Abstract
We used a sub-sample from the Older Australian Twins Study to estimate the heritability of performance on three tests of language ability: Boston Naming Test (BNT), Letter/Phonemic Fluency (FAS) and Category/Semantic Fluency (CFT) Tests. After adjusting for age, sex, education, mood, and global cognition (GC), heritability estimates obtained for the three tests were 0.35, 0.59, and 0.20, respectively. Multivariate analyses showed that the genetic correlation were high for BNT and CFT (0.61), but low for BNT and FAS (0.17), and for FAS and CFT (0.28). Genetic modelling with Cholesky decomposition indicated that the covariation between the three measures could be explained by a common genetic factor. Environmental correlations between the language ability measures were low, and there were considerable specific environmental influences for each measure. Future longitudinal studies with language performance and neuroimaging data can further our understanding of genetic and environmental factors involved in the process of cognitive aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Language or verbal ability is a crucial element of good communication skills, and as with most other cognitive domains, there is often an age-associated change in some aspects of language ability (LA) in late adulthood. Compared to research focused on the LA in children and adolescents, and especially research into its genetic and environmental determinants, LA in older age has received much less attention. Examination of the relative genetic and environmental contributions to LA in older age can help to understand the determinants of aging-related change in LA, which may lead to strategies to improve LA, and in turn may enhance effective communication and promote active social engagement, the latter being a facet of successful aging (Rowe and Kahn 1997). Further, examination of the relative genetic and environmental influences on LA can help to enhance our understanding of the basis of some neurodegenerative disorders.
Seven studies that examined heritability as well as the genetic and environmental contributions to verbal ability in older adults were conducted within the Swedish Adoption/Twin Study of Ageing (SATSA). The heritability estimates obtained from the SATSA were moderate to high, ranging from 0.55 (McClearn et al. 1997) to 0.79 (Finkel et al. 2005). These studies used “Information” (a test of general knowledge), “Synonyms” (a test of word knowledge), and “Analogies” (a test of verbal reasoning ability) to index the “verbal” domain of cognitive functioning (Pedersen et al. 1992).
There are other aspects of verbal ability, such as verbal fluency and confrontation naming ability that involved lexical knowledge and lexical retrieval (Shao et al. 2014). These are sensitive to age-related cognitive decline as well as to the presence of neurodegenerative disorders such as Alzheimer’s dementia (Henry et al. 2004a). Verbal fluency, while commonly used in the clinical setting, has been relatively less examined in the context of behavioral genetics in older adults. It has often been used as a measure of executive function in research, and is considered to be associated with “fluid intelligence” (Roca et al. 2012). In twin studies of older adults, the genetic contribution to category (semantic) fluency was 37%, as reported by McGue and Christensen (2001) in the Longitudinal Study of Aging in Danish Twins (LSADT), with an average age of 80. Swan and Carmelli (2002) reported the genetic influence on letter (phonemic) fluency to be 34% in a study within the National Heart Lung Blood Institute (NHLBI), with participants’ average age 71. In an Italian twin study of older adults, with an average age of 68, the genetic contribution to verbal fluency was substantially higher than these previous studies, estimated to be 62% for letter fluency and 54% for category fluency (Giubilei et al. 2008). Findings from these twin studies have been reviewed in Lee et al. (2010).
In regard to confrontation naming, performance on this task had been shown to be strongly correlated with other aspects of verbal ability, such as reading ability, and in particular reading comprehension and reading fluency in school children (Luoni et al. 2015). The Boston Naming Test (Kaplan et al. 2001) is the most widely used test of this construct, and has been variously defined as a measure of word knowledge, word retrieval, semantic language, and verbal memory (Brouillette et al. 2011). BNT performance is significantly correlated with vocabulary, which is considered a “hold” (crystallised) test, relatively more resistant to the compromise of brain functions (Hawkins et al. 1993). In older adults, naming has been examined in studies focused on the neurocognitive functions of neurodegenerative disorders and other progressive language-associated disorders (Leyton et al. 2011). However, to our knowledge, no published study has explored the heritability or genetic influences on performance on visual confrontation naming in cognitively healthy, older adults.
While there is a substantial literature on the differences in cognitive performances between males and females, there has been very little research into sex differences in the heritability of cognitive abilities. Read et al. (2006) did not find sex differences in the heritability of cognitive abilities in the areas of “verbal”, “fluid” (intelligence), “memory”, and “speed”. In Finkel et al.’s (2006) examination of genetic and environmental contributions to five cognitive measures, the results showed sex differences in the level of performance for all measures, but only “Synonyms” (a verbal test) showed higher heritability in males than in females. Therefore, conclusions regarding sex differences in the heritability of cognitive abilities cannot be drawn from the limited information available. Sex differences in the heritability of verbal ability, as indexed by verbal fluency and naming, have not been investigated.
