Dray C, Valet P, Saulnier-Blache JS, Schanstra JP, Thalamas C, Viguerie N, Moro C, Langin D (2021) Metabolic and cardiovascular adaptations to an 8-wk lifestyle weight loss intervention in younger and older obese men. Am J Physiol Endocrinol Metab 321:E325–E337. https://doi.org/10.1152/ajpendo.00109.2021
Wang ZQ, Bell-Farrow AD, Sonntag W, Cefalu WT (1997) Effect of age and caloric restriction on insulin receptor binding and glucose transporter levels in aging rats. Exp Gerontol 32:671–684. https://doi.org/10.1016/s0531-5565(97)00054-5
Wang P, Liu J, Li Y, Wu S, Luo J, Yang H, Subbiah R, Chatham J, Zhelyabovska O, Yang Q (2010) Peroxisome proliferator-activated receptor delta is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart. Circ Res 106:911–919. https://doi.org/10.1161/CIRCRESAHA.109.206185
Wang H, Arias EB, Cartee GD (2016) Calorie restriction leads to greater Akt2 activity and glucose uptake by insulin-stimulated skeletal muscle from old rats. Am J Physiol Regul Integr Comp Physiol 310:R449–458. https://doi.org/10.1152/ajpregu.00449.2015
Wende AR, Kim J, Holland WL, Wayment BE, O’Neill BT, Tuinei J, Brahma MK, Pepin ME, McCrory MA, Luptak I, Halade GV, Litwin SE, Abel ED (2017) Glucose transporter 4-deficient hearts develop maladaptive hypertrophy in response to physiological or pathological stresses. Am J Physiol Heart Circ Physiol 313:H1098–H1108. https://doi.org/10.1152/ajpheart.00101.2017
Wronska A, Kmiec Z (2012) Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol (Oxf) 205:194–208. https://doi.org/10.1111/j.1748-1716.2012.02409.x
Yang M, Rigdon J, Tsai SA (2019) Association of triglyceride to HDL cholesterol ratio with cardiometabolic outcomes. J Investig Med 67:663–668. https://doi.org/10.1136/jim-2018-000869
Yu W, Qin J, Chen C, Fu Y, Wang W (2018) Moderate calorie restriction attenuates age-associated alterations and improves cardiac function by increasing SIRT1 and SIRT3 expression. Mol Med Rep 18:4087–4094. https://doi.org/10.3892/mmr.2018.9390
Zeng H, He X, Chen JX (2020) Endothelial sirtuin 3 dictates glucose transport to cardiomyocyte and sensitizes pressure overload-induced heart failure. J Am Heart Assoc 9:e015895. https://doi.org/10.1161/JAHA.120.015895
Zhao L, Zou X, Feng Z, Luo C, Liu J, Li H, Chang L, Wang H, Li Y, Long J, Gao F, Liu J (2014) Evidence for association of mitochondrial metabolism alteration with lipid accumulation in aging rats. Exp Gerontol 56:3–12. https://doi.org/10.1016/j.exger.2014.02.001
Zheng J, Chen LL, Zhang HH, Hu X, Kong W, Hu D (2012) Resveratrol improves insulin resistance of catch-up growth by increasing mitochondrial complexes and antioxidant function in skeletal muscle. Metabolism 61:954–965. https://doi.org/10.1016/j.metabol.2011.11.005
Zubrzycki A, Cierpka-Kmiec K, Kmiec Z, Wronska A (2018) The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J Physiol Pharmacol. https://doi.org/10.26402/jpp.2018.5.02
Acknowledgements
The study was supported by National Science Centre (Grant No. N401 038038 to Z.K.) and the Polish Ministry of Science and Higher Education (statutory Grant ST-12 to Z.K., young scientist Grant No. MN 01–0049/08 to A.Ł.).
Author information
Authors and Affiliations
Contributions
AŁ performed the experiments, analysed the data, and prepared the first draft of the manuscript. AW conducted the experiments and data analysis, prepared the final draft of the manuscript, and the revised version of the manuscript. PW designed the primers for qPCR and evaluated the statistical analysis. ZK designed the study and contributed to data interpretation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethical approval
All animal experimental procedures had been authorized by the Local Ethical Committee in Gdansk (protocol no 11/2011) and were conducted in agreement with the institutional and European guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ławniczak, A., Wrońska, A., Wierzbicki, P. et al. Aging and short-term calorie restriction differently affect the cardiac and skeletal muscle expression of genes regulating energy substrate utilization in male rats. Biogerontology 23, 325–340 (2022). https://doi.org/10.1007/s10522-022-09965-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-022-09965-y