Abstract
Curable sexually transmitted infections (STIs) affect millions of people across the world. Besides unacceptably high HIV rates, South Africa also has the highest burden of STIs in the world. The aim of the study was to investigate temporal changes in STI incidence rates using the data from ~ 10,000 women who enrolled in several HIV prevention trials, KwaZulu-Natal, South Africa (2002–2016). We particularly focused on the changes in distribution of the most influential factors and their population-level impacts on STI incidence rates over time. Characteristics of the women were compared across the study periods: 2002–2004, 2005–2008, 2009–2011, and 2012–2016. Besides multivariable Cox regression models, population attributable risks were calculated for the significant factors. Despite the significant progress in prevention, testing, and treatment programs, infection rates increased substantially from 13.6 to 20.0 per 100 person-year over the study period. Our findings provided a compelling evidence for single/non-cohabiting South African women to be the most vulnerable population who consistently and substantially contributed to increasing STI rates during the 15 years of study duration (PAR%: 44%–47%). We also highlighted the impact of women’s lack of knowledge related to their partner, using injectable contraceptives, less parity, and baseline STI positivity which were increased substantially over time. Our findings suggest that a significant proportion of STIs could be prevented by targeting single/non-cohabiting. These results may provide guidance in developing more effective STI prevention programs by targeting women at highest risk of infections and delivering more realistic messages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Curable sexually transmitted infections (STIs) affect millions of people across the world. Based on the World Health Organization (WHO) estimates, in 2016, approximately 400 million people were infected with one of the four infections including chlamydia (chlamydia trachomatis), gonorrhea (Neisseria gonorrhoea), syphilis (Treponema pallidum) or trichomoniasis (trichomonas vaginalis) (WHO, 2016; UNAIDS, 2016; Rowley et al., 2019). Besides causing significant stigma and negative social consequences, STIs are also have been shown to increase the risk of HIV transmission (Moodley et al., 2015). In addition to a biological link through genital inflammation, this association was primarily attributed to risky sexual behaviors (Chersich & Rees, 2008; Lewis, 2012; Mayer & de Vries, 2018; Mayer & Venkatesh, 2011). As a result, testing and treatment of STIs and guidelines often considered in HIV prevention programs (McClelland et al., 2007; Reynolds et al., 2006; Shin & Kaul, 2008).
Besides unacceptably high HIV rates, South Africa also has the highest burden of sexually transmitted infections including chlamydia and gonorrhea in the world (WHO, 2016; UNAIDS, 2016). After decades of research, women continue to be the most vulnerable group and remained at greater risk of infections. As the search for an effective intervention continues, testing and treatment campaigns have been intensified and expanded substantially over time (Abdool Karim et al., 2010). These nationwide efforts primarily focused on the sexual health messages to reduce condomless sex and emphasized clinical and public health benefits of early testing and treatment. However, current evidence suggests high burden of STI-related morbidities with substantial cost to reproductive health care in sub-Saharan Africa (Adachi et al., 2016; Mayer & de Vries, 2018; Mayer & Venkatesh, 2011; Moodley et al., 2015).
The aim of the current study was to identify the temporal changes in significant predictors of STI incidence rates in a large cohort of South African women. We particularly investigated how specific characteristics can contribute to trends in STI rates and attempt to understand their temporal changes and implications over time.
As the infections continue to take its toll in the region, besides identifying the most vulnerable populations, describing the temporal trends in STI prevalence and incidence rates can potentially be crucial to evaluate effectiveness of the prevention, testing and treatment programs in the region. This crucial information may also assist to highlight the changing profile of those at increased risk of STIs. Although there is an extensive research to report prevalence of STIs and their risk factors, to date, there has not been a study to investigate the temporal changes in significant predictors of STI incidence rates among South African women.