The aim of the present study was to explore the genetic and environmental contributions to performance in verbal or language ability tests that are sensitive to cognitive change in normal aging, that have not been commonly examined in older adults. In the context of our current study, we will refer to the performance in the three fluency and naming tests of interest as “language ability” (LA). The specific aims were firstly, to investigate the heritability of performance on three commonly used measures of LA: the Boston Naming Test (Kaplan et al. 2001), Letter Fluency Test (Benton and Hamsher 1976), and the Category Fluency Test (Goodglass and Kaplan 1987) in a cohort of older adult twins. Secondly, we aimed to examine the genetic and environmental influences on the variation and covariation between performances on the three LA measures and their genetic and environmental relationships. Thirdly, we would attempt to explore sex differences in the heritability of performances in these LA measures, to complement the limited research in this aspect of cognitive functioning.
Methods
Participants
Methodology of the Older Australian Twins Study (OATS) was previously described in detail in Sachdev et al. (2009). Briefly, participants aged 65 and above were recruited from the Australian Twin Registry, as well as through advertisements, media, and citizens’ networks. They were residents of the three Eastern states of Australia: New South Wales, Victoria, and Queensland. The inclusion criteria were: ability to consent to participate, having a co-twin who also consented to participate in the study, having completed some education in English, having at least low average intelligence (estimated IQ ≥ 80), and a Mini-Mental Status Examination (Folstein et al. 1975) score of ≥ 24. Exclusion criteria included life-threatening illness, acute psychosis, or inadequate English to complete the assessments.
There were 623 participants in Wave 1 (baseline) assessment. When the individual twins (without co-twins) and siblings were excluded, the present study sample comprised 506 individuals (253 complete twin pairs), 142 monozygotic (MZ) twin pairs and 111 dizygotic (DZ) twin pairs. Table 1 shows the sample demographics. The average age was 70 (range 65–88), average years of education was 11 years (range 6–21 years) and the estimated mean IQ was 106 (range 77–128). Two participants with estimated IQ scores below 80 (77 and 79) were included in the present sample, as their performances on cognitive testing were generally within normal limits. There was an over-representation of female participants, with an approximate ratio of 2:1. There were three pairs of twins who were born overseas, all of whom had nominated English as their first language and were able to participate in the language-based assessments in OATS.
The current study sample was larger than the OATS samples in our two previous studies on the genetic and environmental influences on processing speed and executive functions, with sample sizes of 477 individuals [218 complete pairs, plus 41 individual twins, (Lee et al. 2012a)] and 472 participants [215 twin pairs and 42 individuals (Lee et al. 2012b)], respectively. All data and information across the three studies were drawn from the baseline phase of OATS.
Measures
Participants were administered a comprehensive battery of cognitive tests, which in addition to a measure of overall estimated IQ (NART, Nelson and Willison 1991), included tests that tap into the following specific cognitive domains: attention/concentration, verbal memory, visual memory, verbal/language ability, visuo-spatial ability, executive function, and processing speed. Three measures of LA, a global measure of baseline cognitive performance, and a measure of mood state were of interest in the present study:
-
1.
Boston Naming Test (BNT, Kaplan et al. 2001)—this is a short-form of a test of visual confrontation naming. Participants were to name 30 pictures of line-drawings of everyday objects. The number of correct items spontaneously named or named after semantic cueing was the score. The test–retest reliability of this 30-item version of BNT has been reported to be 0.90 (Dong et al. 2013).
-
2.
Controlled Oral Word Association Test (COWAT, Benton and Hamsher 1976)—this test is also referred to as letter fluency test, phonological verbal fluency test, and phonemic fluency test. Participants were required to generate three word-lists, according to three designated letters (F, A, and S), 1 min for each letter, while abiding by the rules (no proper nouns, no numbers, and no derivative from words already given). This test is referred to as “FAS” from here onwards. The total number of words generated for the three letters (minus errors and repetitions) was the dependent measure.
-
3.
Category Fluency Test (CFT, Goodglass and Kaplan 1987)—this test is also known as semantic fluency test. Participants were asked to name as many “animals” as they could within 1 min. The total number of animals named within the time limit was the score. The test–retest reliability for both the COWAT and CFT in an older adult sample has been estimated to be 0.70 (Harrison et al. 2000).
-
4.
Global Cognition (GC)—This was created by combining the cognitive domain scores, formed as the average of the z-scores of the tests comprising each of the cognitive domains (attention/concentration, verbal memory, visual memory, visuo-spatial ability, executive function, and processing speed). As in our previous studies that examined the genetic and environmental influences in processing speed and executive functions (Lee et al. 2012a, b), the tests that form the cognitive domain being examined (LA in the current study) were not included in forming this composite measure.
-
5.
Geriatric Depression Scale (GDS, Yesavage 1988)—a 15-item short version of the GDS was administered to provide an index of mood state. A score of 5/15 or greater would be suggestive of depression.
Statistical analyses
Since the data for some of the variables were skewed, all variables were inverse normal transformed prior to analyses. T-tests for continuous variables for all other measures were used to compare means between MZ and DZ pairs. Equality of proportions of females between the two groups was compared using the Chi square test. All p-values were obtained using a permutation procedure with N = 10,000 (Fornito et al. 2011).
