Abstract
The objective of this study was to estimate the joint impact of demographic and sexual risk behaviors on HIV acquisition. A total of 2,523 HIV seronegative women were recruited through three community based studies in Durban, South Africa. Point and interval estimates of partial population attributable risk (PAR) were used to quantify the proportion of HIV seroconversions which can be prevented if a combination of risk factors is eliminated from a target population. More than 80% of the observed HIV acquisitions were attributed to five risk factors: lack of cohabitation, frequency of sex, sexually transmitted infections (STIs), incidence of pregnancy and not being employed/no income. Structural factors such as minimizing migratory patterns by ensuring cohabitation of partners, access to treatment of STIs, income generation and safe sex negotiation skills are likely to play an important role in future prevention strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
South African women continue to be the group comprising the largest proportion of new HIV infections [1]. Unprotected heterosexual contact is the single most important risk factor for acquiring HIV in this region. Particularly, KwaZulu-Natal (KZN), largest provincial population in South Africa is the region most in need of an effective HIV prevention method for women [2]. The current HIV incidence in KZN estimated as 3.4 per 100 person-years (95% Confidence Interval (CI) 3.1, 3.7) [3]. In addition to the high-risk sexual behaviors, studies examining risk factors for HIV incidence have found independent associations with increased number of partners; and sexually transmitted infections including chlamydia, syphilis, gonorrhea and herpes simplex virus type 2 [4].
Previously, we used standard epidemiological methods to estimate the relative risks (RRs) of various factors associated with HIV infection by using the data from a cohort of women in Durban, South [5]. Although risk factor analyses may be well-suited to assessment of causality, they do not convey information about the potential impact on disease occurrence by eliminating these risk factor(s). Additional information is necessary to determine the public health implications of risk factor reduction/elimination and population health prevention priorities. The population attributable risk (PAR) provides information as a quantitative assessment of the potential reduction in disease incidence if the risk factor in question were eliminated from the population. It was first formulated by Levin [6] for one exposure at two levels and generalized to the multi-factorial setting [7]. PAR provides information about the public health significance of potentially modifiable risk factor(s) on a disease by accounting for both the strength of the association on the outcome, as well as the prevalence of the risk factor in the population. Identification of measures of attributable risk may assist in guiding policy and prioritizing prevention strategies by understanding the potential impact of interventions that target specific risk factor(s). Reduction or elimination of common risk factors with large relative risks may lead to substantial reductions in the burden of disease associated with HIV infection.
The primary aim of this paper is to extend the method of Spiegelman et al. [8] to estimate PAR for time-to-event data. Briefly, we developed point and interval estimation for the PARs associated with modifiable risk factors and assessed their relative contributions to time to HIV seroconversion. The combined effect of these risk factors was also estimated and compared to the impacts of individual risk factors. In a multifactorial disease setting, at least some key risk factors such as age and sex are not modifiable. This limits the practical utility of the full PAR which is based on modification of all variables of interests. Therefore we derived and used partial PAR which kept unmodifiable variable(s) unchanged. In our analysis, we also addressed the relationship between various risk behaviors and the potential effectiveness of different prevention strategies. Prevention strategies do not only differ in the kinds of behaviors they address, but also with respect to the sub-populations on which they focus.
After more than 20 years of risk factor research, now, we have a complete understanding of how HIV is acquired. The current challenge is to interpret risk factor analysis in terms of prioritizing prevention strategies. The identification and quantification of potentially modifiable risk factors is much more relevant to focus potential prevention strategies. To address these issues, we estimated PAR, i.e. the proportion of HIV seroconversions in the study population attributable to the factors such as demographic, socio–economic, sexual behaviors and clinical when they were considered separately and combined.
Methods
Study Population
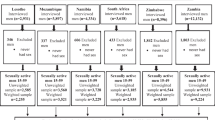
We combined data of 5,748 sexually active women who consented to screening for three studies in Durban, KZN: Methods for Improving Reproductive Health in Africa (MIRA) trial of the diaphragm for HIV prevention (September 2002–September 2005; undertaken in Umkomaas and Botha’s Hill, southern Durban) [9]. The Microbicides Development Programme (MDP) Feasibility Study in Preparation for Phase III Microbicide Trials (August 2002–December 2004; Tongaat and Verulam, northern Durban) [10], and the HIV Prevention Trials Network (HPTN) Site Preparedness study for Future Implementation of Phase2/IIb/III clinical trials (rural district of Hlabisa, and urban Durban) [11]. A total of 2,523 women who were HIV negative and eligible were included in this study.