Method
Participants
We used the data from reproductive age women who consented to enrol in one of the six HIV prevention trials (2002–2016), KwaZulu-Natal, South Africa (Baeten et al., 2016; Marazzo et al., 2015; McCormack et al., 2010; Microbicide Trial Network, 2011; Padian et al., 2007; Skoler-Karpoff et al., 2008). These trials were conducted at 9-clinical sites located within 100 km radius: (1) the MIRA trial (Methods for Improving Reproductive Health in Africa) (n = 1,485) (Padian et al., 2007); (2) Carraguard Trial (n = 1,467) (Skoler-Karpoff et al., 2008); (3) MDP 301 (Microbicides Development Programme 301) (McCormack et al., 2010) (n = 2,389); (4) HPTN 035 (HIV Prevention Trials Network) 11% (n = 1,054) of the women who enrolled (Abdool Karim et al., 2010); (5) The VOICE trial (n = 2,750) (Marrazzo et al., 2015); (6) the ASPIRE trial (n = 803) of the women enrolled in (Baeten et al., 2016).
Measures
We included the characteristics if they were consistently measured in all trials. Participants’ age, marital status, total number of sex partners (in the past 3 months), using condom (at last sex), participants’ age at sexual debut, primary contraceptive method used, parity and knowledge of partner’s other partner and baseline sexually transmitted infections (STIs) positivity.
Primary Outcome and Incidence Cohorts
The primary outcome of this study was STI incidence occurred in one of the six biomedical intervention trials. Women were tested for STIs at every study visits. In order to estimate trends in STI incidence rates, data from the 15 year period were divided into four-study cohorts: 2002–2004, 2005–2009, 2010–2011, and 2012–2016. Since some of these trials were conducted in overlapping years, we used the individual-level data to allocate the study participants into one of the four consecutive study periods based on their enrolment and first infection dates (and/or end of study). We calculated total years of follow-up for each woman. Next, we ensured that participant’s enrolment and last visit dates (or date of STI positivity) occurred within the cohort that woman is allocated. Each woman was followed up at least 24 months from the date of enrolment to STI incidence or end of study (or loss to follow-up), whichever occurred first. In an ancillary analysis, we reran the same models after splitting the data into 5 and 6 STI incidence cohorts (data not shown). These analyses collectively provided a further evidence for the validity of the models presented in Table 2. We also investigated the associations between the time periods (2002–2004 (reference), 2005–2008, 2009–2011, and 2012–2016) and STI prevalence and incidence rates using logistic and Cox regression models, respectively. In this part of the analysis, models were adjusted for all the characteristics differed significantly across the time points. The Mantel–Haenszel test was used to test for trend in adjusted ORs and HRs.
Statistical Analysis
Descriptive statistics (percentages) were used to summarize the characteristics of the study population by study years. Cox proportional models were used to identify the significant predictors of the STI incidence in each time period and study period. We presented the adjusted hazard ratios (aHR) for the risk factors from a multivariable model rather than to assess their causality. We calculated the population-level impacts (PAR%) of the risk factors if they were statistically significant in adjusted models only (Wand & Ramjee, 2011) (Appendix1). In an additional analysis, we investigated the associations between the STI prevalence and incidence rates and the four time periods (2002–2004 (reference), 2005–2008, 2009–2011, and 2012–2016) using logistic and Cox regression models, respectively. Both models were adjusted for all the characteristics differed significantly across the time points. The Mantel–Haenszel test was used to test for trend in adjusted ORs and HRs. Three-dimensional intensity graphs (i.e. “heat-maps”) were presented to investigate the temporal trends in STIs by age groups across the four study periods.
Stata 14.0 (College Station, TX), SAS 9.0 (SAS Institute Inc., Cary, NC, USA) and R-package (version 3.5.3; 2019–03-11) were used to analyze the data.
Results
Women’s baseline characteristics were presented by the study periods (Table 1). We observed substantial differences in sociodemographic and sexual behaviors of the women across the study periods. Over time, study participants were increasingly younger, more likely to be single and more likely to have less than two children. The proportion of women 35 + year old was the highest in the earliest cohort compared to the latest cohort (29% vs 8%, p < 0.001), while the proportion of single women increased from 67% before 2005 to 87% in 2012–2016. Study participants who had less than two children were also steadily increased from 47 to 63% over time (p < 0.001). Our data also showed significant temporal changes in the contraceptive use, with using injectable contraceptives increasing from 39 to 76% (p < 0.001), while oral pills increased from 6 to 20%, respectively. The proportion of women who reported two or more sexual partners also increased over time from 7 to 20% (p-value < 0.001); 31% of the women indicated that their partner had no other partner(s) in 2002–2004; this proportion declined to 14% in 2012–2016. Based on the only available data, most of the women (90%) became sexually active when they were teenagers (i.e. < 20 years old). Baseline STI positivity did not change significantly over time and ranged 17% to 19% (p = 0.239).