Five covariates were used in the analyses for heritability estimates as well as in multivariate genetic modelling. Age was included as a covariate because it is a recognised factor influencing most aspects of cognition (e.g. Glisky 2007). As one of our aims was to explore sex difference in the heritability of LA, sex was included as a covariate. As an individual’s educational attainment can impact on their cognitive performance (e.g. de Azeredo Passos et al. 2015), hence it was included as a covariate. As a low mood can potentially impact on verbal production and fluency (e.g. Henry and Crawford 2005), the GDS score was also included as a covariate. Global Cognition was also used as one of the covariates in some of the analyses. We have only used main effects for these covariates and no interaction terms were used in genetic modelling.
The classic twin design was used to estimate the genetic and environmental contributions to the covariation between the variables. As MZ twins are 100% genetically concordant, and DZ twins only share 50% of their genes on average, this design allows for the proportioning of variance into additive genetics (“A”) and environmental influences. The latter would either be “shared” between the twin pairs (“C”) or unique to one twin of the pair (“E”, and includes measurement error). If an MZ twin correlation is greater than the corresponding DZ twin correlation, “A” influence is suggested. If DZ correlation is more than half the MZ correlation, a “C” (shared environmental) effect is indicated.
Structural equation modelling (SEM, Neale and Cardon 1992) was used to estimate the heritability and genetic correlations. Twin SEM was conducted with different models fitted to the data using the full information maximum likelihood estimation in OpenMx (2.0.1) R package (Boker et al. 2011), making use of paired twins only. In the univariate analysis, a full Cholesky ACE model was fitted first and subsequently AE, CE and E models were fitted and compared with the Cholesky ACE model.
In a post-hoc analysis, we also examined the genetic parameters between male and female samples, using a heterogeneity model. Different set of parameters for the likelihood functions for male, female and opposite sex pairs were considered under this model. The test of homogeneity between male and female samples was examined using the likelihood ratio test, comparing the heterogeneity model against the homogeneity model with the same set of parameters for both genders. Further, to examine heritability as a function of age, the gene-environment interaction model (Purcell 2002) was fitted. In this model, age effect is incorporated in to the A, C and E path coefficients.
The genetic and environmental correlations among the three LA tests were examined using multivariate SEM. We began with the full Cholesky ACE (CholACE) model, followed by independent pathway (IP) and common pathways (CP) models. For the Cholesky, IP and CP models, we have also considered the model without the C component (AE model). The Akaike’s Information Criterion (AIC, Akaike 1987) and the p values from the − 2LL (minus two times log-likelihood) statistics were compared between nested models and the full model to assess model parsimony.
Results
Sample characteristics
As can be seen in Table 1, there were no significant differences between the MZ and DZ twins in their average age, years of education, proportion of females, or their mood status (all p values were > 0.05). Means and standard deviations of the participants’ performance on the three LA variables BNT, FAS, CFT and their GC scores are also shown here. There were no significant differences between the MZ and DZ twins on any of the measures of LA, or on their global cognitive functioning (GC).
Heritability estimates
Table 2 shows the heritability estimates and intraclass correlations under the univariate ACE model. The heritability estimate was high for FAS (0.59), moderate for BNT (0.35) and low for CFT (0.20). Education and GC were significantly associated with performance on all measures. GDS (mood) was not associated with any of the LA measures. Age was significantly associated with CFT and sex was significantly associated with BNT (Supplementary Table 1). Contributions from all the shared environment components were very small, and hence the AE model was found to be the parsimonious model. Education and GC had a significant impact on the heritability of all the LA measures, as the heritability estimates without these covariates were higher than the estimates with these covariates (Supplementary Table 2). Specifically, GC and education had a large impact on the heritability of CFT, as the estimate was doubled without these covariates. The heritability of GC, adjusted for age, sex, education and GDS, was also found to be high (0.70).
In order to investigate the possibility that FAS had a much higher heritability than CFT was due to the former being a total score of three 1-min tests, while the latter is the score of a 1-min test, the scores for the three letters were analysed individually. Overall, the heritability estimates for F (0.46), A (0.43), and S (0.43) individually were lower than the heritability of FAS total (0.59), though these remained more than twice as high as CFT (0.20), as shown in Supplementary Table 3.
Sex heterogeneity and age moderation
A sex heterogeneity model was fitted to contrast the difference in heritability estimates between the male and female samples, with age, education, mood and GC entered as covariates. As shown in Table 3, the heritability estimates were generally higher in women for all LA variables. Except for BNT (p value: 0.03), the test of homogeneity of parameters between male and female samples indicated that the heritability estimates between the two groups were similar (p value > 0.05). Though the test for homogeneity of parameters between pooled samples against separate estimates in male and female samples was rejected for BNT, there was a considerable overlap between the confidence intervals for heritability in both samples.
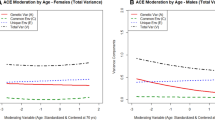
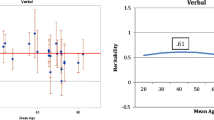
Across the age range of 65–85 years (the approximate age range in our data), the variation of heritability of the LA measures was examined using the age moderation model. The heritability of LA measures as a function of age were plotted in Fig. 1. Heritability of FAS increased with age and approached 75% at 85 years. Although there was a moderate increasing trend for CFT and decreasing trend for BNT across the age range, the heritability of both of these measures were approximately 27% at 85 years.