All study populations have previously been described elsewhere [9–11]. For the MIRA trial and HPTN 055 trials, HIV diagnostic testing was performed using two rapid tests on whole blood sourced from either finger-prick or venipuncture: Determine HIV-1/2 (Abbot Laboratories, Tokyo, Japan) and Oraquick (Orasure Technologies, Bethlehem, PA, USA). The Abbot IMX ELISA test (Abbot Diagnostics, Africa Division) was used for HIV diagnosis during the MDP Feasibility study.
STI testing was accomplished by similar means in all studies. For the MIRA trial: chlamydia and gonorrhea were assessed using PCR (Roche Pharmaceuticals, Branchburg, NJ, USA); syphilis by rapid plasma reagin (RPR) in combination with Treponema pallidum haemagglutination (TPHA) (Randox Laboratories, Crumlin, UK); HSV2 by ELISA (FOCUS Diagnostics, Cypress, CA, USA); and Trichomonas vaginalis by PCR (Roche Pharmaceuticals, Branchburg, NJ, USA). For the MDP feasibility study: chlamydia and gonorrhea were assessed using PCR; syphilis by RPR; HSV2 by ELISA; and bacterial vaginosis, Trichomonas vaginalis by Gram stain, KOH and Whiff test, and wet mount microscopy. For the HPTN feasibility study: chlamydia and gonorrhea were assessed using the BD Probe Tec ET assay (Becton–Dickinson, MD) or ELISA (Murex Biotech) or culture where the assay was not available; syphilis was assessed using RPR plus either TPHA or microhaemagglutination assay-Treponema pallidum (MHA-TP); and Trichomonas vaginalis, KOH and Whiff test, and Gram stain.
Study participants were recruited from the general population in Durban and rural communities in Hlabisa. Family health clinics and health centers were the primary recruitment centers in Umkomaas, Botha’s Hill, Tongaat and Verulam. Briefly, the main eligibility criteria were similar for all studies and included: being sexually active (an average of at least four sex acts per month); HIV negative status at screening; willingness to provide written consent and follow study procedure; not pregnant with intention to maintain non-pregnant status; and residence in and around the study area for a minimum of 1 year. Questions regarding unsafe sex such as condom use, frequency of sexual act were also consistent across the studies. At all visits, participants received risk reduction counseling and access to condoms. Women who were HIV-positive at screening were referred to local health care facilities for care and support. Women who seroconverted during the trials remained in the study and were provided with ongoing counseling. All protocols and informed consent forms were approved by the Biomedical Research Ethics Committee (BREC) at the University of KwaZulu-Natal.
Statistical Analysis
Proportional hazards regression models of HIV seroconversion were used on the discrete time scale of monthly and quarterly visits. With this approach, the behavior variables were assessed at the same time that the blood was drawn for seroconversion testing, and refer to the previous 1-month (for monthly visits) or 3-month time period. Additionally, since the behavioral variables were time-varying, the values of these covariates could change from one 1-month interval (for monthly visits) or 3-month interval (quarterly visits) to the next.
The primary outcome was incident HIV infection defined as time from enrollment to seroconversion, on the basis of a discrete time scale determined by an individual’s monthly/quarterly visit. For women who seroconverted, the time of seroconversion was defined as the time of first positive HIV test result. For cases in which one or more visits were missed in the intervals between the last negative and first positive tests, the time of seroconversion was assumed to be the visit containing the midpoint between these two time points.
Among covariates under consideration: age (≤24, 25–34, 35+), district (urban, semi-urban, rural), education level (less than high school vs. at least high school), employed/earning income (yes/no), district (rural, semi-rural, urban), cohabitation status (single/not cohabitating vs. married/cohabitating), baseline and on study condom used (last seven days) (yes/no), using any contraception (yes/no), frequency of sex acts (last seven days), diagnoses with STIs (chlamydia, gonorrhea, syphilis or Trichomonas vaginalis) (baseline and follow up) and pregnancy (follow-up) were measured consistently across the studies.