Temporal Trends in STI Prevalence and Incidence
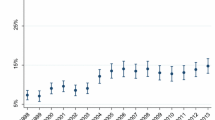
Overall, 15-year STI prevalence and incidence rates were 18% and 14.5 (95%CI 13.7, 15.3) per 100 person-year, respectively. After adjusting for all the characteristics differed significantly across the time periods (i.e. age, marital status, parity, multiple sex partners, knowledge about partner’s partner), compared to 2002–2004, the prevalence of STIs remained relatively stable over time (adjusted ORs 1.11 − 1.16, ptrend = 0.473) (Fig. 1a), while STI incidence rates increased linearly over time (adjusted HRs 1.05 to 2.04, ptrend < 0.001) (Fig. 1b). In age-stratified visual presentation, younger women had the highest STI prevalence (< 25%) (Fig. 2a) and incidence rates (30 per 100 person-year) (Fig. 2b) in all time periods.
Temporal Trends in Predictors of STI Incidence
The predictors of STI incidence in each study period were presented in Table 2. Younger women (< 35 years) were significantly more likely to have STI during the study follow-up in all four time periods with aHR:2.94 in 2002–2004, aHR: 2.44 in 2005–2008, aHR: 5.77 in 2009–2011 and aHR: 5.29 in 2012–2016. Besides younger age, being single (aHRs ranged 2.03–2.94), using hormonal injectables as a contraceptive method (aHRs ranged 1.41–1.82), having less than two children (aHRs ranged 1.43–2.14) and baseline STI positivity (aHRs ranged 2.54–3.22) were also identified as significant predictors of STI incidence in all four cohorts. We did not observe association with STI incidence rates and multiple sex partners in all time periods. However, compared to the women who reported that their partners do not have any other sexual partner, those who reported that their partner has another sexual partner(s) were significantly more likely to be at greater risk of STIs in all three cohorts except 2005–2008. Same risk factors were identified as significant predictors of STIs in all analysis when the analysis was repeated using the 5 and 6 time points (data not shown).
Temporal Trends in Population-Level Impacts of Risk Factors
Table 3 presents the population-level impacts of the significant predictors of STI incidence in each study period. Marital status was the most influential factor for STI incidence in all study periods with PAR%:47%, 44%, 47% and 46% in 2002–2004, 2005–2008, 2009–2011, and 2012–2016, respectively; this substantial impact was primarily due to the high prevalences and hazard ratios in all study periods. Impact of baseline STI positivity on STI incidence rates remained broadly stable over time without an apparent trend (PAR% ranged 21% to 29%). Population attributable risk of parity (i.e. number of children < 2) had the highest impact in 2002–2004 with 34% due to the highest prevalence; this proportion declined to 22% in 2012–2016 due to the reductions in the hazard ratios, while the partnership-related factors including women’s marital status (46%) and their knowledge whether their partner has another partner(s) or not (38%) had the highest impact in the latest time period. These five factors were collectively associated with 65%, 50%, 65% and 69% of the STI incidence rates in four study periods.
Discussion
We described temporal changes in predictors of STI incidence rates in a large cohort of South African women. After decades of research and significant progress in prevention, testing and treatment programs, we observed a substantial increase in STI incidence rates over time (13.6 per 100 person-year in 2002–2004 and 20.0 per 100 person-year in 2012–2016), while STI prevalence remained broadly similar (17% versus 18% in 2002–2004 and 2012–2016, respectively).