Multivariate modelling
The phenotypic correlation matrix, derived from the full saturated model, between traits and between twins is shown in Supplementary Table 4. The correlations of FAS with BNT and CFT were similar, and the correlation between BNT and CFT was slightly higher than their correlations with FAS. GC had high correlations (> 0.5) with the LA measures, with the highest correlation observed with CFT.
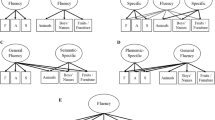
In order to obtain the genetic correlations among the three LA measures, the saturated Cholesky model, followed by IP and CP models were considered. The model fit comparisons with the Cholesky ACE model and the other models are summarised in Table 4. Comparisons of the AIC and p values for model parsimony showed that the common pathway models without the shared environment components provided the best fit for the data. The estimated path coefficients from one latent factor common pathway model (ComAE) are represented in Fig. 2.
Table 5 shows the phenotypic, genetic, and environmental correlations, using one latent factor common pathway model (ComAE). The genetic correlations amongst the LA variables were low for FAS and CFT (0.28) and for FAS and BNT (0.17). High genetic correlation was found for BNT and CFT (0.61). The environmental correlations amongst the three LA measures were all low, ranging from 0.10 to 0.16.
The heritability (additive genetic variance) of the latent factor was 0.60 and the environment variance is 0.40. The proportion of variance accounted for by the additive latent factor for BNT, FAS and CFT to the total additive variance respectively are 38, 8 and 100%. Total additive genetic variance for CFT was explained by the latent genetic factor and there was no specific genetic variance for the CFT. The proportion of variance explained by the additive genetic variance (heritability) for the three tests were 35, 59 and 21% repectively, consistent with the heritability estimates from the univariate heritability analysis.
In a separate analysis, to examine the extent of genetic and environmental correlations of GC with the three LA variables, we fitted a four variable Cholesky ACE model adjusted for age, sex, education and mood score, followed by the AE model. Under the Cholesky AE model, the genetic correlation of GC with BNT, CFT and FAS respectively were 0.62, 0.70, 0.44 and the corresponding environmental correlations were 0.19, 0.14 and 0.16.
Discussion
The primary aim of the present study was to examine the heritability of verbal performances in three commonly used LA tests in a community sample of older adults. We also aimed to explore the variation and covariation amongst performances on these LA tests and their relationships with each other. In addition, we explored the possibility of sex differences in the heritability of performances on these LA tests. A summary of our findings is displayed in Table 6.
Of the three LA tests, heritability was found to be highest for the phonemic fluency test (FAS), estimated to be 59%. This estimate can be considered similar to Giubilei et al.’s (2008) study, but much higher than the estimate reported in the all men NHLBI study (Swan and Carmelli 2002). Discrepancies between different studies could partly be explained by sex differences, as there is a trend for in the heritability estimates in our study to be higher for women for all LA tasks. The heritability estimate for category fluency (CFT) was 20% in the present study, substantially lower than that of Giubilei et al.’s (2008) study but closer to that of the LSADT study (McGue and Christensen 2001). The present study was the first to investigate heritability of confrontation naming (BNT), where a moderate heritability estimate of 35% was found.
While it may not be appropriate to make a direct comparison of the heritability estimates of our findings with those of the SATSA, it is interesting to note the greater (55–79%) genetic influences on their verbal tests (of general knowledge, word knowledge and verbal abstract reasoning) and the lower heritability (20–59%) found in our language test of fluency and naming, when fluency and naming also involved word knowledge and semantic memory. This could be further investigated in the context of “crystallised” intelligence and “fluid” intelligence, given that some of the tests used in the SATSA to represent verbal ability are considered to be associated with the former, and the language tests in our study are more representative of the latter.
Our findings on the heritability of LA performance complement two previous publications from OATS, which reported on the heritability of measures of processing speed (Lee et al. 2012a) and executive functions (Lee et al. 2012b). Comparing the heritability estimates of 35–62% for measures of speed and 29–63% for measures of executive functions, the heritability estimates of LA (20–59%) appear to be within a similar range. It should be noted that the same measure of verbal fluency (FAS) had been used in both our executive functions and the current LA studies, and their heritability estimates are similar at 62 and 59% respectively. It has been suggested that the verbal letter fluency test is a multifactorial measure of cognitive functioning. It has often been used as a measure of fronto-executive functions (Lezak et al. 2012) and letter fluency (such as FAS) has been associated with focal frontal cortical lesions (Henry and Crawford 2004). However, it has also been reported that performance in verbal fluency test is correlated with other cognitive measures such as word knowledge, auditory attention span, and memory (Ruff et al. 1997). Henry et al. (2004) have noted a language component in this task, and more recently, Whiteside et al. (2016) examined the main cognitive structure underlying two verbal fluency tests, and found that letter fluency (FAS) and category fluency (CFT) both loaded completely onto the “language” factor and not the “executive functioning” factor.