To accommodate missing data, we applied the last-value-carried forward convention to the time-varying covariates except for indicators for the pregnancy and STIs. The site preparedness studies didn’t have an intervention, and the randomized trial did not find a statistically significant effect on risk of HIV infection [9, 11].
Assessment of Modifiable and Non-Modifiable Risk Factors
Age groups, not being employed/having no income, lack of cohabitation, frequency of number of sex act (last seven days), diagnoses with STIs (baseline and follow up) and pregnancy (follow-up) were all significantly associated with increased risk of HIV seroconversion in univariate analysis [5]. The current study classified these risk factors as modifiable (by public health intervention) and non-modifiable (even theoretically) and/or background risk factors (all persons similarly exposed or potential confounders for the HIV transmission). Among the risk factors, frequency of sex was classified as a modifiable risk factor. Generally, since diagnoses with STIs and pregnancy occur with unprotected sex, they were assumed to be an indication of inconsistent condom use and considered as theoretically modifiable risk factors. Among the socio–economic factors, cohabitation and employment/income status were also classified as potentially modifiable risk factors. Age at infection was considered to be non-modifiable background risk factor and assumed to be unchanged. All decisions regarding classifications of the risk factors were made prior to conducting the analyses.
Estimation of Relative and Population Attributable Risks
To evaluate associations between potential risk factors and HIV seroconversion, we calculated the hazard ratios and 95% confidence intervals (CIs) for HIV seroconversion using Cox regression models. Results with a P < 0.05 were considered to be significant.
The method for calculating the PAR is based on estimated individual risk factors and their various combinations by using Cox regression models for the incidence rate of HIV seroconversion. Prevalences for the combinations of the risk factors were estimated as multinomial probabilities from the person-time under follow up in the cohort as the empirical fraction of person-time of follow-up among cohort members at each unique level. The principle of the approach for PAR was to determine the joint impact of several theoretically modifiable risk factors on HIV transmission while keeping non-modifiable and/or background risk factors unchanged. Further details are given in Appendix. Analyses were performed using SAS statistical software, version 9 (SAS Inc, Cary, NC, USA).
Results
Baseline characteristics of the study populations were similar in terms of age and sexual risk behaviors (data not shown). The median age (inter-quartile range (IQR)) of the 2,523 participants was 28 (22–36) years and 39% were 24 years of age or younger. Almost 80% of them were not employed (or not earning income). The majority of women (58%) were single/not cohabiting and 70% of them were residing in a rural area (data not shown). Over two-thirds (69%) reported at least three sexual acts in the last seven days. The majority of women (88%, data not shown) reported using condom consistently in the last seven days. At baseline, 12% of them were diagnosed with at least one sexually transmitted infections (STIs) (chlamydia, gonorrhea, syphilis or Trichomonas vaginalis), 41% reported using contraception. Approximately, one third (32%) of them were diagnosed with at least one STIs and 22% of them had positive pregnancy test prior to the seroconversion.
Risk Factors and Their Joint Impacts on HIV Seroconversion
A total of 211 HIV seroconversions were observed during the follow-up with an overall incidence rate of 6.6 per 100 women-years. Lack of cohabitation, average of three or more sex acts (in the seven days) had higher rates of HIV seroconversion (7.8 and 6.4 per 100 women-years respectively). In addition to the high-risk sexual behaviors, demographic and socio–economic variables such as younger age (<35 years), not being employed/having no income, as well as clinical variables such as being diagnosed with STIs at baseline, incidence of STIs and pregnancy were also significantly associated with HIV seroconversion. Table 1 provides the frequency distributions and hazard ratios for these factors. The present study categorized all these risk factors as potentially modifiable (at least theoretically) risk factors according to the literature and data at hand. Age at infection was considered to be non-modifiable (even theoretically) background risk factor and left unchanged. Table 2 presented estimates of the proportion of HIV seroconversions attributable to the five theoretically modifiable and one unmodifiable risk factors which were determined to be related with increased risk of HIV seroconversion. In the overall study population, two factors, namely, lack of cohabitation and reporting average of three or more sexual acts in a week were accounted for 64% (95% CI 0.57,0.72) of the seroversions. The proportion increased to 71% (95% CI 0.65, 0.77) when the, STIs (baseline/on study) were considered. Finally current analysis suggested that 82% (95% CI 0.78–0.86) of the HIV seroconversions were attributed to the five risk factors listed in Table 2 when age remain unchanged in the target population.