Women’s marital status was the most effective factor on increasing STI incidence rates in all time periods. Approximately 50% of the STIs in 2004–2004 were attributed to the single women. This high impact remained almost unchanged over time. Indicating that after many years of prevention research, single/non-cohabiting women still contribute substantially to increasing rates of infections. This impact provides evidence for substantial proportion of unprotected sex in this group. A range of factors may impact single/non-cohabiting women’s decision not to use condoms consistently including cultural/religious beliefs, self-perceived risk and intimate partner violence which are consistent with the published literature (Jewkes et al., 2010). Studies also reported a strong link between women in a stable relationship and financial stability in South Africa where single/non-cohabiting women were more likely to expose income poverty (Jewkes et al., 2004; Street et al., 2016; Wand et al., 2017). This may lead them to engage transactional and/or intergenerational sex and reduce their ability to negotiate for condom (Luke, 2003; Ott et al., 2011). These results were further confirmed by the high proportion of women who do not know their partners’ sexual partner status, which was increased substantially over time (45% in 2002–2004 to 64% in 2012–2016). In fact, this characteristic was the second most influential risk factor in the most recent cohort and associated with 38% of the STIs which exceeded that of the other three factors. Considering the other factors, namely hormonal contraceptives (i.e. injectables), women with none/one child and those who were diagnosed with at least one STIs at baseline were collectively associated with approximately 70% of the STIs during the study follow-ups. This impact was primarily due to the substantial increases in the prevalences of these risk factors over time, while their hazard ratios remained relatively stable. For example, the prevalence of using injectable contraceptives increased from 39% (earliest cohort) to 76% (recent cohort), while their relevant adjusted hazard ratios associated with this characteristic ranged from 1.41 to 1.82, respectively. Although hormonal contraceptives such as injectables are one of the most effective methods to prevent unintended pregnancies, they do not protect against sexually transmitted infections including HIV (Heffron et al., 2017; Wand & Ramjee, 2011). Similar increases were observed in the prevalence of single/non-cohabiting women over time (67% to 87%). Most importantly, these factors were more common among single/non-cohabiting women. For example, compared to those married/cohabiting, single/non-cohabiting women were more likely to: use injectable contraceptives (62% vs 44%, data not shown), have less than two children (66% vs 19% data not shown) and be diagnosed with other STIs (at baseline) (18% vs 11%, data not shown); and significantly higher proportions of single/non-cohabiting women also did not know whether their partner had another partner(s) (60% vs 45% data not shown). Taken together, our findings provide a compelling evidence for single/non-cohabiting South African women to be the most vulnerable population who contribute to increasing STI rates during the 15 years of study duration. However, in the overall study population, compared to the married/cohabiting women, distribution of condom used at last sex was significantly higher among the single/non-cohabiting women (58% versus 72% p < 0.001). In fact, women who reported using condoms at last sex were identified as at increased risk of STIs compared to those who reported that they did not use condoms at last sex in the earliest time period. Since STIs usually occur unprotected sex, these counterintuitive findings can be an indicator for high level of unprotected sex in this population which was also reported previously in African populations (Brown et al., 2012; DiClemente et al., 2013). Despite intensive condom counseling sessions at every study visit, women enrolled in these trials were unable to negotiate safe sex. Therefore, in our study population, women were more likely to overestimate their condom use to avoid further counseling and education sessions as required by the study protocol.
The current study has several limitations due to the nature of the study population which included women who participated in various HIV biomedical intervention trials with certain eligibility criteria. Therefore, they may be at increased risk of STIs compared to the general population. Baseline characteristics were self-reported as a result they would be subject to recall bias. Although these trials used similar inclusion and exclusion criteria, observations related to STI incidence and prevalence over time might be driven by differences in the trials' samples rather than true population-level changes. Despite these limitations, our findings are concurrent with the past and current studies conducted in South Africa. STI rates have steadily increased over time (Kharsanya et al., 2020). These results are consistent with the nationwide estimates in KwaZulu Natal (South African National Department of Health, 2015). Due to the high levels of labor migration, approximately 50% of the households were headed by single women (Census, 2011). Lack of marriage as well as high levels of HIV/AIDS-related deaths were also contributed to this high rates of “female headed households” (Leah, 2016; Posel & Rogan, 2009). Taken together, these results indicate low socioeconomic conditions which may increase South African women’s vulnerabilities in the region.