As the heritability estimates were 59, 35, and 20% for FAS, BNT, and CFT respectively, 41, 65 and 80% respectively of the variance in the performance in these three LA tests can be attributed to a combination of environmental factors and measurement error. Some potential environmental factors influencing performance in the LA measures were suggested in a discordant MZ co-twin study of this cohort (Lee et al. 2014). Specifically, greater participation in cognitive activities and better odour identification ability were associated with better performance in the LA tests, whereas poorer performance was associated with higher body mass index. It should be emphasized that these environmental factors have been identified from discordant MZ twin pairs, and there are also genetic influences in odor identification (Finkel et al. 2001), participation in cognitive activities (Lykken et al. 1993) and body mass index (Pérusse et al. 2000). In our study, the environmental correlations between the three LA measures were very low, suggesting that there is little shared environmental influence between the performances on these tests.
The phenotypic correlations between the three LA measures are low to moderately low (0.13–0.28). These may partly be explained by the fact that performance in these tasks involved different brain regions. Letter fluency (FAS) has reportedly been associated with activation in the frontal structures (Abrahams et al. 2003), and specifically for strategic word retrieval (Baldo et al. 2006). However, in the latter study of lesion symptom mapping, the temporal cortex was found to be involved in category fluency (CFT) when retrieval of words was restricted by semantics. Consistent with this view, in their meta-analysis which focused on the pattern of impairment observed following focal lesions, Henry and Crawford (2004b) argued that category fluency may impose greater demands on temporal neural substrates than letter fluency. As for confrontation picture naming (BNT), when substantial demands are placed on semantic memory and semantic knowledge, areas of the temporo-occipital cortices and the inferior frontal gyrus appear to be particularly activated (Abrahams et al. 2003). However, in a more recent study of older adults (in another cohort within our Centre), which examined cerebral gray matter correlates of the same three LA measures in our study, found that either unilateral or bilateral frontal and temporal lobes volumes were associated with performance in these three tests (Zhang et al. 2013).
The genetic correlations between the three LA measures were low to moderately low for FAS and BNT (0.17) and for CFT and FAS (0.28), but moderately high for BNT and CFT (0.61). These findings on genetic correlations, together with the results of genetic modelling, which demonstrated that one common factor could explain the covariation between performances in these three tasks, suggest that performance on these tests share the same genes or the same set of genes. In addition, under this one common factor model, there is relatively less genetic influence to the performance in CFT and BNT, with less than half of their genetic variance (none in CFT and 22% in BNT) due to genetic influence specific to itself. In contrast, for the performance on FAS, approximately half of the genetic influence was specific to itself. This disparity of high and low genetic correlations between performance on the three tests could be due to the fact that performance on both the BNT and CFT involves “semantics”, which is subserved by temporal lobe structures, and FAS is relatively more associated with frontal lobe structures, as discussed above. In addition, there appears to be relatively lower heritability for CFT and BNT, and their heritability is primarily shared through the common latent language factor. The influence of the latent language factor on FAS is comparatively low. Therefore, it would seem that performance on these three tests might have different underlying brain structures or brain networks, with BNT and CFT showing greater overlap.
One of our aims was to explore the possible effect of sex difference on the heritability of LA in older adults. A sex heterogeneity model (with age, education, mood and GC as covariates) demonstrated a trend for greater heritability estimates for women than men for all the three verbal measures, with the sex difference significant for confrontation naming (BNT: 0.19 and 0.38, for men and women respectively). If the present results are shown to be robust, the finding of sex difference in the genetic influence in language performance would have practical implications for maintaining cognitive health in older age. For instance, some intervention strategies for enhancing LA may provide more benefit for men, as there is relatively less genetic influence on LA for men than women. Nevertheless, the differences in the heritability estimates in women and men in FAS and CFT were not significant, and should only be regarded as a trend. Also, our sample has a high proportion of female and therefore, a larger sample in an independent cohort would be necessary to definitively address the question of sex difference.
Performances in CFT and BNT were shown to have large environmental contributions, at 80 and 65%, respectively (including measurement error). Level of education was a significant factor in the performance in these two tasks in our study, and higher education has been considered a factor associated with verbal fluency tasks in several studies (e.g. Tombaugh et al. 1999). As mentioned above, we have previously reported that odor identification ability, participation in cognitive activities, and body mass index are associated with language ability (Lee et al. 2014). In a study of cognitively intact older adults, social isolation and self-reported feelings of loneliness were found to be associated with greater cortical amyloid burden, an indicator of pre-clinical Alzheimer’s disease (Donovan et al. 2016). Further exploration of the mechanisms underlying these associations and other environmental factors in older age could be informative. In particular, these could be translated into interventions to improve or optimise verbal communication through amelioration of word-finding difficulty as detected in performances in the LA tests, thereby promoting social engagement and interaction, contributors to successful aging.