Table 3 presents the frequency distributions and hazard ratios for the risk factors of interests stratified by three age categories: ≤ 24, 25–34 and 35 years or older. Lack of cohabitation, frequency of sex were significantly associated with increased risk of seroconversion among women under the age of 35 years old, while not being employed/having no income showed increased risk (not significant) for those older than 35 years of age. Incidence of pregnancy and STIs were consistently associated with increased risk of HIV seroconversions in all age groups. Age-specific theoretical prevention strategies were presented in Table 4. According to this, lack of cohabitation accounted for 41% (95% CI 0.27–0.58) and 64% (95% CI 0.54–0.73) of the infections for women younger than 25 years of age and women aged between 25 and 34 years respectively. The high prevalence and high hazard ratios of cohabitation status (85% and 1.85 and 54% and 4.41 for women younger than 25 years and between 25–34 years of age respectively [Table 3]) were responsible for this impact among women. Mean while not being employed/having no income appeared to have the largest potential impact on the risk of HIV seroconversion among women who were 35 years or older (PAR p =43%, 95% CI 0.23–0.65). Women older than 25 years of age were similar in terms of impact of incidence of pregnancy (PAR p =15%, 95% CI 0.09–0.24 and PAR p =15%, 95% CI 0.08–0.28 for 25–34 years and 35 + years old respectively). On study pregnancy only accounted for 6% (95% CI 0.03–0.13) of the seroconversions in youngest age groups. Average of three or more sexual acts in last seven days had the highest reduction among the women younger than 25 years and 25–34 years old compared to the women older than 35 years of age (PAR p=23%, 95% CI 0.14–0.36; PAR p=18%, 95% CI 0.08–0.36 and PAR p=5%, 95% CI 0.01–0.37 respectively).
Discussion
Our analyses suggest that majority of HIV seroconversion is attributed to single/not cohabitating women, reported to have more frequent sex and had high incidence of STIs and pregnancy. Our analyses also suggest that to have a very substantial effect on HIV prevention, a range of risk factors particularly related with unsafe sex need modifying. The most efficient use of scarce resources in reducing HIV infections will require complex balancing between the PAR for a given risk factor(s), the efficacy of interventions to modify the risk factor, and the cost of these interventions.
Our findings indicate that overall more than 80% of the observed cases of incident HIV infection among women are associated with five modifiable and one unmodifiable risk factors. Current study failed to determine inconsistent condom use as a predictor for HIV infection. However, generally, since STIs and particularly pregnancy can only occur with unprotected sex, these two risk factors can give hard evidence for high rate of inconsistent condom use. Although, number of male sexual partners was not collected in this study, however, being single/not cohabiting combined with high frequency of sexual acts gives strong evidence for those women having multiple partners as well as possibly engaging in transactional sex.
If these results are applied to the underlying population, majority of the cases among women could potentially have been prevented by effective public health interventions, particularly measures aimed at reducing the frequency of unprotected sex through intensive and aggressive condom counseling with couples and keeping couples together through changes in labor migrating patterns whereby the family unit moves with the job holder. Employers need to take cognizance of individual needs in order for disease prevention as well reducing the impact of HIV at the workplace.
Further analyses illustrated the age-specific impacts of risk factors on HIV transmission. Overall, high-risk sexual behaviors played important roles in HIV infection among the younger women, while socio–economic characteristic such as not being employed/not having income was the single most important factor for those 35 years or older. Results implied that improving socio–economic conditions for women along with low-risk sexual behaviors may reduce the infections considerably. There is an urgent need to focus on new HIV prevention intervention strategies that will not only address the needs of women but encourage safe sex behavior among couples.