Our combined data provided a unique opportunity to estimate STI incidence rates across the four consecutive cohorts among South African women. As there are no routine STI surveillance programs to monitor the infections in South Africa, our results can provide the crucial estimates for the number of STI infections and changing the profile of the most influential factors to identify those at highest risk of infection over time. Although our study has common elements with the previous research, it is unique in several ways; this is the first study (1) to estimate temporal trends in risk factors and their population-level impacts of on STI incidence rates using a large cohort of women, and (2) to show single/non-cohabiting women to be the most vulnerable population for the infections.
Conclusion
Given the significant progress in prevention, testing and treatment programs in the region, it is important to investigate the temporal changes in STI incidence rates. During the 15-years of research, single/non-cohabiting women were consistently identified to be the most vulnerable group with the highest risk of infections. These results suggest that significant proportion of the STIs could be prevented by targeting single/non-cohabiting and certain partnership characteristics. Despite decades of efforts to reduce unprotected sex, social and cultural values influence individuals’ decisions not to engage unprotected sex. Therefore, effective strategies should be developed by targeting those at highest risk of STIs. These strategies should focus on social and cultural influences on sexual practices aimed at increasing women’s knowledge for safer sexual practices.
References
Adachi, K., Nielsen-Saines, K., & Klausner, J. D. (2016). Chlamydia trachomatis infection in pregnancy: The global challenge of preventing adverse pregnancy and infant outcomes in Sub-Saharan Africa and Asia. BioMed Research International. https://doi.org/10.1155/2016/9315757.
Baeten, J.M., Palanee-Phillips, T., Brown, E.R., Schwartz, K., SotoTorres, L.E., Govender, V., Mgodi, N.M., Kiweewa, F.M., Nair, G., Mhlanga, F., Siva, S., Bekker, L.G. for the MTN-020-ASPIRE Study Team. (2016). Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. New England Journal of Medicine. https://doi.org/10.1056/NEJMoa1506110.
Brown, J. L., Sales, J. M., DiClemente, R. J., Salazar, L. F., Vanable, P. A., Carey, M. P., Brown, L. K., Romer, D., Valois, R. F., & Stanton, B. (2012). Predicting discordance between self-reports of sexual behavior and incident sexually transmitted infections with African American female adolescents: Results from a 4-city study. AIDS and Behavior, 16(6), 1491–1500.
Census. (2011). Statistical release. Statistics South Africa. 2012 (p. 14) Retrieved from http://www.statssa.gov.za.
Chersich, M. F., & Rees, H. V. (2008). Vulnerability of women in southern Africa to infection with HIV: Biological determinants and priority health sector interventions. AIDS, 22, S27–S40.
DiClemente, R. J., Swartzendruber, A. L., & Brown, J. L. (2013). Improving the validity of self-reported sexual behavior: No easy answers. Sexually Transmitted Diseases, 40, 111–112. https://doi.org/10.1097/OLQ.0b013e3182838474.
Dunkle, K. L., Jewkes, R. K., Brown, H. C., Gray, G. E., McIntryre, J. A., & Harlow, S. D. (2004). Transactional sex among women in Soweto, South Africa: Prevalence, risk factors and association with HIV infection. Social Science and Medicine, 59(1581–1592), 39.
Heffron, R., Parikh, U. M., Penrose, K. J., Mugo, N., Donnell, D., Celum, C., Mellors, J. W., & Baeten, J. (2017). Objective measurement of inaccurate condom use reporting among women using depot medroxyprogesterone acetate for contraception. AIDS and Behavior, 21, 2173–2179. https://doi.org/10.1007/s10461-016-1563-y
Jewkes, R. K., Dunkle, K., Nduna, M., & Shai, N. (2010). Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: A cohort study. Lancet, 376, 41–48.
Joint United Nations Program on HIV/AIDS (UNAIDS). (2016). Global AIDS update. Geneva, Switzerland. Retrieved from https://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf
Karim, Q. A., Karim, S. S. A., Frohlich, J. A., Grobler, A. C., Baxter, C., Mansoor, L. E., Kharsany, A. B., Sibeko, S., Mlisana, K. P., Omar, Z., & Gengiah, T. N. (2010). Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science, 329(5996), 1168–1174.