Our analysis using the age moderation model revealed an increase in heritability of FAS and a trend for increasing heritability of CFT with age, whereas the heritability for BNT decreases with age. These findings do not support the observation that there is a trend for heritability of cognitive functions to decrease with age in cross-sectional samples in a selective review of genetic influence on cognitive functions in older twins (Lee et al. 2010). Our findings are also incongruent with expectations from a meta-analysis of cognition across the lifespan with longitudinal twin and adoption studies (Tucker-Drob and Briley 2014), which found that genetic stability of cognition to be very low in very early life but markedly increases in childhood, with their exponential model suggesting ultimately “nearly perfect” longitudinal stability from early adulthood to beyond age of 80. Nonetheless, the heritability of CFT and BNT, both approximating 27% at age 85, is consistent with the heritability of verbal ability (as measured with other verbal tests) reported in the meta-analytic studies in Reynolds and Finkel (2015).
Findings from this study have implications for exploring genetic influences in neurodegenerative disorders. Language difficulties, such as in word-finding, impaired naming ability, and loss of semantic knowledge or memory, have commonly been reported in a clinical setting. These are also prominent features in language-associated neurodegenerative disorders, such as variants of Primary Progressive Aphasia in Fronto-temporal dementia, as well as Alzheimer’s disease. Our findings can be informative for gene discovery, in that performances in CFT and BNT are likely to share many of the same genes or the same set of genes. Performance in these LA measures can potentially be useful endophenotypes for language-associated neurodegenerative disorders, mediating between genotype and phenotype (cerebral changes). This is especially so given that it has long been recognised that clinical manifestations of cognitive decline can precede dementia, such as due to Alzheimer’s disease, by several years (Amieva et al. 2005).
It is important to acknowledge the cross-sectional nature of this study, which limits interpretation of findings in relation to causality. Future longitudinal studies are therefore needed, as these would prove informative on the possible change in genetic influence along the trajectory of cognitive aging. The fact that the fluency and naming ability examined in this study represent only some facets of language and verbal ability, limits the generalizability of our findings to other aspects of verbal ability. If there were more measures, such as vocabulary and verbal abstract reasoning ability included in this study, it is possible that more than one genetic factor would be found. It is also possible that FAS and GC, and FAS and executive functions may share some genetic variation, although we have not attempted to separate the sources of genetic variation from these factors. Future investigations with genetic modelling on performance on the three LA tests and their neuroanatomical correlates (frontal and temporal lobe structures) in this cohort of OATS would further our understanding of the relationship between genetics, brain function, and brain structure. Further exploration into sex differences in LA with a larger sample would help to clarify the difference in heritability of verbal, language functions between sexes.
References
Abrahams S, Goldstein LH, Simmons A, Brammer MJ, Williams SC, Giampietro VP, Andrew CM, Leigh PN (2003) Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp 20(1):29–40. https://doi.org/10.1002/hbm.10126
Akaike H (1987) Factor analysis and AIC. Psychometrika 52(3):317–332. https://doi.org/10.1007/bf02294359
Amieva H, Jacqmin-Gadda H, Orgogozo JM, Le Carret N, Helmer C, Letenneur L, Dartigues JF (2005) The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain 128(Pt 5):1093–1101. https://doi.org/10.1093/brain/awh451
Baldo JV, Schwartz S, Wilkins D, Dronkers NF (2006) Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc 12(6):896–900. https://doi.org/10.1017/S1355617706061078
Benton AL, Hamsher KS (1976) Multilingual aphasia examination: manual of instruction. University of Iowa, Iowa City
Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estarbook R, Kenny S, Bates T, Mehta P, Fox J (2011) OpenMx: an open source extended structural equation modeling framework. Psychometrika 76(2):306–317. https://doi.org/10.1007/s11336-010-9200-6
Brouillette RM, Martin CK, Correa JB, Davis AB, Han H, Johnson WD, Foil HC, Hymel A, Keller JN (2011) Memory for names test provides a useful confrontational naming task for aging and continuum of dementia. J Alzheimers Dis 23(4):665–671. https://doi.org/10.3233/JAD-2011-101455
de Azeredo Passos VM, Giatti L, Bensenor I, Tiemeier H, Ikram MA, de Figueiredo RC, Chor D, Schmidt MI, Barreto SM (2015) Education plays a greater role than age in cognitive test performance among participants of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). BMC Neurol 15(1):191