Our study has some limitations that need to be considered in the interpretation of our results. First, because of the nature of the research conducted in these trials, populations selected were known to be at moderate-to-high risk of HIV infection. Although, we were able to target women from different communities in different settings (rural, semi-rural and urban), the women in this study may not necessarily representative of women in KwaZulu-Natal province. Second, we cannot completely rule out the possibility that our findings may be due in part to unmeasured characteristics such as multiple or concurrent sex partners and commercial sex. Lastly, no data concerning migration were included in these analyses. We were also unable to collect any sexual behavior data from male partners of the women. Nevertheless, the models were adjusted for the high risk sexual behaviors such as frequency of sex which can be used as “proxy” for multiple partners/commercial sex. STIs and pregnancy are the evidence for unprotected sex therefore; it is not clear whether they are acting as true risk factors for HIV acquisition or whether they are a proxy for having more unprotected sex. Nonetheless, baseline STIs were also associated with increased risk of HIV infection.
In this study, we used the partial PAR where both the hazard ratios and population prevalences were estimated from the same cohort study. In order to interpret a PAR as the proportion of cases caused by a risk factor and thus that could be prevented by its elimination from the population, causality needs to be proven. PARs are usually estimated for well-established risk factors whether causality is proven or not. For more speculative risk factors, PAR estimates can be regarded as measuring potential impact on disease incidence and the potential reduction in disease incidence that could be attained from their elimination were they later proven to be causal. The relative risks for the background risk factors can typically be most validly estimated in a well designed epidemiologic cohort study, for the evaluation of public health interventions it is often of greatest interest to estimate the joint prevalences of the risk background factors and exposures in a more general population to which these interventions may be applied. Although our estimates should be interpreted with caution, overall they point to a potential explanation for the excess HIV incidence observed. In light of the potential impact on HIV transmission, more comprehensive prevention strategies based on these risk factors and their prioritization, as well as further exploratory studies evaluating their role in HIV transmission are needed. The 95% confidence intervals for the partial PARs estimated under different prevention scenarios and for each risk factors took fully into account all sources of variability in the estimates given in Tables 2, and 4, both from estimating the hazard ratios as well as from estimating the prevalence of the risk behaviors. Despite the limitations outlined above, we present a robust methodology for calculating quantitative epidemiological measures of disease burden that provides policy makers and health service administrators with an important tool to prioritise health services and prevention strategies.
References
UNAIDS. Report on the global AIDS epidemic. 2008. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. Accessed 18 Nov 2008.
Coovadia H, Jewkes R, Barron P, Sanders D, McIntyre D. The health and health system of South Africa: historical roots of current public health challenges. Lancet. 2009;374(9692):817–34. doi:10.1016/S0140-6736(09)60951-X.
Barnighausen T, Tanser F, Newell ML. Lack of a decline in HIV incidence in a rural community with high HIV prevalence in South Africa, 2003–2007. AIDS Behav. 2009;25(4):405–9.
Ramjee G, Williams B, Gouws E, et al. The impact of incident and prevalent herpes simplex virus-2 infection on the incidence of HIV-1 infection among commercial sex workers in South Africa. J Acquir Immune Defic Syndr. 2005;39:333–9.
Ramjee G, Wand H, Whitaker C, et al. Women and HIV incidence—is it too late in Kwazulu Natal, Durban, South Africa. (unpublished article, under the review).
Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9(3):531–41.
Walter SD. The distribution of Levin’s measure of attributable risk. Biometrika. 1975;62:371–4.
Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18(5):571–9.
Padian NS, van der Straten A, Ramjee G, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007;370:251–61.
Gappoo S, Naidoo S, Ramjee G, Guddera V, Raju E. HSV2 prevalence amongst women participating in HIV prevention studies in rural communities in Durban, South Africa—urgent need for microbicide product to be active against HSV2 [Abstract AB14]. Microbicides 2006 conference. Durban, South Africa. 2006.
Ramjee G, Kapiga S, Weiss S, et al. The value of site preparedness studies for future implementation of phase 2/IIb/III HIV prevention trials: experience from the HPTN 055 study. J Acquir Immune Defic Syndr. 2008;47:93–100.