Kharsanya, A. B. M., McKinnona, L. R., Lewisa, L., Cawoodd, C., Khanyiled, D., Masekoe, D. V., & Toledog, C. (2020). Population prevalence of sexually transmitted infections in a high HIV burden district in KwaZulu-Natal, South Africa: Implications for HIV epidemic control. International Journal of Infectious Diseases, 98(2020), 130–137. https://doi.org/10.1016/j.ijid.2020.06.046.
Leah, G. (2016). The mercurial piece of the puzzle: Understanding stigma and HIV/AIDS in South Africa. SAHARA-J. Journal of Social Aspects of HIV/AIDS, 13(1), 8–16.
Lewis, D. A. (2012). Detection and management of acute HIV infections in patients with sexually transmitted infections: A window of opportunity for HIV prevention within South Africa? Southern African Journal of Epidemiology and Infection, 27(4), 149–155.
Luke, N. (2003). Age and economic asymmetries in the sexual relationships of adolescent girls in sub-Saharan Africa. Studies in Family Planning, 34(2), 67–86.
Marrazzo, J. M., Ramjee, G., Richardson, B. A., Gomez, K., Mgodi, N., Nair, G., Palanee, T., Nakabiito, C., Van Der Straten, A., Noguchi, L., & Hendrix, C. W. (2015). Tenofovir-based preexposure prophylaxis for HIV infection among African women. New England Journal of Medicine, 372(6), 509–518.
Mayer, K. H., & de Vries, H. (2018). HIV and sexually transmitted infections: Responding to the “newest normal.” Journal of the International AIDS Society, 21(7), e25164.
Mayer, K. H., & Venkatesh, K. K. (2011). Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. American Journal of Reproductive Immunology, 65, 308–316.
McClelland, R. S., Sangaré, L., Hassan, W. M., Lavreys, L., Mandaliya, K., Kiarie, J., Ndinya-Achola, J., Jaoko, W., & Baeten, J. M. (2007). Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. Journal of Infectious Diseases, 195(5), 698–702.
McCormack, S., Ramjee, G., Kamali, A., Rees, H., Crook, A. M., Gafos, M., Jentsch, U., Pool, R., Chisembele, M., Kapiga, S., Mutemwa, R., Vallely, A., Palanee, T., Sookrajh, Y., Lacey, C. J., Darbyshire, J., Grosskurth, H., Profy, A., Nunn, A., & Weber, J. (2010). PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): A phase 3, randomised, double-blind, parallel-group trial. Lancet, 376, 1329–1337.
Microbicide Trials Network (MTN). (2011). MTN statement on decision to discontinue use of Tenofovir gel in VOICE, a major HIV prevention study in women. http://www.mtnstopshiv.org/ node/3909. Accessed 19 December 2019.
Moodley, D., Moodley, P., Sebitloane, M., Deepak, S., McNaughton-Reyes, H. L., Groves, A. K., & Maman, S. (2015). High prevalence and incidence of asymptomatic sexually transmitted infections during pregnancy and postdelivery in KwaZulu Natal, South Africa. Sexually Transmitted Diseases, 42(1), 43–47. https://doi.org/10.1097/OLQ.0000000000000219.
Ott, M. Q., Bärnighausen, T., Tanser, F., Lurie, M. N., & Newell, M. L. (2011). Age-gaps in sexual partnerships: Seeing beyond ‘sugar daddies.’ AIDS, 25, 861–863.
Padian, N. S., van der Straten, A., Ramjee, G., Chipato, T., de Bruyn, G., Blanchard, K., Shiboski, S., Montogomery, E. T., Fancher, H., & Cheng, H. (2007). Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: A randomised controlled trial. Lancet, 370, 251–261.
Posel, D., & Rogan, M. (2009). Women, income and poverty: Gendered access to resources in post-apartheid South Africa. Agenda, 81, 25–34.