Dong Y, Gan DZ, Tay SZ, Koay WI, Collinson SL, Hilal S, Venketasubramanian N, Chen C (2013) Patterns of neuropsychological impairment in Alzheimer’s disease and mixed dementia. J Neurol Sci 333(1):5–8. https://doi.org/10.1016/j.jns.2013.05.011
Donovan NJ, Okereke OI, Vannini P, Amariglio RE, Rentz DM, Marshall GA, Johnson KA, Sperling RA (2016) Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry 73(12):1230–1237. https://doi.org/10.1001/jamapsychiatry.2016.2657
Finkel D, Pederson NL, Larsson M (2001) Olfactory functioning and cognitive abilities: a twin study. J Gerontol: Ser B 56(4):226–233. https://doi.org/10.1093/geronb/56.4.P226
Finkel D, Reynolds CA, McArdle JJ, Pedersen NL (2005) The longitudinal relationship between processing speed and cognitive ability: genetic and environmental influences. Behav Genet 35(5):535–549. https://doi.org/10.1007/s10519-005-3281-5
Finkel D, Reynolds CA, Berg S, Pedersen NL (2006) Surprising lack of sex differences in normal cognitive aging in twins. Int J Aging Hum Dev 62(4):335–357. https://doi.org/10.2190/C39X-9QHY-49DM-X9GJ
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Fornito A, Zalesky A, Bassett DS, Meunier D, Ellison-Wright I, Yücel M, Wood SJ, Shaw K, O’Connor J, Nertney D, Mowry BJ, Pantelis C, Bullmore ET (2011) Genetic influences on cost-efficient organization of human cortical functional networks. J Neurosci 31(9):3261–3270. https://doi.org/10.1523/JNEUROSCI.4858-10.2011
Giubilei F, Medda E, Fagnani C, Bianchi V, De Carolis A, Salvetti M, Sepe-Monti M, Stazi MA (2008) Heritability of neurocognitive functioning in the elderly: evidence from an Italian twin study. Age Ageing 37(6):640–646. https://doi.org/10.1093/ageing/afn132
Glisky EL (2007) Changes in cognitive function in human aging. In: Riddle DR (ed) Brain aging: models, methods, and mechanisms. CRC Press, Boca Raton, pp 3–20
Goodglass H, Kaplan E (1987) Boston diagnostic aphasia examination (BDAE), 2nd edn. Lea & Febiger, Philadelphia
Harrison JE, Buxton P, Husain M, Wise R (2000) Short test of semantic and phonological fluency: normal performance, validity and test-retest reliability. Br J Clin Psychol 39(Pt 2):181–191. https://doi.org/10.1348/014466500163202
Hawkins KA, Sledge WH, Orleans JF, Quinlan DM, Rakfeldt J, Huffman RE (1993) Normative implications of the relationship between reading vocabulary and Boston naming test performance. Arch Clin Neuropsychol 8(6):525–537. https://doi.org/10.1016/0887-6177(93)90053-4
Henry JD, Crawford JR (2004) A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology 18(2):284–295. https://doi.org/10.1037/0894-4105.18.2.284
Henry J, Crawford JR (2005) A meta-analytic review of verbal fluency deficits in depression. J Clin Exp Neuropsychol 27(1):78–101. https://doi.org/10.1080/138033990513654
Henry JD, Crawford JR, Phillips LH (2004) Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia 42(9):1212–1222. https://doi.org/10.1016/j.neuropsychologia.2004.02.001
Kaplan E, Goodglass H, Weintraub S (2001) Boston naming test, 2nd edn. Lea & Febiger, Philadelphia
Lee T, Henry JD, Trollor JN, Sachdev PS (2010) Genetic influences on cognitive functions in the elderly: a selective review of twin studies. Brain Res Rev 64(1):1–13. https://doi.org/10.1016/j.brainresrev.2010.02.001
Lee T, Mosing MA, Henry JD, Trollor JN, Lammel A, Ames D, Martin NG, Wright MJ, Sachdev PS (2012a) Genetic influences on five measures of processing speed and their covariation with general cognitive ability in the elderly: the older Australian twins study. Behav Genet 42(1):96–106. https://doi.org/10.1007/s10519-011-9474-1
Lee T, Mosing MA, Henry JD, Trollor JN, Ames D, Martin NG, Wright MJ, Sachdev PS, OATS Research Team (2012b) Genetic influences on four measures of executive functions and their covariation with general cognitive ability: the Older Australian Twins Study. Behav Genet 42(4):528–538. https://doi.org/10.1007/s10519-012-9526-1
Lee T, Lipnicki DM, Crawford JD, Henry JD, Trollor JN, Ames D, Wright MJ, Sachdev PS, OATS Research Team (2014) Leisure activity, health, and medical correlates of neurocognitive performance among monozygotic twins: The Older Australian Twins Study. J Gerontol B 69(4):514–522. https://doi.org/10.1093/geronb/gbt031
Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, Burrell JR, Rowe CC, Hodges JR (2011) Subtypes of progressive aphasia: application of the international consensus criteria and validation using β-amyloid imaging. Brain 134(Pt 10):3030–3043. https://doi.org/10.1093/brain/awr216
Lezak MD, Howieson DB, Bigler E, Tranel D (2012) Neuropsychological assessment (fifth edition). Oxford University Press Inc., Oxford
Luoni C, Balottin U, Rosana L, Savelli E, Salini S, Termine C (2015) Confrontation naming and reading abilities at primary school: a longitudinal study. Behav Neurol 2015:981548. https://doi.org/10.1155/2015/981548
Lykken DT, Bouchard TJ Jr, McGue M, Tellegen A (1993) Heritability of interests: a twin study. J Appl Psychol 78(4):649
McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R (1997) Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science 276:1560–1563
McGue M, Christensen K (2001) The heritability of cognitive functioning in very old adults: evidence from Danish Twins aged 75 years and older. Psychol Aging 16(2):272–280. https://doi.org/10.1037/0882-7974.16.2.272
Neale MC, Cardon LR (1992) Methodology for genetic studies of twins and families. Kluwer Academic Publishers, Dordrecht
Nelson HE, Willison JR (1991) National adult reading test (NART) test manual, 2nd edn. NFER-Nelson, Windsor
Pedersen NL, Plomin R, Nesselroade J, McClearn GE (1992) A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychol Sci 3(6):346–352. https://doi.org/10.1111/j.1467-9280.1992.tb00045
Pérusse L, Rice T, Chagnon YC, Tremblay A, Rao DC, Bouchard C (2000) A genome-wide linkage analysis of genes related to energy and macronutrient intake in the Quebec Family Study. Obes Res 8:20S-20S
Purcell S (2002) Variance components models for gene–environment interaction in twin analysis. Twin Res 5:554–571
Read S, Pedersen NL, Gatz M, Berg S, Vuoksimaa E, Malmberg B, Johansson B, McClearn GE (2006) Sex differences after all those years? Heritability of cognitive abilities in old age. J Gerontol B 61(3):P137–P143. https://doi.org/10.1093/geronb/61.3.p137
Reynolds CA, Finkel D (2015) A meta-analysis of hertitability of cognitive aging: minding the "missing heritability" gap. Neuropsychol Rev 25(1):97–112
Roca M, Manes F, Chade A, Gleichgerrcht E, Gershanik O, Arévalo GG, Torralva T, Duncan J (2012) The relationship between executive functions and fluid intelligence in Parkinson’s disease. Psychol Med 42(11):2445–2452. https://doi.org/10.1017/s0033291712000451
Rowe JW, Kahn RL (1997) Successful aging. Gerontologist 37(4):433–440. https://doi.org/10.1093/geront/37.4.433
Ruff RM, Light RH, Parker SB, Levin HS (1997) The psychological construct of word fluency. Brain Lang 57(3):394–405. https://doi.org/10.1006/brln.1997.1755
Sachdev PS, Lammel A, Trollor JN, Lee T, Wright MJ, Ames D, Wen W, Martin NG, Brodaty H, Schofield PR, OATS Research Team (2009) A comprehensive neuropsychiatric study of eldeerly twins: the Older Australian Twins Study. Twin Res Hum Genet 12(6):573–582. https://doi.org/10.1375/twin.12.6.573
Shao Z, Janse E, Visser K, Meyer AS (2014) What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol 5:772. https://doi.org/10.3389/fpsyg.2014.00772
Swan GE, Carmelli D (2002) Evidence of genetic mediation of executive control: a study of aging male twins. J Gerontol B 57(2):P133–P143. https://doi.org/10.1093/geronb/57.2.p133
Tombaugh TN, Kozak J, Rees L (1999) Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 14(2):167–177. https://doi.org/10.1016/S0887-6177(97)00095-4
Tucker-Drob EM, Briley DA (2014) Continuity of genetic and environmental influences on cognition across the life span: a meta-analysis of longitudinal twin and adoption studies. Psychol Bull 140(4):949–979. https://doi.org/10.1037/A0035893
Whiteside DM, Kealey T, Semla M, Luu H, Rice L, Basso MR, Roper B (2016) Verbal fluency: language or executive function measure? Appl Neuropsychol Adult 23(1):29–34. https://doi.org/10.1080/23279095.2015.1004574
Yesavage JA (1988) Geriatric Depression Scale. Psychopharmacol Bull 24(4):709–711
Zhang H, Sachdev PS, Wen W, Kochan NA, Crawford JD, Brodaty H, Slavin MJ, Reppermund S, Kang K, Trollor JN (2013) Grey matter correlates of three language tests in non-demented older adults. PLoS ONE 8(11):e80215. https://doi.org/10.1371/journal.pone.0080215
Acknowledgements
This research was facilitated through the Australian Twin Registry, a national research resource in part supported by a Centre of Research Excellence Grant from the NHMRC (ID No. 1079102). We thank the participants for their generous contributions to this study. We also thank and acknowledge the editorial assistance of Dr Sophia Dean in the preparation of this manuscript.
Funding
The Older Australian Twins Study was supported by the National Health and Medical Research Council (NHMRC, ID No. 401162).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
T. Lee, A. Thalamuthu, J. D. Henry, J. N. Trollor, D. Ames, M. J. Wright, P. S. Sachdev and OATS Research Team declare that they have no conflict of interest.
Ethical approval
All procedures/assessments performed in this study involving human participants were in accordance with, and approved by the Human Research Ethics Committee (HC No. 12599) of the University of New South Wales, Australia.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5).
Informed consent
Informed consent was obtained from all individual participants included in the Older Australian Twins Study.
Additional information
Edited by Deborah Finkel.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, T., Thalamuthu, A., Henry, J.D. et al. Genetic and Environmental Influences on Language Ability in Older Adults: Findings from the Older Australian Twins Study. Behav Genet 48, 187–197 (2018). https://doi.org/10.1007/s10519-018-9897-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-018-9897-z