Acknowledgments
The authors would like to thank the sponsors of the MIRA trial (Gates Foudation) and PI Dr Nancy Padian, The division of AIDS (NIH) for their support for the HPTN 055 Study. Cliff Kelly and Ben Masse the statisticians for the HPTN 055 study, THE MDP feasibility PI and Clinical trials Unit (CTU), Dr Sheena McCormack and Prof Andrew Nunn the MRC (UK) and DIFID for financial support of the feasibility study.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The PAR is formulated as a function of hazard ratio (HR) (s) and the prevalence (p)(s) of the risk factor(s). When there is only one risk factor at two levels (1 vs. 0)
Where HR is the hazard ratios, p is the prevalence of the risk factor in the population and sindexes the two strata determined by the value of the risk factor. Equation 1 can be generalized to the multi-factorial setting when there are more than one risk factors at multiple levels, as
where HR s and p s , s = 1,…, S, are the hazard ratios and the prevalences in the target population for the sth combination of the risk factors. Full PAR (PAR F ) can be estimated by using Eq. 2 and interpreted as the percent reduction expected in the number of HIV seroconversion if all the known risk factors were eliminated from the target population.
In a multifactorial disease setting, at least some key risk factors such as age and sex are not modifiable. This limits the practical utility of the full PAR which is based on modification of all variables of interests. In an evaluation of a preventive intervention in a multifactorial disease setting, the interest is in the percent of cases associated with the exposures to be modified, when other risk factors, particularly non-modifiable, exist but do not change as a result of the intervention. Therefore we derived and used partial PAR which kept unmodifiable variable(s) unchanged.
Under the assumption of no interaction between the modifiable and non-modifiable risk factors of interest, the partial PAR(PAR p ) is formulated as
where t denotes a stratum of unique combinations of levels of all background risk factors which are not modifiable and/or not under study, t = 1, …, T and HR 2t is the hazard ratio in combination t relative to the lowest risk level, where HR 2, 1=1. As previously, s indicates a risk factor defined by each of the unique combinations of the levels of the modifiable risk factors, that is, those risk factors to which the PAR p applies, s = 1,…, S, and HR 1s is the relative risk corresponding to combinations relative to the lowest risk combination, HR 1,1 = 1. The joint prevalence of exposure group s and stratum tis denoted by p st , and\( p_{.t} = \Upsigma_{s = 1}^{S} p_{st} \). The PAR p represents the difference between the number of cases expected in the original cohort and the number of cases expected if all subsets of the cohort who were originally exposed to the modifiable risk factor(s) had eliminated their exposure(s) so that their relative risk compared to the unexposed was 1, divided by the number of cases expected in the original cohort.
Derivation of the Variance of PAR
Where
where
\( {\mathbf{HR}}_{1} = \left({HR_{1,1}, HR_{1,2}, \ldots, HR_{1,s}} \right)^{\prime} \) and \( {\mathbf{HR}}_{ 2} = \left({HR_{2,1}, HR_{2,2}, \ldots, HR_{2,T}} \right) \) are the vectors of the hazard ratios corresponding to the modifiable and unmodifiable risk factors respectively. Under the proportional hazards model, \( HR_{1s} = e^{{\beta^{\prime}_{1} x_{s} }} \) where \( x_{s} \) is the vector of values of binary indicators corresponding to the sth combination of modifiable exposure variables, of which there are S combinations, and \( HR_{2t} = e^{{\beta^{\prime}_{2} x_{t} }} \) where \( x_{t} \) is the vector of values of the tth combination of unmodifiable background risk, of which there are T combinations.
The PAR estimates for individual risk factors and combination of risk factors based on a multiplicative model therefore the may total more than that 100%. Consequently, they can be interpreted as estimating the relative importance of individual and combination of risk factors.
Rights and permissions
About this article
Cite this article
Wand, H., Ramjee, G. Combined Impact of Sexual Risk Behaviors for HIV Seroconversion Among Women in Durban, South Africa: Implications for Prevention Policy and Planning. AIDS Behav 15, 479–486 (2011). https://doi.org/10.1007/s10461-010-9845-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-010-9845-2