Reynolds, S. J., Risbud, A. R., Shepherd, M. E., Rompalo, A. M., Ghate, M. V., Godbole, S. V., Joshi, S. N., Divekar, A. D., Gangakhedkar, R. R., Bollinger, R. C., & Mehendale, S. M. (2006). High rates of syphilis among STI patients are contributing to the spread of HIV-1 in India. Sexually Transmitted Infections, 82(2), 121–126.
Rowley, J., Vander Hoorn, S., Korenromp, E., Low, N., Unemo, M., Abu-Raddad, L. J., Chico, R. M., Smolak, A., Newman, L., Gottlieb, S., & Thwin, S. S. (2019). Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bulletin of the World Health Organization, 97(8), 548–562.
Shin, L., & Kaul, R. (2008). Stay it with flora: Maintaining vaginal health as a possible avenue for prevention of human immunodeficiency virus acquisition. Journal of Infectious Diseases, 197, 1355–1357.
Skoler-Karpoff, S., Ramjee, G., Ahmed, K., Altini, L., Plagianos, M. G., Friedland, B., Govender, S., De Kock, A., Cassim, N., & Palanee, T. (2008). Efficacy of Carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet, 372, 1977–1987.
South African National Department of Health (DOH). (2015). National Antenatal Sentinel HIV & Syphilis Survey Report. Retrieved from https://www.hst.org.za/publications/NonHST%20Publications/Antenatal_Sentinel_survey_Report2012_final.pdf
Street, R., Reddy, T., & Ramjee, G. (2016). The generational effect on age disparate partnerships and the risk for human immunodeficiency virus and sexually transmitted infections acquisition. International Journal of STD & AIDS, 27, 746–752.
Wand, H., & Ramjee, G. (2011). Combined impact of sexual risk behaviors for HIV seroconversion among women in Durban, South Africa: Implications for prevention policy and planning. AIDS and Behavior, 15(2), 479–486.
Wand, H., Reddy, T., Naidoo, S., Moonsamy, S., Siva, S., Morar, N. S., & Ramjee, G. (2018). A simple risk prediction algorithm for HIV transmission: Results from HIV prevention trials in KwaZulu Natal, South Africa (2002–2012). AIDS and Behavior, 22(1), 325–336. https://doi.org/10.1007/s10461-017-1785-7.
World Health Organization. (2016). Global health sector strategy on sexually transmitted infections 2016–2021. Towards ending STIs. Report No. WHO/RHR/16.09. Geneva: Author. Retrieved from http://www.who.int/reproductivehealth/publications/rtis/ghss-stis/en/
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Ethical approval for the trials, including all study protocols and informed consent forms, was received from the University of KwaZulu-Natal Biomedical Research Ethics Committee and the South African Medical Research Council Ethics Committee as well as the various study-specific Institutional Review Boards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1: Population Attributable Risk
Appendix 1: Population Attributable Risk
We estimated population attributable risk (i.e. \(PAR\%\)) for the factors in the combined study population using a methodology which can handle complex and correlated nature of the several risk factors in a multivariable model setting (Wand & Ramjee, 2011) where \(PAR\%\)( 95% CIs) were estimated by combining the prevalence of an exposure and its strength with STI incidence (i.e. HRs). For a binary risk factor:
\({\text{HR}}\) is the hazard ratio, \(p\) is the prevalence of the risk factor in the population and \(s\) indexes the two strata determined by the value of the risk factor. In multi-factorial setting:
where \({\text{HR}}_{s}\) and \(p_{s}\), \(s = 1,...,S\), are the hazard ratios and the prevalence of an exposure in the population for the \(s\) th combination of the risk factors.
where \(t\) denotes a stratum of unique combinations of levels of all background risk factors which are not modifiable and/or not under study, \(t = 1,...,T,\) and \(HR_{2t}\) is the hazard ratio in combination \(t\) relative to the lowest risk level, where \(HR_{2,1}\) = 1.
Rights and permissions
About this article
Cite this article
Wand, H., Ramjee, G. & Reddy, T. Quantifying Vulnerabilities of Single Women and Sexually Transmitted Infections in South Africa (2002–2016): Is It Getting Better (or Worse)?. Arch Sex Behav 50, 3237–3245 (2021). https://doi.org/10.1007/s10508-021-01998-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10508-021-01998-7